Abstract
Differences in morningness-eveningness among humans are commonly ascribed to circadian parameters, such as circadian period and responsivity to environmental time cues, as well as homeostatic sleep drive. Light is the primary synchronizer of the human biological clock, and if circadian differences exist between morning and evening types, they should have different phase angles of entrainment to the light/dark cycle; that is, morning and evening types should have different patterns of light exposure relative to endogenous circadian phase (ECP). When phase angle of entrainment is strictly defined as the relationship between a marker of ECP and the timing of light exposure, such differences have been demonstrated in the laboratory under controlled light/dark cycles and have recently been shown under conditions of spring and summer light exposure outside the laboratory, taking into account the variable intensity of light. Here, we report similar results from a large (n = 66), diverse cohort of morning and evening types across the age span studied at all different times of the year. Differences between morning and evening types in light exposure relative to ECP, indicative of a difference in the phase angle of entrainment to the external light/dark cycle, were found. Specifically, evening types, compared to morning types, had a higher ratio of phase advancing to phase delaying by light. We interpret this as indicating a longer circadian period (τ) in evening types.
Keywords: Circadian, Human, Light, Morningness-eveningness, Melatonin
INTRODUCTION
Chronotype (morningness-eveningness) in humans likely has multiple causes. It has been hypothesized that differences in chronotype are due to homeostatic sleep drive (i.e., accumulation or dissipation of sleep pressure) as well as circadian parameters, such as circadian period and responsivity to zeitgebers. In addition to increased dissipation in some morning types, they may also have more rapid accumulation of homeostatic sleep drive (Mongrain et al., 2005, 2006; Taillard et al., 2003). These would be expected to result in earlier wake- and bedtimes in morning types. There is abundant evidence for circadian differences between chronotypes as well: circadian phase has been found to occur about 2 h later in evening types (Baehr et al., 2000; Duffy et al., 1999; Liu et al., 2000; Mongrain et al., 2004). Although differences in phase could be due to differences in self-selected light/dark cycles, an association between circadian period (τ) and morningness-eveningness has been demonstrated at both a physiological (Duffy et al., 2001) and molecular level (Brown et al., 2008), with evening types having longer periods.
Phase angle of entrainment is defined as the relationship between the timing of the biological clock and the timing of an external time cue (i.e., zeitgeber; see Pittendrigh & Daan, 1976), and if the primary explanation for morningness-eveningness is circadian, then a difference in phase angle of entrainment to the light-dark cycle would be expected between the two types (Duffy & Wright Jr., 2005; Pittendrigh & Daan, 1976; Roenneberg et al., 2003a). Indeed, under the uniform light conditions of the laboratory, a difference in phase angle of entrainment to the light-dark cycle between morning and evening types has in fact been found (Duffy et al., 1999). Outside of the laboratory, a difference in the timing of the core body temperature rhythm, relative to the timing of sleep, has been found between morning and evening types (Baehr et al., 2000), as well as correlations between chronotype, defined in this case by the timing of sleep, and the timing of the solar light/dark cycle (Randler, 2008; Roenneberg et al., 2007) and between self-reported outdoor light exposure and the timing of sleep (Roenneberg et al., 2003b).
More recently, Goulet et al. (2007) assessed light exposure outside the laboratory and endogenous circadian phase (ECP) in 24 young morning and evening types studied between May and September. As expected, the chronotypes differed in their light exposure across the 24 h day. However, no difference between the two groups was found in light exposure relative to phase. Goulet and colleagues (2007) hypothesized that this was due to either insufficient sample size or because only a subset of extreme chronotypes have primarily a circadian cause for their morningness-eveningness. In fact, when they examined only those subjects with the earliest and latest circadian phases (i.e., “non-overlapping” or “extreme” phases), a difference in light exposure relative to ECP was found between the two types, indicating a difference in phase angle of entrainment to the light/dark cycle (Goulet et al., 2007). We sought to replicate and extend the findings of Goulet and colleagues in a larger and demographically more heterogeneous cohort of subjects studied at all different times of year.
