Abstract
Lipid derived desiccation resistance in membranes is a rare and unique ability previously observed only with trehalose dimycolate (TDM), an abundant mycobacterial glycolipid. Here we present the first synthetic trehalose glycolipids capable of providing desiccation protection to membranes of which they are constituents. The synthetic glycolipids consist of a simple trehalose disaccharide headgroup, similar to TDM, with hydrophobic tail groups of two 15 or 18 carbon chains. The synthetic trehalose glycolipids protected supported monolayers of phospholipids against dehydration even as minority components of the overall membrane, down to as little as 20 mol % trehalose glycolipid, as assessed by assays of membrane fluidity. The dependence of the desiccation protection on synthetic trehalose glycolipid fraction is nearly identical to that of TDM. The striking similarity of the desiccation resistance observed with TDM and the synthetic trehalose glycolipids, despite the variety of hydrophobic tail structures employed, suggests interactions between the trehalose headgroup and surrounding molecules are the determining factor in dehydration protection.
Introduction
Tuberculosis is a major public health concern. The pathogen Mycobacterium tuberculosis (MTb) infects one third of the global population, and the disease kills about two million people annually (1). MTb is remarkably robust, able for example, to survive extended periods of desiccation (2–4). This and other physical properties are believed to derive from the unusual structure and composition of the mycobacterial membrane. Beyond the plasma membrane, all mycobacteria have a dense outer envelope that consists of a cross-linked network of glycans and galactans outside of which sits a nonfluid, hydrophobic layer of mycolic acid. Beyond this nonfluid layer is a fluid, lipid outer leaflet rich in unusual glycolipids such as trehalose dimycolate (TDM), the most abundant extractable lipid in the MTb envelope (Figure 1)(5). (The fraction of TDM found in various strains of MTb is highly variable (5–7).) Though glycolipids are ubiquitous in the living world, glycolipids like TDM that feature trehalose as the headgroup are exceedingly rare, found only in the mycobacteria and a few related groups (8). Free trehalose disaccharides are known to reduce damage to biomolecules under stresses such as freezing and desiccation (9–11). Motivated by this, and by the uniqueness of trehalose glycolipids in the mycobacteria, we recently examined whether TDM can confer stress resistance to lipid membranes of which it is a component. Using an experimental model of mycobacterial membrane structure that allows control of molecular composition (Figure 2), we found that TDM does confer dehydration resistance to membranes, and can do so even as a minority membrane component (12). To the best of our knowledge, this was the first reported discovery of a dehydration-resistant lipid.
Figure 1.
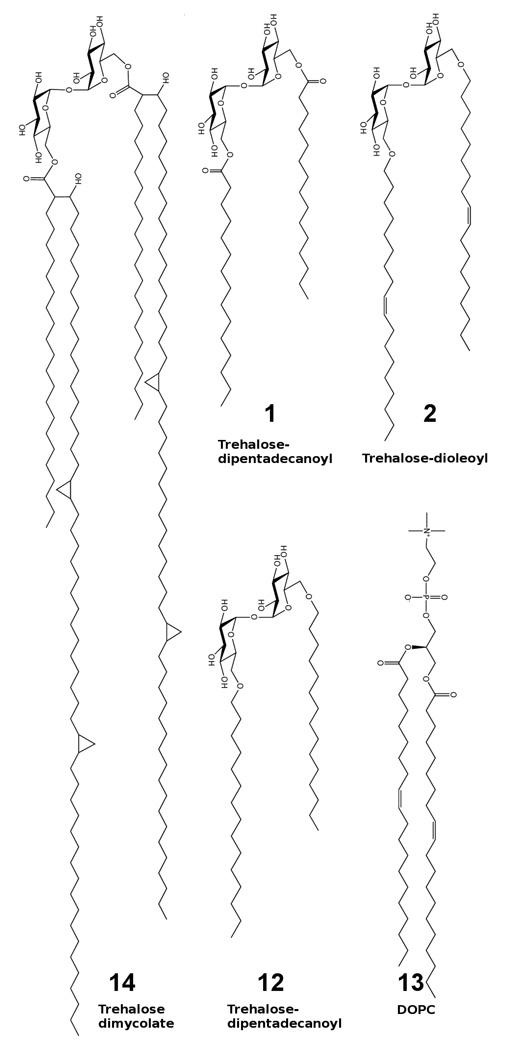
Schematic of trehalose glycolipid and lipid structures. (1,2,12) Synthetic trehalose glycolipids designed to provide desiccation protection. (1) Ester linked trehalose-dipentadecanoyl. (2) Trehalose-dioleoyl. (12) Ether linked trehalose-dipentadecanoyl. (13) DOPC, a common phospholipid with similar hydrophobic tail structure to synthetic compounds 1, 2, and 12. (14) Trehalose dimycolate, desiccation resistant lipid found in all mycobacteria and investigated previously in Ref. (12).
