Abstract
We present two cases of clinically extensive bilateral DVTs associated with inferior vena caval thrombosis. Young patients presenting with symptoms of DVT should be investigated not only to establish any thrombophilic pre-disposition, but to ascertain the proximal extent of thrombus which may itself influence treatment.
Keywords: Deep vein thrombosis, inferior vena cava thrombus, retroperitoneal haematoma, congenital malformation
INTRODUCTION
Thrombosis of the inferior vena cava (IVC) has similar aetiological factors to lower limb deep venous thrombosis (DVT)1. Hypercoagulability related to haematological or neoplastic abnormalities, venous stasis secondary to extraluminal pressure from tumours or inflammatory processes and vessel injury due to trauma have all been implicated as primary mechanisms in the pathophysiology of IVC thrombosis1.
We present two cases of spontaneous bilateral IVC thrombosis in previously active young men. Case-A was a 23-year old patient who presented with an IVC / bilateral iliac vein thrombosis thought to be secondary to a retroperitoneal haematoma of benign origin. Case-B was a 25-year old patient diagnosed with a significant IVC / bi-iliac / bi-femoral venous thrombus secondary to a previously undiagnosed congenital IVC malformation.
CASE SERIES
Patient A
A 23-year old male student presented with a 1-week history of severe lower back pain initially felt when climbing stairs. The pain radiated to both thighs, being worse on the right side. He had associated anorexia, nausea, night sweats and mild diarrhoea. He had been previously fit and healthy with a history of mild asthma. There was no significant family history and he was a non-smoker.
On examination, he was haemodynamically stable but had a temperature of 38.2°C. Cardio-respiratory examination was unremarkable and his abdomen was soft with mild lower abdominal tenderness. There was tenderness in the medial aspect of each thigh and no evidence of erythema, oedema or trauma. Haematological analysis confirmed a mild normocytic anaemia with haemoglobin 11.3 g/dl and mean corpuscular volume of 95 fl. The lactacte dehydrogenase level was elevated at 661 U/L and the C-reactive protein was markedly elevated at 210 mg/L. All other haematological, coagulation, and biochemical analyses were normal. Plain X-rays of the chest, abdomen and lumbar spine were normal.
A CT (computerised tomography) scan of the abdomen and pelvis revealed a 5.5 × 4.7cm mass in the right retroperitoneum located anterior to and indenting the psoas muscle between the IVC and the right kidney. The adjacent IVC was compressed, and inferior to this, the IVC and bilateral iliac veins were significantly distended and probably thrombosed. Numerous collateral vessels were demonstrated within the pelvis and on the right side of the abdomen. A single 5mm aorto-caval node was also identified. The initial differential diagnosis included a lymphoma or connective tissue tumour. CT-guided biopsies of the mass confirmed the presence of skeletal muscle, haematoma and normal caval wall. Repeat biopsies demonstrated the same with no evidence of lymphoma or sarcoma. An ultrasound of the testes was normal. Tumour markers carcinoembryonic antigen (CEA) and alpha feto-protein (AFP) were normal at less than 4 ng/ml and 10 kU/L respectively.
Doppler ultrasound confirmed bilateral common femoral vein thrombus. An IVC venogram via the right jugular vein demonstrated occlusion of the IVC inferior to the right atrium. Magnetic resonance imaging (MRI) suggested that the retroperitoneal mass was a haematoma which had been compressing the adjacent IVC. MRI also demonstrated intraluminal thrombus extending proximally up to the confluence of the hepatic veins immediately inferior to the right atrium with distal extension to the femoral veins bilaterally (Figures 1a & b). Thrombophilia screen did not reveal any abnormality.
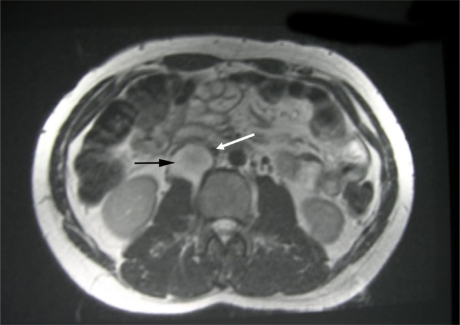
Fig 1a.