MATERIALS AND METHODS
Subjects
Seventy-one self-identified morning-and evening-type subjects were recruited for study by advertisement. Subjects were included for study if their Horne-Östberg score was ≥59, indicative of moderate to definite morning type, or ≤41, indicative of moderate to definite evening type. Data from sixty-six subjects—47 women and 19 men, 19–64 yrs of age—are reported here (see Table 1 for subject demographics). One subject was excluded because she failed to complete a sleep diary, two subjects were excluded because they failed to wear their actiwatch, and two subjects were excluded because of technical problems with their acti-watch. All subjects were in good general physical and mental health at the time of study, as verified by physical and psychiatric examination plus EKG and laboratory screening tests. Subjects were not taking any medications that would affect plasma melatonin concentrations. Subjects doing night shift work were excluded, and subjects were not studied within two months of time-zone travel. Thirty-nine (59%) of the subjects were employed, six (9%) were self-employed, six (9%) were students, and 15 (23%) were unemployed. Subjects (morning = AM/evening = PM types) were studied in Portland, Oregon (45°, 31′ N) at all times of the year: 7 (3 AM/4 PM) in January, 8 (4 AM/4 PM) in February, 4 (2 AM/2 PM) in March, 3 (1 AM/2 PM) in April, 7 (4 AM/3 PM) in May, 8 (6 AM/2 PM) in June, 3 (1 AM/2 PM) in July, 3 (2 AM/1 PM) in August, 2 (0 AM/2 PM) in September, 5 (3 AM/2 PM) in October, 9 (6 AM/3 PM) in November, and 7 (3 AM/4 PM) in December. All subjects provided written informed consent. The Institutional Review Board of Oregon Health & Science University (OHSU) approved the protocol and the consent forms, and the study conformed to the ethical standards of this journal (Portaluppi et al., 2008).
TABLE 1.
Demographic, sleep, and circadian variables
| All subjects |
Overlapping phases |
Non-overlapping phases |
||||
|---|---|---|---|---|---|---|
| Morning types ± SD (n = 35) |
Evening types ± SD (n = 31) |
Morning types ± SD (n = 24) |
Evening types ± SD (n = 21) |
Morning types ± SD (n = 11) |
Evening types ± SD (n = 10) |
|
| Horne-Östberg Score | 72.0 ± 4.2‡ | 30.1 ± 3.8 | 72.1 ± 4.3‡ | 30.7 ± 3.5 | 71.7 ± 4.2‡ | 29.0 ± 4.4 |
| Age (yrs) | 42.9 ± 14.1† | 33.3 ± 10.3 | 40.1 ± 13.3 | 34.1 ± 9.5 | 49.1 ± 14.5† | 31.7 ± 12.1 |
| Women/men | 24/11 | 23/8 | 18/6 | 16/5 | 6/5 | 7/3 |
| Employment status (E/ SE/S/U) |
21/3/3/8 | 18/3/3/7 | 14/2/3/5 | 13/0/2/6 | 7/1/0/3 | 5/3/1/1 |
| Daylength (h) | 12.04 ± 2.65 | 11.68 ± 2.46 | 12.19 ± 2.70 | 12.02 ± 2.48 | 11.71 ± 2.61 | 10.96 ± 2.38 |
| Bedtime (clock h) | 22:09 ± 00:32‡ | 00:21 ± 1:36 | 22:17 ± 00:34† | 23:50 ± 1:04 | 21:53 ± 00:21* | 01:27 ± 2:00 |
| Midsleep (clock h) | 02:03 ± 00:34‡ | 04:14 ± 01:33 | 02:12 ± 00:35‡ | 03:44 ± 00:57 | 01:44 ± 00:22* | 05:16 ± 02:04 |
| Waketime (clock h) | 05:57 ± 00:41‡ | 08:06 ± 01:36 | 06:06 ± 00:43‡ | 07:38 ± 01:00 | 05:36 ± 00:27* | 09:05 ± 02:10 |
| Sleep duration (h) | 7.79 ± 0.52 | 7.75 ± 0.71 | 7.82 ± 0.57 | 7.81 ± 0.80 | 7.72 ± 0.38 | 7.63 ± 0.47 |
| DLMO (clock h) | 20:06 ± 01:14‡ | 22:03 ± 01:35 | 20:42 ± 00:59 | 21:14 ± 00:54 | 18:47 ± 00:26‡ | 23:45 ± 01:22 |
| DLMO to bedtime (h) | 2.02 ± 1.20 | 2.23 ± 1.30 | 1.59 ± 1.15† | 2.53 ± 1.11 | 3.06 ± 0.44† | 1.70 ± 1.49 |
| DLMO to mid-sleep, PAD (h) |
5.96 ± 1.16 | 6.18 ± 1.21 | 5.50 ± 1.09† | 6.50 ± 0.89 | 6.96 ± 0.46* | 5.52 ± 1.55 |
| DLMO to wake-time (h) | 9.80 ± 1.15 | 9.98 ± .29 | 9.41 ± 1.10‡ | 10.34 ± 0.90 | 10.76 ± 0.53* | 9.33 ± 1.64 |
Difference (morning versus evening type):
p < 0.05
p < 0.01
p < 0.001.