Figure 2.
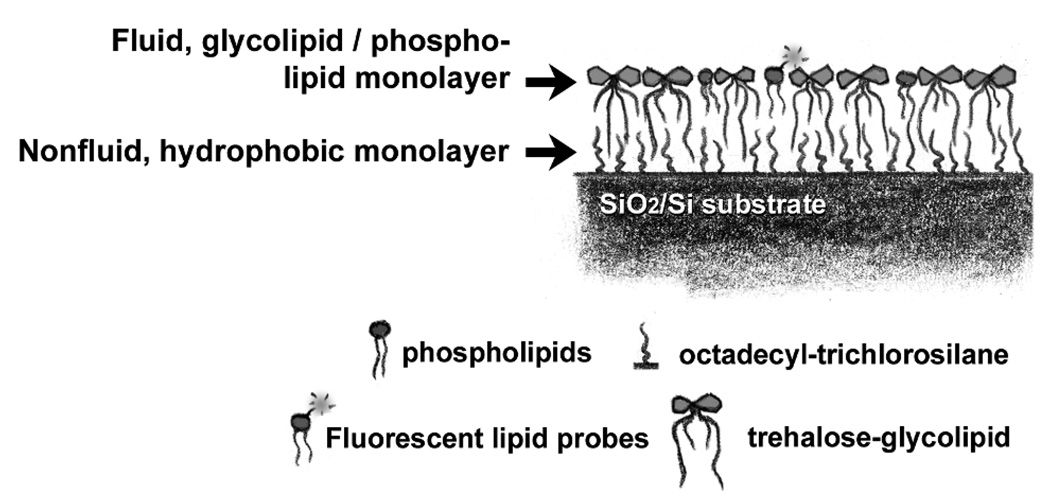
Illustration of a synthetic, supported lipid and glycolipid monolayer. The system consists of a silicon/silicon oxide substrate with covalently bound octadecyltrichlorosilane on top of which sits a fluid, lipid and glycolipid monolayer. The upper monolayer contains a small fraction (1%) of fluorescently labeled lipids that permit measurements of monolayer integrity and fluidity.
The mechanism of TDM's dehydration resistance remains unclear. Though the trehalose headgroup is the likely candidate for stabilizing desiccated membranes, one may suspect a role for the molecules' four large hydrophobic chains as well, mediating interactions in the hydrophobic membrane interior. The observed sharp onset of TDM-derived protection above a critical TDM concentration in the membrane suggests a mapping of protection onto a geometric percolation transition (12), but the appropriateness of this model requires further quantitative tests. Beyond its importance for microbiology, the desiccation resistance observed with TDM points out a previously unrealized physical capability of lipids. Understanding its nature, for example the relative importance of the conjugated disaccharide versus the acyl chains, will broadly impact physical chemistry and soft condensed matter physics.
Synthetic trehalose glycolipids can provide a powerful tool for addressing mechanistic questions. Control of molecular architecture can delineate the structural features responsible for the behaviors exhibited by TDM, illuminating the biophysical chemistry employed by an important pathogen. The control afforded by synthetic lipids can also open doors to the engineering of desiccation resistance into lipid membranes used for a wide variety of biotechnological applications, for example supported-membrane-based sensors (13, 14) and liposome-based drug delivery (15, 16).
We have designed and created a set of synthetic trehalose glycolipids that incorporate a single trehalose disaccharide conjugated to different lipid chain structures (Figure 1). The only persistent structural feature among these glycolipids is the trehalose headgroup. We incorporated these trehalose glycolipids into two-dimensionally fluid supported lipid monolayers that structurally resemble the outer envelope of mycobacteria, as in previous studies of TDM (12). We find that the synthetic trehalose glycolipids confer desiccation resistance to membranes of which they are a component. Moreover, the concentration dependence of membrane recovery is identical to that of TDM. Therefore, the dehydration resistance properties exhibited by natural TDM and these new synthetic mimics are determined by the trehalose headgroup and are insensitive to the hydrophobic chain architecture or linkage between the disaccharide and the chains.