T2-weighted axial MRI demonstrating the mass (predominantly high signal) in the right retroperitoneal space anterior to psoas muscle between the IVC and right kidney (Black Arrow) compressing the overlying IVC (White Arrow).
Fig 1b.
Coronal MRI post-gadolinium enhancement showing the retroperitoneal lesion with a high signal rim (Black Arrow).
The patient was treated conservatively with subcutaneous low molecular weight heparin followed by oral warfarin and the application of compression hosiery. Subsequent MRI imaging demonstrated complete resolution of the mass and return of full patency of the IVC at 4-months (Figure 2). It remains unclear whether the IVC thrombus was preceded by the haematoma or vice versa. It was felt on balance that treatment should be directed towards the thrombus, especially in view of the early scans indicating speedy resolution of the haematoma. His bilateral lower limb pain resolved at an early stage and the patient remains well two years later with regular vascular and haematological clinical review. Warfarin was discontinued after one year. Subsequent haematological evaluation did not reveal any thrombophilic predisposition.
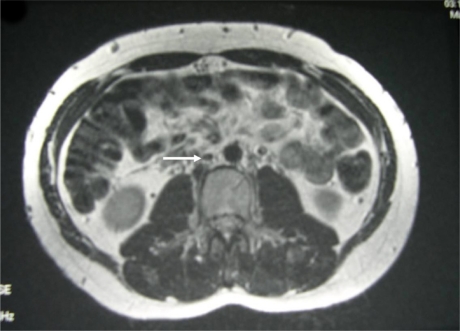
Fig 2.
T2-weighted axial MRI comparable in position and image acquisition to Figure 1a demonstrating complete resolution of haematoma and IVC (White Arrow) without thrombus after 4-months of oral anticoagulation therapy.
Patient B
A 25-year old male office employee was admitted with a 3-day history of recent onset low back pain radiating to both thighs and bilateral lower limb swelling. The pain increased in severity over the preceding 48-hours and resulted in the patient having difficulty mobilising. There were no precipitating factors. He was a non-smoker with no other co-morbidities or significant family history.
On examination, he had a low-grade pyrexia of 37.8°C with a pulse rate of 110 beats/min and blood pressure of 128/73 mmHg. He had significant lower back discomfort and bilateral lower limb swelling with pitting oedema extending to above the inguinal ligaments. Venous distension was visible on the lower anterior abdominal wall. Both lower limbs appeared dusky in the supine position with further exacerbation of the dark discolourisation on standing. Bilateral lower limb arterial examination was normal. Both lower limbs were tender on palpation. Haematological analysis showed a platelet count of 93 × 109/L, white cell count of 11.0 × 109/l, C-reactive protein of 92 mg/l and a d-dimer >20 μg/ml (reference range 0.01-0.5). Bilirubin was 76 μmol/l and lactacte dehydrogenase was elevated at 566 U/L. All other haematological and biochemical analyses were normal. Plain X-rays of the chest, abdomen and lumbar spine were normal.
Doppler ultrasound of the femoral veins demonstrated marked expansion of both vessels with intra-luminal thrombus. A CT scan of the chest/abdomen/pelvis revealed atypical venous anatomy where the IVC appeared slit-like between the hepatic and renal segment associated with marked dilatation of the infra-renal IVC, both common iliac veins and both external iliac veins. MRI imaging confirmed the CT findings and revealed a well developed collateral pathway through lumbar, azygous, hemi-azygous and subcutaneous anterior abdominal wall veins suggestive of long-standing caval obstruction (Figures 3 and 4). MRI also demonstrated IVC stenosis between the renal and hepatic segments, with a large thrombosed tortuous left renal vein, and no evidence of haematoma (Figure 5). The superficial renal portion of the IVC was narrowed thereby consistent with a congenital malformation of the IVC. A transthoracic echocardiogram did not reveal any intra-cardiac or aortic root anomaly. Thrombophilia screens, anti-cardiolipin antibodies, serum electrophoresis, direct Coomb's, auto-immune, complement, anti-neutrophil and immunoglobulin screens were normal.