Abbreviations: E = employed, SE = self-employed, S = student, U = unemployed.
Assessment of Sleep and Light Exposure
Subjects were asked to maintain a consistent sleep schedule (±. 5 h) of 8 h in bed for one week. Naps were limited to a maximum of one per day for no more than 30 min and no less than 6 h before bedtime. Caffeine intake was limited to a maximum of two cups of coffee, or caffeine equivalent, before noon. A maximum of one alcoholic drink per day was allowed. During the week, subjects kept a written sleep diary from which average bedtimes, waketimes, midsleep, and sleep duration were calculated, and they wore a wrist actigraph with an integrated light meter on the non-dominant wrist (Actiwatch-L ®, Mini-Mitter Co., Bend, Oregon, USA). Data were collected using a 30 s sampling epoch for a total of seven 24 h periods. Sixty (91%) of the subjects used one of three actigraphs. Four additional actigraphs were used in the remaining six subjects. The same actigraphs were used in morning and evening types. No additional calibration beyond that done by the manufacturer was performed. Subjects were asked to ensure that the actigraph was not covered by clothing and shielded from light.
Light data during periods of wakefulness were edited to remove points that did not correspond with actual light exposure: data were removed during reported naps and periods of wakefulness when no activity was recorded for greater than 15 min (when the watch was presumably off), and any values ≤0.1 lux during periods of wakefulness (when the watch was thought to be inadvertently covered) were removed (Goulet et al., 2007). An average of 8.52% of the light data was removed per subject as a result of these procedures. We did not exclude whole days of data because of missing data. Data were base 10 log-transformed [Log10(Light intensity +1)], and an average intensity was calculated for each 30 s epoch of the 24 h day. As also done by Goulet and colleagues (2007), we included light data from across the 24 h day. This was done to be more consistent with the animal literature regarding phase angle of entrainment to the light/dark cycle (Pittendrigh & Daan, 1976) and to avoid excluding potentially relevant light exposure during brief periods of wakefulness during sleep episodes. Even brief periods of ocular light exposure during the night could be relevant, given that they might occur on the most sensitive regions of the light PRC (Czeisler et al., 1989; Khalsa et al., 2003) and what is now known about the sensitivity of the pacemaker to even low levels of light (Wright Jr. et al., 2001; Zeitzer et al., 2000) in the context of dim light or dark adaptation (Chang et al., 2008; Hebert et al., 2002; Jasser et al., 2006; Smith et al., 2004).
Assessment of Circadian Phase
At the end of the week, subjects were admitted to the Clinical and Translational Research Center (CTRC) at Oregon Health & Science University (OHSU) approximately 7 h before habitual bedtime (Thursday through Saturday evenings). For each admission, blood was sampled every 15 min for 8 h in dim light (≤10 lux) for a high resolution determination of the plasma dim light melatonin onset (DLMO). The DLMO was operationally defined as the interpolated time when levels continuously rose above the threshold of 10 pg/ml (Lewy & Sack, 1989; Voultsios et al., 1997). This threshold was chosen to allow comparison to previous studies conducted in our laboratory (Lewy et al., 2006). During each admission, no restrictions were placed on posture, as there do not appear to be any significant effects of posture on the timing of the DLMO (Cajochen et al., 2003). Activity was not controlled with the exception that subjects were not allowed to exercise or sleep during admissions.
Plasma melatonin concentrations were measured by radioimmuno-assay with an antibody raised in the laboratory of Kennaway and coworkers and reagents supplied by American Laboratory Products (Windham, New Hampshire, USA) (Voultsios et al., 1997). The lower limit of sensitivity of this assay is ≤0.5 pg/ml. This assay was validated by GCMS (Lewy et al., 1997).