Synthesis
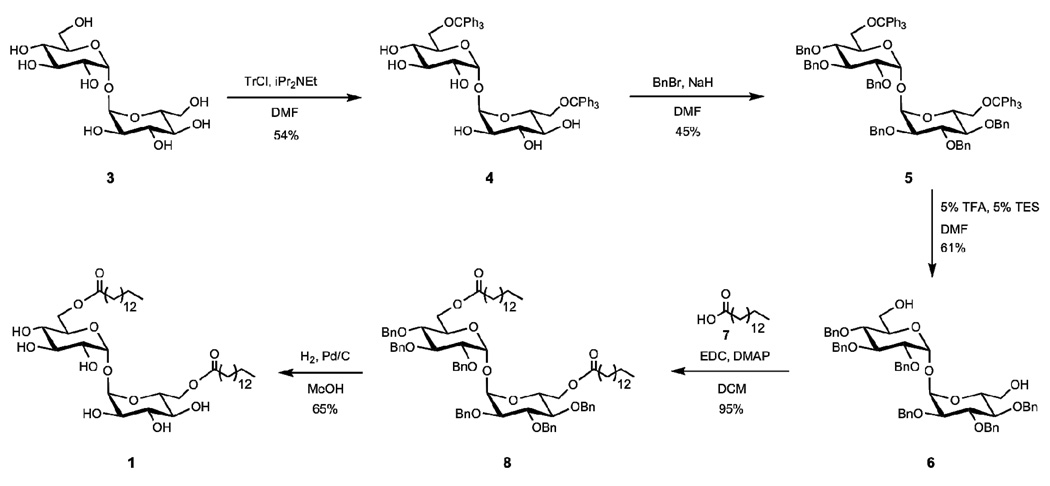
Detailed synthesis procedures can be found in the supplementary materials. In brief, trehalose glycolipid 1 (trehalose-dipentadecanoyl) was synthesized in five steps from trehalose (3) (Scheme 1). The 6 and 6’ hydroxyl groups of trehalose were selectively protected with trityl chloride to yield compound 4. The remaining hydroxyl groups were converted to benzyl ethers under standard conditions to result in fully protected trehalose 5. Treatment of 5 with trifluoroacetic acid yielded the 6,6’-trehalose diol 6. Pentadecanoic 7 acid was coupled to trehalose 5 through an EDC coupling to result in glycolipid 8 in excellent yield. Global deprotection under hydrogenation conditions removed the benzyl ethers to result in desired trehalose glycolipid 1.
Scheme 1.
Synthesis of C-15 trehalose glycolipid.
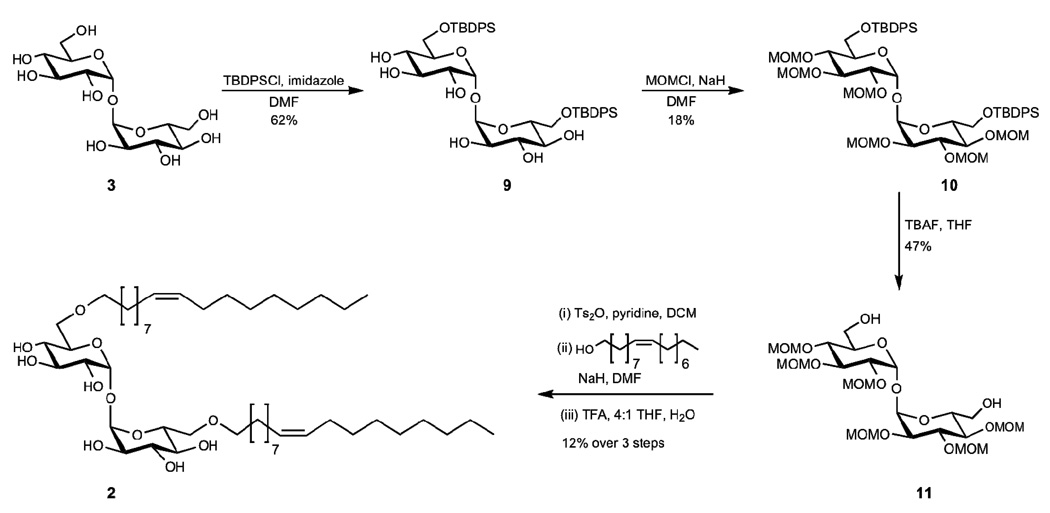
Trehalose glycolipid 2 (trehalose-dioleoyl) was also synthesized from trehalose (3); however, the unsaturation within the lipids required a different protecting group strategy (Scheme 2). The 6 and 6’ hydroxyl groups of 3 were protected as silyl ethers using tert-butyl(chloro)diphenylsilane to afford 9. The remaining hydroxyl groups were then protected as methoxymethyl ethers. We attribute the low yield of this reaction to decomposition of the compound during purification on deactivated silica gel. Slightly better yields were obtained by purification on basic alumina. The 6 and 6’ hydroxyl groups of protected trehalose 10 were deprotected by treatment of 10 with TBAF to yield compound 11. In order to obtain the desired ether linkage between the trehalose and the lipids, the 6 and 6’ hydroxyl groups were activated for coupling with fatty alcohols. The 6 and 6’ alcohols were converted to triflates, tosylates, and mesolates; however, the tosylate resulted in the highest yields upon coupling to oleyl alcohol. After coupling oleyl alcohol to the trehalose, global deprotection of the methoxymethylethers was performed to result in desired trehalose glycolipid 2.