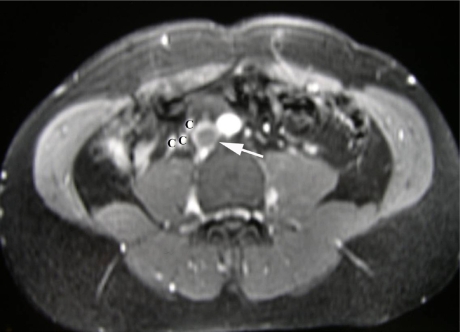
Fig 3.
T2-weighted MRI demonstrating iliofemoral thrombosis extending proximally into the infrarenal vena cava (White Arrow) with extensive collateralisation (C) around the upper retroperitoneum.
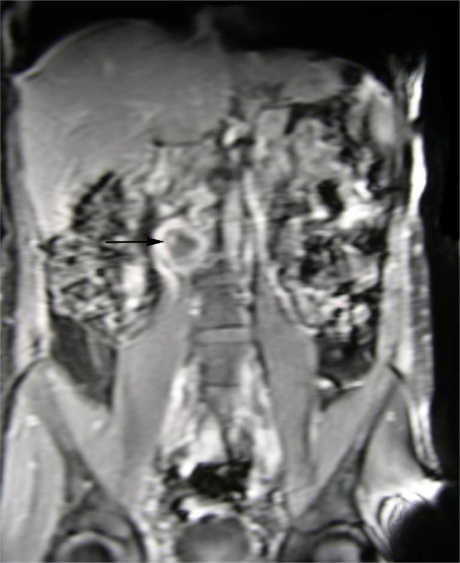
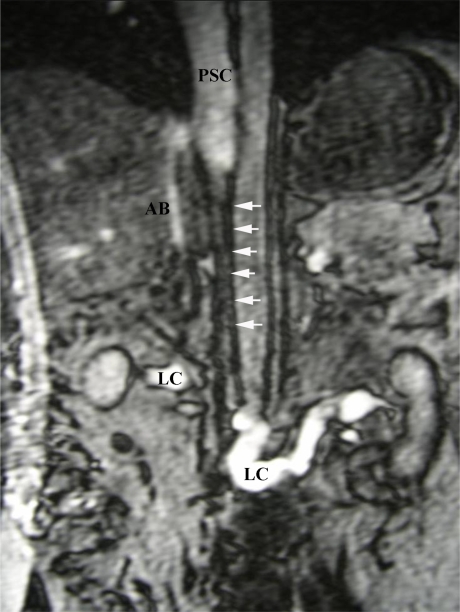
Fig 4.
Coronal gradient echo MRI showing atresia of IVC between renal and hepatic segments (Sequential White Arrows) with a patent hepatic and suprahepatic IVC (PSC). Extensive, well developed collateralisation through ascending lumbar veins, azygous system and anterior abdominal wall subcutaneous veins (LC).
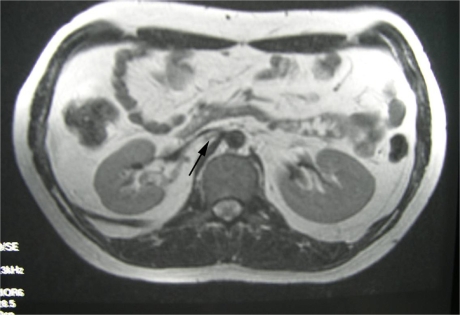
Fig 5.
T2-weighted axial MRI showing apparent stenosis of IVC at renal level (Black Arrow).
The patient was commenced on low-molecular weight heparin and after 72-hours of treatment, his symptoms had significantly improved and his platelet count had normalised. The lower limb swelling resolved weeks later, aided by compression hosiery. The patient remains well 12-months later and is to continue with life-long warfarin.
DISCUSSION
Although the lifetime incidence of venous thrombosis is 0.1%, it still remains a rare condition especially in patients below 30 years of age2-4. Predisposing factors include alterations in blood flow (stasis), injury to the vascular endothelium and abnormalities in the constitution of blood hypercoagulability (Virchow's Triad)5. Endothelial damage is invariably an acquired phenomenon whereas hypercoagulability may result from both congenital and acquired risk factors (especially in the peri-operative period). The classical presentation of IVC thrombus varies according to the level of the thrombosis with up to 50% of patients presenting with bilateral lower extremity swelling and dilatation of superficial abdominal vessels. Whilst some patients remain asymptomatic, lower back pain, nephrotic syndrome, hepatic engorgement, cardiac failure and pulmonary embolus have also been described6. Tsuji et al reported a series of 10 patients where 40% were pyrexic at presentation with an associated elevation in d-dimer levels and inflammatory markers (white cell count, C-reactive protein)7. The majority of these classical features were present in both our patients, however only the second patient had lower limb swelling.