Statistical and Data Analysis
Analyses were conducted with SPSS for Windows (SPSS Inc., Chicago, Illinois, USA), SAS (SAS Inc., Cary, North Carolina, USA), and STATA (StataCorp LP, College Station, Texas, USA). Log-transformed average light data for morning and evening types were binned into six 4 h bins centered at the clock hours of 02:00, 06:00, 10:00, 14:00, 18:00, and 22:00 h, and average values within each bin were obtained for each subject. For a separate analysis, log-transformed light data were also binned relative to the DLMO into six 4 h bins centered 2 and 6 h after the DLMO and 2, 6, 10, and 14 h before the DLMO. The bin size was chosen as a compromise between achieving adequate resolution across the circadian day, and therefore across the light PRC, while also minimizing the risk of type I errors due to multiple comparisons. A single marker of the timing of light exposure (e.g., lights on or lights out) was not used due to the non-uniform pattern of light exposure across the circadian day.
Mixed model analysis (Brown & Prescott, 2003) was used to compare light levels between chronotypes over the time bins. This method allows for correlations between observations on the same individuals and also allows for different variances at different time points. Separate analyses were conducted for light levels relative to DLMO and light levels relative to clock hour. For each analysis, we first examined the interaction between time and morning vs. evening type. Given evidence of interaction, follow-up comparisons were made between evening and morning types separately for each 4 h bin with a Holm adjustment (Wright, 1992) for multiple comparisons. For all analyses, sex and employment status were included as categorical factors, and age and daylength (on the day of circadian phase assessment; see http://aa.usno.navy.mil/data/docs/RS_OneDay.php) were included as continuous covariates. Daylength was included to control for time-of-year effects.
Chi-square analysis was done to compare the employment status and sex in evening and morning types. Analysis of variance (ANOVA) was used to compare bedtime, midsleep, waketime, sleep duration, DLMO clock hour, and the interval (phase angle difference, PAD) between the DLMO and average midsleep time in morning and evening types (see Table 1). Sex and employment status were again included as categorical factors and age and daylength were included as continuous covariates. ANOVA was also used to compare daylength in chronotypes, with sex and employment status included as categorical factors and age as a continuous covariate.
All of the above analyses were repeated after the data set had been subdivided, analogous to the method of Goulet and colleagues, into morning and evening types with the earliest and latest phases (“extreme” or “non-overlapping” phases), respectively, and those chronotypes with similar phases (“intermediate” or “overlapping” phases) (Goulet et al., 2007; Mongrain et al., 2004). To do this, subjects were ranked in order according to the clock hour of the DLMO. The first consecutive morning types (n = 11) without any interspersed evening types were designated as extreme, as were the last consecutive evening types (n = 10) without any interspersed morning types. The remaining subjects were designated overlapping.
RESULTS
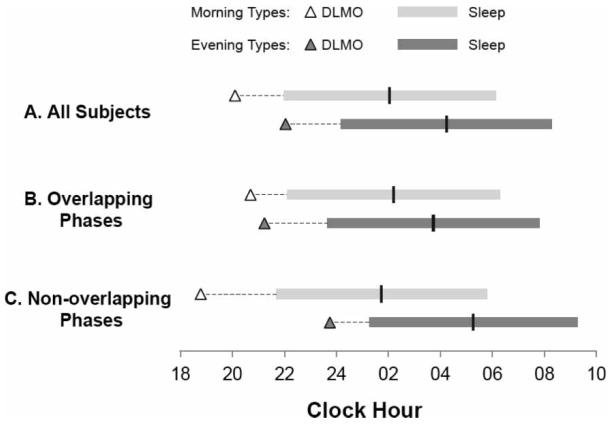
Subjects, on average, were rated as “definite” morning or evening types on the Horne-Östberg questionnaire (see Table 1, all subjects). There was no difference in sex, employment status, or daylength between chronotypes for either the data set as a whole or when the data were subdivided (see Table 1). Morning types were older than evening types, went to bed and woke up about 2 h earlier, and had DLMOs that occurred about 2 h earlier than evening types (see Table 1, all subjects, and Figure 1a). There was a significant correlation between the timing of sleep (midsleep) and circadian phase (DLMO) (r = 0.75, p < 0.001). In the overlapping phase group, age differences were no longer significant, there was a smaller (∼1.5 h) but still significant difference in the timing of sleep, and (as expected) there was no significant difference in the timing of the DLMO (see Table 1, overlapping phases, and Figure 1b). In the non-overlapping group, age differences were significant; there was a greater (∼3.5 h) difference in the timing of sleep, and an almost 5 h difference in the timing of the DLMO (see Table 1, non-overlapping phases, and Figure 1c).