Scheme 2.
Synthesis of C-18 unsaturated trehalose glycolipid.
Results
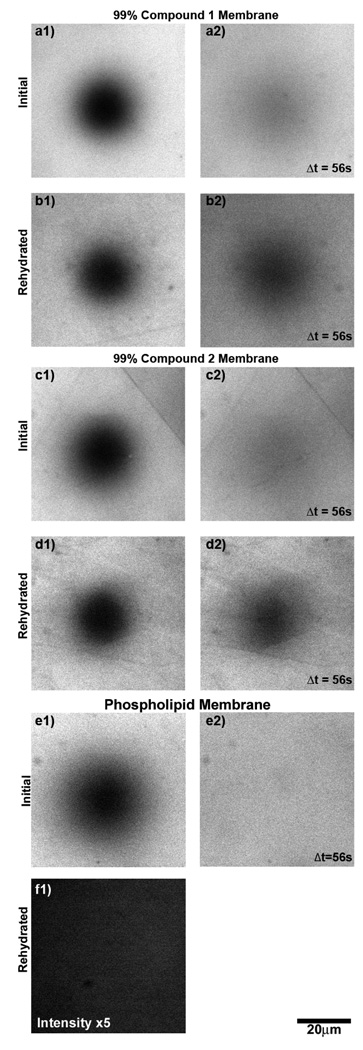
Supported monolayers consisting of 0.99-X:X:0.01, DOPC:trehalose glycolipid:Texas Red DHPE were deposited using the supported monolayer architecture used for earlier investigations of TDM (12), described also in Materials and Methods. (See Materials and Methods for lipid abbreviations.) In brief, lipid solutions were mixed in chloroform and deposited at the air/water interface of a small chamber filled with phosphate buffered saline (PBS). The lipid monolayers were transferred onto silicon/silicon oxide/octadecyltricholorosilane substrates. All samples from X = 0 to 0.99 trehalose glycolipid showed bright, uniform fields of fluorescence and yielded diffusion coefficients on the order of 1µm2/s as measured by fluorescence recovery after photobleaching (FRAP, see Materials and Methods), indicating intact and well formed monolayers (Figure 3a and c).
Figure 3.
Membrane fluidity and dehydration resistance quantified by fluorescence recovery after photobleaching (FRAP). (a-e) Fluorescence images of supported monolayers containing 1 mol % Texas Red DHPE. Initially, images of 99 mol % synthetic trehalose glycolipids (a and c) and 99 mol % DOPC (e) display intact monolayers with characteristically bright and uniform fields of fluorescence. (a2,c2,e2) When the monolayers are photobleached in a defined circular region, they recover a uniform field of intensity, indicating fluidity. (b and d) After dehydration and rehydration, FRAP images of 99 mol % trehalose glycolipid display a similarly intact, bright, and fluid monolayers. (e) Monolayers of 99 mol % DOPC show no measurable intensity above background noise after dehydration and rehydration and are destroyed by the desiccation process. The intensity of f has been increase by a factor of 5 relative to e.