Idiopathic IVC thrombosis is extremely rare. Chikaraishi et al described a case of apparent idiopathic IVC thrombosis in a 57-year old woman who presented with chest pain secondary to pleurisy with a background history of pyelonephritis but no other pro-thrombotic risk factors8. Kaneko et al described a further idiopathic IVC thrombosis in a 73-year old man who presented with a pulmonary embolism complicated by bronchopneumonia. There were no specific thrombotic risk factors except for age and dehydation9. Following exclusion of May Thurner syndrome and other pathologies, it is possible that in Patient A's circumstances the thrombus was an idiopathic primary event, and the surrounding retroperitoneal haematoma a secondary phenomenon. Idiopathic spontaneous retroperitoneal haematoma has also been previously described10-12. The work of Chia et al and the initial investigative work-up of Patient-A in this series demonstrate the difficulties associated with a diagnosis of a retroperitoneal haematoma10. The condition presents a significant diagnostic challenge both due to its similarity to more sinister pathologies, and the requirement to achieve a tissue diagnosis. This distracts the clinician from allowing time for the “Mass” to resolve and the less sinister nature of the underlying pathology to reveal itself. We hypothesise that in the case of Patient-A, trauma to the iliopsosas muscle from the simple act of climbing stairs caused the retroperitoneal haematoma which led, via mass effect, to compression of the IVC, hence intraluminal thrombus.
Shrestha et al described an endemic variant of IVC thrombosis in Nepalese patients leading to hepatic venous outflow obstruction (HVOO) which caused obstruction or stenosis of the IVC hepatic segment near the cava-atrial junction13. Ostia of one or more of the hepatic veins were commonly occluded. This chronic disease is characterised by upper abdominal pain, hepatosplenomegaly, ascites with a high protein content and dilated superficial veins in the trunk having cephalad blood flow. Previously, this variation of IVC thrombosis was endemic in Japan, however it is now only seen in developing countries, most notably Nepal13-15. This condition was originally thought to be caused by a congenital vascular malformation due to the observation of a membrane within the obstructing lesion14. Considerable evidence now suggests an acquired infective aetiology which results in thrombophlebitis leading to thrombus formation and a subsequent fibrotic stenosis15. Organisms thought to be involved are staphalococcus aureus and gram-negative enteric organisms with the resultant bacteraemia causing a transient protein-S deficiency15.
The IVC is created by the fusion of three sets of paired veins, specifically the posterior cardinal, subcardinal and supracardinal veins during weeks six to eight of embryonic development16 It is failure of these paired veins to fuse into a unilateral right–sided venous system which leads to an anomalous IVC16. Congenital malformations or interruptions of the IVC are unusual with a 0.3% to 0.6% prevalence in the general population17. These interruptions or absence of the IVC are usually limited to the intrahepatic segment and include an interruption in the IVC with azygos and hemi-azygous continuation, the transposition or duplication of the IVC, circum-aortic venous rings, and a retroaortic left renal vein16. The prevalence of these defects increases to 2% in patients with other congenital cardiovascular defects such as dextrocardia, transposition of the great vessels, pulmonary artery stenosis and a single atrium17. Co-existent visceral anomalies include situs inversus, polysplenia, asplenia and hypoplasia of the kidney7.
Caval aberrancy has been reported to occur in 5% - 16.2% of young patients presenting with DVT.5,18-20. A lower limb DVT is three to eight times more frequent on the left side, whereas bilateral iliofemoral thromboses are uncommon, occurring in less than 10% of cases21. However in the presence of caval aberrancy, bilateral iliofemoral thrombosis, has been reported in 66-75% of patients5,22. An aberrant IVC may also remain asymptomatic as alternative pathways of collateralisation develop through the azygous / hemiazygous and portal circulations to counteract venous stasis, as identified in the second patient7,23. Raju et al state that common iliac vein patency is the crucial link to the rich potential collateralisation via the retroperitoneal venous network which is usually only of importance in embryological development. Concurrent occlusion of the common iliac(s) as well as the IVC, with distal extension of thrombus leads to clinical symptomatology in patients with both normal and abnormal IVC anatomy23.