FIGURE 1.
Average timing of sleep and circadian phase. Triangles represent the timing of the DLMO in morning (△) and evening (▲) types. Light and dark gray horizontal bars represent the timing of sleep in morning and evening types, respectively. The vertical bars indicate the timing of midsleep. Dashed lines connect the DLMO to the beginning of sleep. (A) all morning and evening types; (B) those subjects with overlapping phases; (C) those subjects with non-overlapping phases.
The interval (phase-angle difference, PAD) between the DLMO and average midsleep time was approximately 6 h, and there was no difference between chronotypes (see Table 1, all subjects, and Figure 1a; F = 0.002, p = 0.968). There was no effect of age (F = 1.037, p = 0.315), employment status (F = 2.565, p = 0.068), sex (F = 2.296, p = 0.137), or daylength (F = 0.064, p = 0.802) on PAD. Although PAD was longer in women (6:13 ± 1:12 h versus 5:41 ± 1:03 h in men), this was not significant (F = 2.296, p = 0.137). PAD was longest in employed individuals and became progressively shorter in students, self-employed individuals, and unemployed individuals (6:08 ± 1:07 h, 6:04 ± 1:43 h, 6:00 ± 0:52 h, 5:54 ± 1:17 h, respectively), although this also did not quite reach statistical significance (F = 2.565, p = 0.068). There was not a significant interaction between chronotype and either sex (F = 0.429, p = 0.516) or employment status (F = 0.641, p = 0.593) for PAD.
There was a significant chronotype × overlap status interaction for PAD (F = 13.935, p = 0.001). In the overlapping phase group, PAD was about 1 h shorter in morning types (see Table 1, overlapping phases, and Figure 1b), while in the non-overlapping group, PAD was almost 1.5 h longer among morning types (see Table 1, non-overlapping phases, and Figure 1c).
Light Exposure Relative to Clock Hour
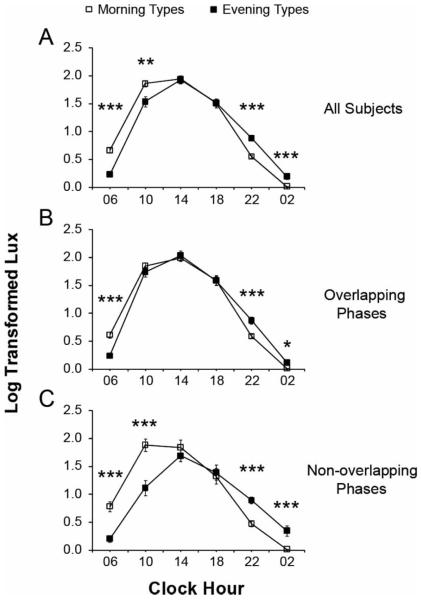
Average light data for morning and evening types plotted according to clock hour are shown in Figure 2a. Mixed model analysis showed a significant interaction between chronotype and clock hour bin (F = 18.99, p < 0.0001). The interaction implies the difference in light levels between chronotypes varies over the time bins. As seen in Figure 2a, follow-up comparisons with the Holm-adjustment for multiple comparisons showed, as expected based on their differences in clock hours of wakefulness, that evening types had higher light levels than morning types during the 4 h bins centered at 22:00 h (t = 6.61, p < 0.001) and 02:00 h (t = 4.52, p < 0.001), while morning types had higher light levels during the 4 h bins centered at 06:00 h (t = 7.07, p < 0.001) and 10:00 h (t = 3.14, p = 0.008). A significant effect of daylength on light level (F = 11.55, p = 0.001) was also found, with longer daylengths associated with greater intensities: each additional hour was associated with an approximate 5% increase in lux. There was no difference in light levels based on chronotype (F = 0.32, p = 0.572) or sex (F = 0.02, p = 0.878). There was a small difference in light levels based on age (F = 4.70, p = 0.034): each additional year was associated with an approximate 0.5% increase in lux.
FIGURE 2.