Desiccation resistance was probed as in Ref. (12), by drying the supported monolayers, rehydrating, and examining the existence and fluidity of the monolayer by fluorescence imaging and FRAP. Two-dimensional fluidity is a crucial membrane property that is sensitive to both continuity and membrane structure; measuring fluidity provides a robust and quantitative measure of membrane integrity. All samples were dehydrated and rehydrated as described in Materials and Methods. At X = 0.99, the trehalose glycolipids 1 and 2 (Figure 1) showed uniform, bright fields of fluorescence and 2D mobility after rehydration. In contrast, DOPC (X = 0) membranes were destroyed by the desiccation process (Figure 3e and f), their absence indicated by near-zero fluorescence intensity, as expected. The ratio of the diffusion coefficient after rehydration relative to the initial value before dehydration gives a quantitative measure of recovery, which we refer to as the recovery fraction (Dr). Samples with no discernible fluorescence after rehydration are assigned Dr = 0. For all X = 0.99 trehalose glycolipid samples examined, Dr was greater than 0.50 indicating the synthetic trehalose glycolipids successfully reproduce the desiccation resistance that, until now, has only been observed with TDM.
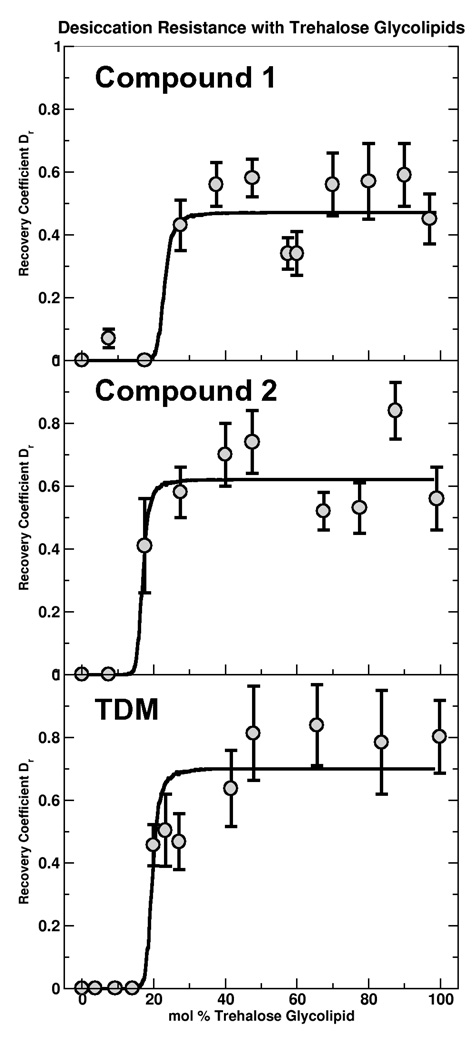
A concentration series with synthetic trehalose glycolipids 1 and 2 over the range X = 0 to 0.99 reveals strikingly similar recovery curves to those measured with TDM (12) (Figure 4). The synthetic lipids provide no protection below a well defined critical fraction, pc, and, above pc, Dr rises indicating protection of the membrane against dehydration. The saturated step behavior has a form similar to many percolation phenomenon and a fit to a percolation model (described in (12)) gives values of pc that are very similar for the three lipids, 0.23 for 1, 0.17 for 2, and 0.20 for TDM, each with an estimated uncertainty of ± 3%.
Figure 4.
The ratio, Dr, of the membrane diffusion coefficient after rehydration to its initial value (circles) shows recovery of the membrane above a critical fraction. The curves represent simulated percolation on a triangular lattice with the saturation, psat, and critical fraction, pc, as fit parameters. In the fit, the mapping from site occupation probability to mole fraction includes the ratio of the area per trehalose glycolipid to the area per DOPC. Synthetic trehalose glycolipids (a,b) show the same recovery behavior as the previously investigated TDM (c) despite their structural differences.
The robustness of the protecting ability was examined by subjecting synthetic trehalose glycolipid monolayers with X = 0.90 to an extended dehydration time of two weeks. The samples were dehydrated using the normal process and left exposed to ambient air for a further two weeks. Upon rehydration, the samples recovered with Dr greater than 0.6 (figures provided as Supplemental Material).
We examined synthetic trehalose glycolipids with two 8 carbon and single 15 carbon hydrophobic chains, otherwise identical in structure to compound 1. Over the full range X = 0 to 0.99 we found no protection against desiccation (data not shown). However, pressure versus area isotherms of monolayers containing the 8 carbon lipids at the air/PBS interface prior to deposition showed a strong decrease in surface pressure over time indicating that glycolipids were leaving the interface. The critical micelle concentration (CMC), for 8 carbon lipids is 0.3mM while for the single chain 15 carbons lipids the CMC is ≈ 0.1mM (17), well above the lipid concentrations present in this studies. The short chain and single chain lipids are incapable of stable incorporation into the membrane, thereby, explaining the lack of desiccation protection.