Anomalies of the IVC are also linked to thrombophilia disorders such as Factor V Leiden, prothrombin gene mutation, low protein S levels, high homocysteine concentration, methylenetetrahydrofolate reductase gene mutation and antiphospholipid antibodies18,24-28. It is unclear whether IVC thrombosis is purely related to an anomalous IVC or whether an interaction between an anatomical abnormality and thrombophilia tendency is required22. Gayer et al state that further research is required to assess this interaction due to considerations regarding anticoagulant therapies and treatment durations.
Recent advances in the utilisation of ultrasound, CT and MRI imaging as well as endovascular procedures have resulted in an increase in detection rates of IVC anomalies, as well as the incidental discovery of such abnormalities during unrelated investigations, therapeutic endovascular or surgical procedures7. Contrast venography remains the standard for diagnosis of IVC thrombosis with a low false-positive rate and the advantage of access for immediate treatment if required. However, it is an invasive procedure associated with a 2%-10% incidence of post-procedural DVT1. Duplex ultrasound scanning has become an accurate non-invasive method of diagnosing IVC thrombosis and is often the first-line investigative modality1. However, duplex USS is operator dependant and can be limited by body habitus or the presence of bowel gas and may occasionally fail to identify any IVC anomaly1,29. CT imaging is a rapid non-invasive method which can accurately diagnose and assess the extent of thrombus as well as delineate any associated abdominal or pelvic abnormality1. MRI imaging is now replacing CT as the optimal investigative tool avoiding radiation and giving more accurate delineation of thrombus as well as any IVC anomaly. MRI is also used to follow-up patients to determine morphological changes in the thrombus following therapy30.
Treatment options in the case of IVC thrombus without anatomical variance include anticoagulation, mechanical thrombectomy, systemic thrombolytic therapy, transcatheter regional thrombolysis, pulse-spray pharmacomechanical thrombolysis and angioplasty1,31. There is no specific literature describing the ideal duration of anticoagulation in these instances, however, case evidence identifies a trend toward treatment for a minimum of one year with the interplay of hypercoagulability disorders needing to be factored into any decision. Surgical reconstruction of the IVC and bypass of an aberrant section are both recognised modalities reserved for the most severe cases and are associated with morbidity and mortality risk32. Endovascular stent placement in combination with angioplasty is recommended in the cases of residual stenosis and chronic IVC occlusion32.
In the case of IVC thrombus associated with an aberrant IVC, with no other predisposing factors, treatment involves anti-coagulation. The duration of this treatment is widely debated with no extensive literature to provide an evidence-based approach. Dean et al take a similar view to us, that a caval anomaly is a permanent risk factor for venous stasis and thrombosis and that anticoagulant treatment should be lifelong21.
CONCLUSION
IVC thrombosis is associated with a significant acute and chronic morbidity. A high index of suspicion is warranted for IVC thrombus in the young patient with lower back and limb pain, swelling of the lower limbs, dilatation of superficial abdominal veins, with a concurrent rise in inflammatory markers and pyrexia. In these cases, further investigational modalities are mandatory following ultrasonic identification of an ileo-femoral thrombosis especially when bilateral. CT or preferably MRI imaging, are required to delineate IVC anatomy and ascertain proximal extent of the thrombus. Although invasive therapeutic modalities exist, long-term and commonly life-long anticoagulation is often required.
Acknowledgments
Written informed patient consent was obtained from the patient for the publication of this study.
The authors have no conflict of interest.