Light exposure in morning types (open squares) and evening types (closed squares) plotted relative to clock hour. The average log-transformed light data are plotted and binned into six 4 h bins. Results are presented for (A) the entire data set, (B) subjects with overlapping phases, and (C) subjects with non-overlapping phases. Error bars represent the standard error of the mean. *p < 0.05, **p < 0.01, ***p < 0.0001.
There was a significant three-way interaction between chronotype, clock hour bin, and overlap status (F = 3.26, p = 0.011), indicating the effect of chronotype on light levels relative to clock hour (time bin) depended upon overlap status. Therefore, the analysis was repeated with the data subdivided into overlapping and non-overlapping subjects (see Figures 2b and 2c). Among the overlapping subjects, evening types again had higher light levels than morning types during the 4 h bins centered at 22:00 h (t = 5.08, p < 0.001) and 02:00 h (t = 2.75, p = 0.032), while morning types had higher light levels during the 4 h bin centered at 06:00 h (t = 4.93, p < 0.001). Among the non-overlapping subjects, evening types similarly had higher light levels than morning types during the 4 h bins centered at 22:00 h (t = 5.18, p < 0.001) and 02:00 h (t = 5.40, p < 0.001), while morning types again had higher light levels during the 4 h bins centered at 06:00 h (t = 5.11, p < 0.001) and 10:00 h (t = 4.70, p < 0.001).
Light Exposure Relative to Circadian Phase
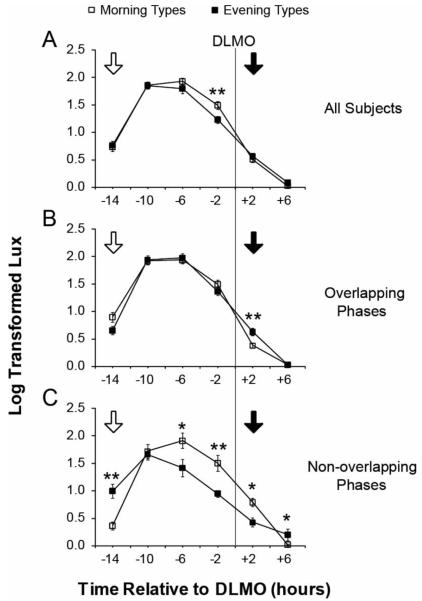
Average light data plotted relative to the DLMO for all morning and evening types is shown in Figure 3a. A significant interaction was found between chronotype and time relative to DLMO bin (F = 4.24, p = 0.002). Morning types, compared to evening types, had higher light intensities in the early biological evening; a significant difference in light levels was found between chronotypes only in the bin 0–4 h before the DLMO (t = 2.85, p = 0.036; see Figure 3a). There was again a significant effect of daylength on light levels (F = 20.42, p < 0.001): each additional hour was associated with an approximate 5% increase in lux. There was no difference in light levels based on chronotype (F = 0.81, p = 0.371), age (F = 0.52, p = 0.474), sex (F = 1.44, p = 0.236), or employment status (F = 0.83, p = 0.483).
FIGURE 3.
Light exposure in morning types (open squares) and evening types (closed squares) plotted relative to the 10 pg/ml dim light melatonin onset (DLMO). The average log-transformed light data are binned into six 4 h bins. Results are presented for (A) the entire data set, (B) subjects with overlapping phases, and (C) subjects with non-overlapping phases. The approximate timing of maximal phase advances and phase delays are indicated by white and black arrows, respectively. Error bars represent the standard error of the mean. *p < 0.05, **p < 0.01.
There was a significant three-way interaction between chronotype, time relative to DLMO bin, and overlap status (F = 6.97, p < 0.001), indicating that the effect of chronotype on light levels relative to the DLMO (time bin) depended upon overlap status. Therefore, the analysis was repeated with the data subdivided into overlapping and non-overlapping subjects (see Figures 3b and 3c). Among the overlapping subjects, evening types had slightly higher light levels in the biological night: a small but significant difference was found between chronotypes in the bin 0–4 h after the DLMO (t = −3.39, p = 0.001; see Figure 3b). Among the non-overlapping subjects, differences were found among all bins except during the late biological morning, the bin 8–12 h before the DLMO. Morning types had greater light levels in the biological afternoon and evening, the bins 0–4 and 4–8 h before the DLMO and 0–4 h after the DLMO (see Figure 3c). Evening types had greater light levels during in the late biological night and biological morning, the bins 4–8 after and 12–16 h before the DLMO (see Figure 3c).