Incorporation of a synthetic trehalose lipid identical to compound 1, but with an ether rather than ester linkage between the trehalose disaccharide and the hydrophobic tail also showed desiccation resistance with Dr > 0.5 at X = 0.99. The membrane recovery results are summarized in Table 1.
Table 1.
Summary of lipid and glycolipid desiccation protection results; the pc values have an estimated uncertainty of ± 0.03.
| Lipid | Initially mobile | Survives Desiccation | Critical Molar Recovery Fraction (pc) | Average Recovery Fraction (above pc) |
|---|---|---|---|---|
| DOPC | Yes | No | n/a | n/a |
| TDM | Yes | Yes | 0.20 | 0.70 |
| Compound 1 | Yes | Yes | 0.17 | 0.62 |
| Compound 2 | Yes | Yes | 0.23 | 0.50 |
| Compound 12 | Yes | Yes | n/a | > 0.50 |
In addition to assessing recovery via lipid mobility, we also measured the overall fluorescence intensity. Compared to mobility, this is not a reliable marker of membrane integrity due to the sensitivity of fluorescence intensity to the environment. We find varying degrees of photodamage while in the “dry” state. Still, the overall brightness for the membranes that do not recover after dehydration and rehydration is small, less than 5% of the original brightness whereas membranes that survive dehydration and rehydration recover over 60% of their original brightness. This result corroborates the recovery behavior indicated by the mobility, and also confirms that the fluorescent probes are not incorporating into the solid-anchored OTS monolayer.
Discussion
The synthetic trehalose glycolipids confer dehydration resistance to membranes, with very similar behaviors as natural TDM. The synthetic lipids and TDM have identical trehalose headgroups but different hydrophobic chains in terms of chain number, length, and linkage. Therefore, the dehydration resistance conferred by TDM appears to be determined by trehalose, with no apparent role for the chains in this process other than enabling stable membrane anchoring. The protective behavior must be derived from interactions mediated by the trehalose headgroup and surrounding molecules. Exploring the molecular underpinnings of these interactions promises to be a fascinating avenue for the future studies.
Free trehalose has been studied extensively, and its mechanism of protection likely involves the disaccharide affecting the formation of a glassy state or replacing hydrating water molecules via the formation of hydrogen bonds with the protected species (18). However, the relative importance of these effects remains qualitatively and quantitatively undetermined (19–21). A major difference between the activities of free trehalose and the trehalose glycolipid is that in the latter the disaccharide is of course not free but bound. This will undoubtedly constrain the interactions between the sugar, whose rotational and translational freedom is limited, and the nearby phospholipids. Many questions related to this remain open: Is there a "mapping" that can be constructed between 2D concentrations of trehalose glycolipids and 3D concentrations of free trehalose that lead to similar behaviors? How many phospholipids can one lipid-conjugated trehalose interact with? Can this be modulated via modification of the sugar-acyl chain linkage? Notably, both linkages probed in this study connect to trehalose to the hydrophobic chains at two sites; a singly connected linkage, though synthetically challenging, may confer more degrees of freedom to the disaccharide.
The membrane recovery versus trehalose glycolipid fraction appears well fit by the percolation form described in our earlier work (12), but this apparent agreement illuminates an important flaw in the percolation model. The essence of the percolation model is as follows: we hypothesized that desiccation resistance occurs if, at any instant in time, the trehalose glycolipid forms a connected network spanning the membrane. As with all percolation phenomena, there is a critical area fraction associated with the existence of this spanning network, which translates to a critical composition in a manner determined by the relative molecular areas of the trehalose glycolipid and the phospholipid (DOPC) (12). Simply by virtue of geometry, larger glycolipids are able to form networks spanning the membrane at lower molar fractions than smaller glycolipids. The synthetic lipids have much smaller hydrophobic chains, and presumably smaller areas, than TDM (Figure 1). This would lead us to expect larger pc values for the synthetic trehalose lipids than for TDM. Specifically, the similarity in size between compounds 1, 2, and DOPC would suggest pc ≈ 0.5. However, we find that the critical synthetic trehalose glycolipid fractions, pc, are the same as that of TDM to within our estimated composition uncertainty of ± 3% (Figure 4). Not only does the invariance of pc with lipid structure argue against a percolation transition, it also strongly implies that structural transitions in lipid packing do not determine pc, as these would also show strong dependence on the glycolipids' molecular structure.