REFERENCES
- 1.Giordano P, Weber K, Davis M, Carter E. Acute thrombus of the inferior vena cava. Am J Emerg Med. 2006;24(5):640–6. doi: 10.1016/j.ajem.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Anderson FA, Wheeler HB, Golberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism, The Worcester DVT study. Arch Intern Med. 1991;151(5):933–8. [PubMed] [Google Scholar]
- 3.Nordstrom M, Lindblad B, Bergqvist D, Kjellstrom T. A prospective study of the incidence of deep-vein thrombosis with a defined urban population. J Intern Med. 1992;232(2):155–160. doi: 10.1111/j.1365-2796.1992.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosendaal FR. Thrombosis in the young: epidemiology and risk factors, a focus on venous thrombosis. Thromb Haemost. 1997;78(1):1–6. [PubMed] [Google Scholar]
- 5.Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol. 2001;114(4):878–80. doi: 10.1046/j.1365-2141.2001.03025.x. [DOI] [PubMed] [Google Scholar]
- 6.Jackson BT, Thomas ML. Post-thrombotic inferior vena caval obstruction. A review of 24 patients. Br Med J. 1970;1(5687):18–22. doi: 10.1136/bmj.1.5687.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji Y, Inoue T, Murakami H, Hino Y, Matsuda H, Okita Y. Deep vein thrombosis caused by congenital interruption of the inferior vena cava – a case report. Angiology. 2001;52(10):721–5. doi: 10.1177/000331970105201010. [DOI] [PubMed] [Google Scholar]
- 8.Chikaraishi T, Kobayashi S, Harada H, Komaki T, Koyanagi T. Idiopathic and spontaneously regressing thrombus in right renal vein and inferior vena cava. Int J Urol. 1997;4(1):83–5. doi: 10.1111/j.1442-2042.1997.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko T, Higuchi A, Takii T, Ishigatsubo Y. A case of pulmonary thromboembolism due to idiopathic thrombosis of inferior vena cava, which was initially misdiagnosed as pneumonia. [Japanese] Nihon Kokyki Gakki Zasshi. 2003;41(11):636–40. [PubMed] [Google Scholar]
- 10.Chia SH, Torosian MH. Spontaneous pelvic hematoma simulating neoplasm: case report and literature review. Oncol Rep. 1999;6(1):189–91. [PubMed] [Google Scholar]
- 11.Dattola P, Barbuscia M, Di Pietro N, Rizzo AG, Rifatto P, Randazzo C. Spontaneous retroperitoneal hematoma from ileopsoas muscle bleeding. A case report. [Italian] G Chir. 1995;16(11-12):503–6. [PubMed] [Google Scholar]
- 12.Alberty A, Jarvinen P. Spontaneous rupture of the iliacus muscle with retroperitoneal haematoma. Ann Chir Gynaecol. 1983;72(2):80–1. [PubMed] [Google Scholar]
- 13.Shrestha SM. Hepatic venous outflow obstruction in Nepal. Trop Gastroenterol. 1996;17(3):165–71. [PubMed] [Google Scholar]
- 14.Shrestha SM, Joshi BL, Shrestha S, Maharajan KG. Cavographic study of an early stage of obstruction of the hepatic portion of the inferior vena cava. J Gastroenterol Hepatol. 2000;15:202–10. doi: 10.1046/j.1440-1746.2000.02043.x. [DOI] [PubMed] [Google Scholar]
- 15.Shrestha S, Shrestha S. Bacterial peritonitis in hepatic inferior cava disease: a hypothesis to explain the cause of infection in high protein ascites. Hepatol Res. 2002;24(1):42–9. doi: 10.1016/s1386-6346(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 16.Salgado Ordóñez F, Gavilán Carrasco JC, Bermúdez Recio FJ, Aguilar Cuevas R, Fuentas Lopez T, González Santos P. Absence of the inferior vena cava causing repeated deep venous thrombosis in an adult – a case report. Angiology. 1998;49(11):951–6. doi: 10.1177/000331979804901113. [DOI] [PubMed] [Google Scholar]
- 17.Chuang VP, Mena CE, Hoskins PA. Congenital anomalies of the inferior vena cava. Review of embryogenesis and presentation of a simplified classification. Br J Radiol. 1974;47(556):206–13. doi: 10.1259/0007-1285-47-556-206. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Fuster MJ, Forner MJ, Flor-Lorente B, Soler, Campos S. Inferior vena cava malformations and deep venous thrombosis. [Spanish] Rev Esp Cardiol. 2006;59(9):171–5. [PubMed] [Google Scholar]