DISCUSSION
In a large cohort of morning and evening types, we have found a difference in the timing of ambulatory light exposure relative to circadian phase. This replicates and extends the findings of Goulet and colleagues (2007) with the inclusion of a broader age range and more heterogenous demographic mix of morning and evening types as well as assessments conducted at all times of year. Our data further indicate that there is a difference in phase angle of entrainment to the light/dark cycle (i.e., a difference in the timing of the clock relative to the timing of light) between the two groups under everyday conditions, similar to what has been found in the laboratory (Duffy et al., 1999). We interpret this as indicating a longer τ in evening types, consistent with what has been reported previously in animal and human studies (Pittendrigh & Daan, 1976; Wright Jr. et al., 2005).
Light Exposure Relative to Clock Hour
As expected, based on past literature (Goulet et al., 2007; Roenneberg et al., 2003b, 2007), we found morning types had higher light levels in the morning, while evening types had higher light levels in the evening and night, in keeping with the fact that the timing of sleep and wakefulness was about 2 h earlier in morning types (see Figure 2a).
Light Exposure Relative to Circadian Phase
When we plot light exposure data relative to circadian phase, as done by Goulet and colleagues (2007), the two profiles overlap across much of the biological day and night (see Figure 3a). The one exception occurs in the 0–4 h before the DLMO. Morning types had higher light levels later in the biological day (see Figure 3a) despite having lower light levels late in the 24 h day (see Figure 2a).
In trying to understand this pattern of light exposure, it is helpful to review the variables that contribute to maintaining stable entrainment. Steady-state entrainment to the 24 h day occurs when the tendency for phase to drift to a later (or earlier) time due to the non-24 h τ is balanced by the net effect of daily phase shifts (Duffy & Wright Jr., 2005; Pittendrigh & Daan, 1976; Roenneberg et al., 2003a): the longer the τ, the greater the ratio of phase advances to phase delays necessary to maintain entrainment; the shorter the τ, the smaller the ratio. During steady-state entrainment, the phase angle of entrainment depends upon how much τ differs from 24 h, the strength of the relevant zeitgeber(s), and an individual's responsivity to the relevant zeitgeber(s), the latter two constituting what is termed coupling strength (Gronfier et al., 2007; Pittendrigh & Daan, 1976; Moore-Ede et al., 1982).
Given the correlation between τ and chronotype (Duffy et al., 2001), it is logical to hypothesize that evening types have longer periods than morning types, and that they require a greater ratio of phase advances to phase delays to maintain entrainment. Our data confirm a corresponding pattern of light exposure across the circadian day in all chronotypes (see Figure 3a). Evening types had the same amount of light exposure on the advance zone of the light phase-response curve (PRC) (see Figure 3a) but had less light exposure on the delay zone (i.e., 0–4 h before the DLMO) (Czeisler et al., 1989; Khalsa et al., 2003; Shanahan et al., 1999). Therefore, evening types had a greater ratio of phase advances to phase delays, presumably due to their longer τ. These results are consistent with measurements of phase angle of entrainment in morning and evening types under the uniform light conditions in the laboratory (Duffy et al., 1999).
Furthermore, our data replicate the finding of Goulet and colleagues (2007) that this pattern of light exposure is explained by those morning and evening types with more “extreme” phases who presumably have mainly a circadian explanation for their chronotype. Among chronotypes with extreme or non-overlapping phases (see Figure 3c), evening types had more light exposure on the advance zone of the PRC, 12–16 h before the DLMO, and less light exposure on the delay zone of the light PRC, 0–4 h before and 0–4 h after the DLMO. Therefore, the non-overlapping evening types had an even greater ratio of phase advancing to phase delaying light, again consistent with the hypothesis that evening types have a longer τ.