The similarity of pc across trehalose glycolipids suggest a different mechanism. The headgroup, a single trehalose disaccharide, is the same for all the compounds examined. The sugar may be forming connections with a specific number of neighboring lipids within the membrane, most probably through hydrogen bonding. Dehydration resistance of the membrane may emerge when this local bonding interaction is sufficient to involve the overall lipid population. In this picture, the onset of protection should be independent of the molecular size or chain structure, consistent with the observations. The steepness of the rise of Dr above its threshold remains surprising, and may indicate some degree of cooperativity in the glycolipid-phospholipid interactions.
The trehalose glycolipids described here are the first reported synthetic lipids that confer desiccation resistance to membranes. This behavior not only illuminates biophysical properties relevant to mycobacteria, but also opens doors to exploiting these unique properties in various contemporary ares of lipid research. The formation of liposomes and DNA-lipid complexes, for example, both very important to drug and gene delivery applications, depends sensitively on lipid structure. The advent of synthetic, dehydration resistant trehalose glycolipids may allow the creation of desiccation resistant liposomes, bilayers, and other structures. The independence of our data on hydrophobic chain architecture reveals that molecular structure can be tuned for specific applications independent of the trehalose-derived protection. Furthermore, it suggests routes to enhanced protection by engineering multiple disaccharides per molecule or by developing a better molecular-level understanding of trehalose-phospholipid interactions.
Materials and Methods
Lipids
DOPC (1,2-Dioleoyl-sn-Glycero-3-Phosphocholine) was purchased from Avanti Polar Lipids. Texas Red DHPE (Texas Red 1,2-dihexadecanoyl-sn-glycero-3- phosphoethanolamine) was purchased from Invitrogen.
Glycolipid Synthesis
Detail of the synthesis of the trehalose glycolipids 1 and 2, as well as structural characterizations, are provided as Supplemental Material.
Substrates
SiO2 /Si wafers were purchased from the UC Berkeley Microfabrication Laboratory and consisted of 54 nm thermal oxide on Si. The substrates were made hydrophobic by covalent linkage of octadecyltrichlorosilane (Sigma-Aldrich) (22); in brief: wafers were cleaned in piranha solution (3:1 concentrated sulfuric acid/30% hydrogen peroxide), dried, and incubated 3 hours in 3 mM OTS dissolved in toluene. After the incubation, the chips were rinsed in toluene and baked in air for 4 hours at 100 °C. The chips were then rinsed again with toluene. Hydrophobicity was verified by observing a contact angle of greater than 80° for water droplets on representative substrates indicating dense surface packing.
Monolayer Deposition
Supported lipid monolayers were formed by Langmuir-Blodgett deposition techniques (23, 24). In brief, a small chamber with 3 ml of phosphate buffered saline (PBS), pH 7.4, was heated to the desired temperature with a Warner Instruments QE-1 temperature controlled stage. Lipid solutions of the desired composition were then mixed in chloroform and deposited on surface of the PBS with a micro-syringe to form a surfactant monolayer. The surface tension was continuously monitored using by a Kibron Microtrough tensiometer, which measures the capillary force acting on a metal surface probe. Lipids were added until the surface tension value saturated (typically between 25 and 35 mN/m, depending on composition), after which the monolayer was transferred to an OTS coated SiO2 /Si substrate by Langmuir-Blodgett deposition at 6mm/min, remaining in PBS buffer. At all stages of sample handling (monolayer preparation, transfer to substrates, rehydration, and imaging), the samples were maintained at 37 ± 2 °C.
Microscopy
Samples were examined and images obtained using a Nikon TE2000-E inverted fluorescence microscope with a 60× objective lens (NA = 0.9) and Hamamatsu ORCA-ER (C4742-80) charged-coupled device camera and Nikon Elements acquisition software. Illumination was provided by a mercury arc lamp. Filter cubes from Chroma Technology were used to examine the fluorescence from Texas Red (excitation band 530–580 nm; emission band 605–675 nm).
Monolayer Dehydration/Rehydration
Monolayers were dehydrated by removing the SiO2 /Si substrates from the PBS buffer followed by 1 minute of drying under a flow of nitrogen gas. The chips were then allowed to sit in open air for 10 minutes. Monolayer rehydration was achieved by submerging the chip face down in a clean PBS solution. During dehydration and rehydration the relative humidity was 25 – 35%. Monolayers were allowed to equilibrate for 5 minutes before any images were taken to ensure thermal equilibration at 37 °C and consistency of experimental parameters. Measurements of membrane mobility conducted immediately after rehydration (within one minute) showed no discernible difference in membrane recovery beyond a slight reduction in the diffusion coefficients that disappeared once brought to 37 °C.