- 19.Ruggeri M, Tosetto A, Castaman G, Rodeghiero F. Congenital absence of the inferior vena cava: a rare risk factor for idiopathic deep-vein thrombosis. Lancet. 2001;357(9254):441. doi: 10.1016/S0140-6736(00)04010-1. [DOI] [PubMed] [Google Scholar]
- 20.Siragusa S, Anastasio R, Falaschi F, Bonalumi G, Bressan MA. Congenital absence of inferior vena cava. Lancet. 2001;357(9269):1711. doi: 10.1016/S0140-6736(00)04845-5. [DOI] [PubMed] [Google Scholar]
- 21.Dean SM, Tytle TL. Acute right lower limb extremity iliofemoral deep venous thrombosis secondary to an anomalous inferior vena cava: a report of two cases. Vasc Med. 2006;11(3):165–9. doi: 10.1177/1358863x06074829. [DOI] [PubMed] [Google Scholar]
- 22.Gayer G, Luboshitz J, Hertz M, Zissin R, Thaler M, Lubetsky A, et al. Congenital anomalies of the inferior vena cava revealed on CT in patients with deep vein thrombosis. AJR Am J Roentgenol. 2003;180(3):729–32. doi: 10.2214/ajr.180.3.1800729. [DOI] [PubMed] [Google Scholar]
- 23.Raju S, Hollis K, Neglen P. Obstructive lesions of the inferior vena cava: clinical features and endovenous treatment. J Vasc Surg. 2006;44(3):820–7. doi: 10.1016/j.jvs.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 24.Vidra T, Szomor A, Battyani I, Muhl D, Losonczy H. Agenesis of inferior vena cava combined with multiple genetic predisposition in the case of deep venous thrombosis in a young male. [Hungarian] Orv Hetil. 2003;144(46):2283–6. [PubMed] [Google Scholar]
- 25.Yun SS, Kim JI, Kim KH, Sung GY, Lee DS, Kim JS, Moon IS, Lim KW, Koh YB. Deep venous thrombosis caused by congenital absence of inferior cava, combined with hyperhomocysteinemia. Ann Vasc Surg. 2004;18(1):124–9. doi: 10.1007/s10016-003-0087-x. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann A, Dirrigl A, Heider P, Metz S, Eckstein HH. Thrombosis of the inferior vena cava and the iliac and femoral veins in a 24-year old man. [German] Dtsch Med Wochenschr. 2007;132(1-2):21–4. doi: 10.1055/s-2007-959282. [DOI] [PubMed] [Google Scholar]
- 27.Parma M, Belotti D, Marinoni S, Pogliani EM. Congenital absence of the inferior vena cava and genetic coagulation abnormalities: a rare associated risk factor for recurrent idiopathic thrombosis. Clin Appl Thromb Hemost. 2003;9(4):347–8. doi: 10.1177/107602960300900412. [DOI] [PubMed] [Google Scholar]
- 28.Schneider JG, Eynatten MV, Dugi KA, Duex M, Nawroth PP. Recurrent deep venous thrombosis caused by congenital interruption of the inferior vena cava and heterozygous factor V Leiden mutation. J Intern Med. 2002;252(3):276–80. doi: 10.1046/j.1365-2796.2002.01034.x. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Lee JB, Han MC, Choi BI, Im CK, Chang KH, et al. Sonographic evaluation of inferior vena caval obstruction: correlative study with vena cavography. AJR Am J Roentgenol. 1985;145(4):757–62. doi: 10.2214/ajr.145.4.757. [DOI] [PubMed] [Google Scholar]
- 30.Soler R, Rodriguez E, Lopez MF, Marini M. MR imaging in inferior vena cava thrombosis. Eur J Radiol. 1995;19(2):101–7. doi: 10.1016/0720-048x(94)00587-3. [DOI] [PubMed] [Google Scholar]
- 31.Yamada N, Ishikura K, Ota S, Tsuji A, Nakamura M, Ito M, Isaka N, Nakano T. Pulse-spray pharmacomechanical thrombolysis for proximal deep vein thrombosis. Eur J Vasc Endovasc Surg. 2006;31(2):204–11. doi: 10.1016/j.ejvs.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Robbins MR, Assi Z, Comerota AJ. Endovascular stenting to treat chronic long-segment inferior vena cava occlusion. J Vasc Surg. 2005;41(1):136–40. doi: 10.1016/j.jvs.2004.10.024. [DOI] [PubMed] [Google Scholar]