Lipid mobility and FRAP
Lipid monolayer mobility was measured by examining fluorescence recovery after photobleaching (FRAP). For each measurement, fluorescent probes in a region defined by a small aperture were bleached by intense illumination for 10 s. Images were acquired at discrete time points after bleaching and analyzed to determine the lipid diffusion coefficient as described in Ref. (12).
Supplementary Material
Detailed explanation of synthetic schemes used to generate synthetic trehalose glycolipids 1 and 2 and FRAP images of long time scale dehydration experiments.
Acknowledgements
This work was supported by the National Institutes of Health (grant R01-AI51622 to C.R.B.), the Engineering and Technology Industry Council of Oregon (C.W.H.), the Office of Naval Research through the Oregon Nanoscience and Microtechnologies Institute (R.P. and C.W.H.), and the Alfred P. Sloan Foundation (R.P.).
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Journal of the American Medical Association. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Volk WA, Benjamin DC, Kadner RJ, Parsons JT. Essentials of Medical Microbiology. Philadelphia: JP Lippincott; 1986. [Google Scholar]
- 3.Van der Hoeden J. Zoonoses. Elsevier; 1964. [Google Scholar]
- 4.Fraise A, Lambert P, Maillard JY. Principles and Practice of Disinfection Preservation and Sterilisation. Blackwell Publishing; 2004. [Google Scholar]
- 5.Almog R, Mannella CA. Biophysical Journal. 1996;71:3311–3319. doi: 10.1016/S0006-3495(96)79523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retzinger GS, Meredith SC, Takayama K, Hunter RL, Kezdy FJ. J. Biol. Chem. 1981;256:8208–8216. [PubMed] [Google Scholar]
- 7.Crowe LM, Spargo BJ, Ioneda T, Beaman BL, Crowe JH. Biochim. Biophys. Acta. 1994;1194:53–60. doi: 10.1016/0005-2736(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 8.Minnikin DE. In: The Biology of the Mycobacteria. Ratledge C, Stanford J, editors. 1982. [Google Scholar]
- 9.Crowe J, Crowe L, Chapman D. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 10.Crowe JH, Hoekstra FA, Crowe LM. Annual Reviews in Physiology. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- 11.Albertorio F, Chapa VA, Chen X, Diaz AJ, Cremer PS. J. Am. Chem. Soc. 2007;129:10567–10574. doi: 10.1021/ja0731266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harland CW, Rabuka D, Bertozzi CR, Parthasarathy R. Biophysical Journal. 2008;94:4718. doi: 10.1529/biophysj.107.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sackmann E. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 14.Holden MA, Jung S, Yang T, Castellana ET, Cremer PS. Journal of the American Chemical Society. 2004;126:6512–6513. doi: 10.1021/ja048504a. [DOI] [PubMed] [Google Scholar]
- 15.Torchilin VP. Crit Rev Ther Drug Carrier Syst. 1985;2:65–115. [PubMed] [Google Scholar]
- 16.Torchilin VP. Cellular and Molecular Life Sciences (CMLS) 2004;61:2549–2559. doi: 10.1007/s00018-004-4153-5. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Kimura Y, Adachi S. LWT-Food Science and Technology. 2007;40:412–417. [Google Scholar]
- 18.Crowe JH, Crowe LM, Carpenter JF, Rudolph AS, Wistrom CA, Spargo BJ, Anchordoguy TJ. BBA-Reviews on Biomembranes. 1988;947:367–384. doi: 10.1016/0304-4157(88)90015-9. [DOI] [PubMed] [Google Scholar]
- 19.Elbein AD, Pan YT, Pastuszak I, Carroll D. Glycobiology. 2003;13:17–27. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- 20.Pereira CS, Hunenberger PH. Biophysical Journal. 2008 doi: 10.1529/biophysj.108.131656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennun SV, Faller R, Longo ML. Langmuir. 2008;24:10371–10381. doi: 10.1021/la8016694. [DOI] [PubMed] [Google Scholar]
- 22.Moaz R, Sagiv J. J. Colloid Interface Sci. 1984;100:465–496. [Google Scholar]
- 23.Barnes G, Gentle I. Interfacial science. Oxford University Press; 2005. [Google Scholar]
- 24.von Tscharner V, McConnell HM. Biophysical Journal. 1981;36:421–427. doi: 10.1016/S0006-3495(81)84741-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed explanation of synthetic schemes used to generate synthetic trehalose glycolipids 1 and 2 and FRAP images of long time scale dehydration experiments.