Although we also found that non-overlapping evening types had greater light levels during part of the phase delay region, 4–8 h after the DLMO, and lower levels during part of the phase advance region, 4–8 h before the DLMO, we think the net effect is still a higher ratio of phase advancing to phase delaying light in these evening types. The ratio of mean log-lux in the three phase advance zone bins 4 to 16 h before the DLMO to the mean log-lux in the three phase delay zone bins 4 h before to 8 h after the DLMO (Figure 3c) was greater in the non-overlapping evening versus morning types (2.68 versus 1.79, F = 15.994, p = 0.003). Of note, this ratio was not significantly different in the overlapping types: 2.42 and 2.66 in the evening and morning types, respectively (F = 3.601, p = 0.067). Of course, this division of the circadian day into single phase-delay and phase-advance regions is an oversimplification; the magnitude of phase shifts varies across the circa-dian day (Khalsa et al., 2003). When we examine just those bins corresponding to the greatest phase advances (i.e., 12–16 h before the DLMO; see Figure 3c, white arrow), and phase delays (i.e., 0–4 h after the DLMO; see Figure 3c, black arrow) in non-overlapping chronotypes, the ratio of phase-advancing to phase-delaying light exposure is even higher in evening types, although it is no longer significant (4.49 in evening types and 0.51 in morning types, F = 1.990, p = 0.192).
It should be acknowledged that our data could also be explained by greater sensitivity of evening types to the phase-delaying effects of evening light and/or less sensitivity to the phase-advancing effects of morning light, and/or the converse in morning types—that is, differences in coupling strength. This would explain the tendency in evening types for a later phase as well as the decreased “need” for light exposure on the phase-delay region or increased “need” for light exposure on phase-advance region of the light PRC that we observed. Of course, differences in τ and coupling strength are not mutually exclusive.
Tau is greater than 24 h in most humans (Burgess & Eastman, 2008; Czeisler et al., 1999; Duffy & Wright Jr., 2005; Duffy et al., 2001). Accordingly, we might expect an average pattern of light exposure in most individuals that is slightly biased toward the phase-advance region of the light PRC, which would offset the tendency for phase to delay to a later time (see Figure 3) (Czeisler et al., 1989; Khalsa et al., 2003; Shanahan et al., 1999). Indeed, as noted above, we found the ratio of phase-advancing to phase-delaying light was greater than a value of one in both chronotypes, indicating a bias toward the phase-advance zone. However, we acknowledge that a significant minority, approximately 25%, of sighted subjects have been shown to have periods <24 h, that they would therefore require a pattern of light exposure biased toward the phase-delay region, and that a disproportionate number of these individuals might be expected to be morning types (Duffy & Wright Jr., 2005; Duffy et al., 2001). Further analysis of the light data may indicate which morning types not only have short circadian periods but also have periods <24 h.
Relationship between Circadian Phase and Sleep
In many human studies, the phase angle of entrainment has been measured using the time interval between circadian phase and some measure of sleep timing, typically sleep onset, sleep offset, or midsleep (Baehr et al., 2000; Duffy et al., 1999; Liu et al., 2000; Mongrain et al., 2004). However, such a measure is valid only to the extent that the timing of sleep accurately reflects the entraining light/dark cycle (Duffy & Wright Jr., 2005). Differences in light exposure across the day may make sleep an inadequate proxy for light. We could not demonstrate a difference between all chronotypes in the timing of sleep relative to phase: although sleep occurred later relative to the DLMO in evening types compared to morning types, this difference was small and statistically insignificant (see Table 1, all subjects, and Figure 1a). However, differences were found when the data were subdivided. Among chronotypes with overlapping or intermediate phases, the habitual timing of sleep occurred earlier relative to phase (shorter PAD) in morning types (see Table 1, overlapping phases, and Figure 1b), while among chronotypes with non-overlapping or extreme phases, the habitual timing of sleep occurred later relative to phase (longer PAD) in morning types (see Table 1, non-overlapping phases, and Figure 1c). This replicates what has been found in young men and women (Goulet et al., 2007; Mongrain et al., 2004) studied under similar non-uniform light conditions. In comparison, studies under uniform light conditions have found the habitual timing of sleep occurred later relative to phase in young male morning types, similar to the non-overlapping or extreme phase chronotypes (see Table 1 and Figure 1c) but opposite of the overlapping or intermediate phase chronotypes (see Table 1 and Figure 1b) (Duffy et al., 1999).
One elegant explanation for differences in the timing of sleep relative to circadian phase, both within and between studies, has been proposed by Mongrain and colleagues (2004) and is consistent with our findings. They hypothesized that morning and evening types may be divided into those with primarily a circadian versus those with primarily a homeostatic sleep-drive component to their chronotype. In one subset of morning and evening types (that is, those with extreme phases, as in Figure 1c), they demonstrated differences consistent with a circadian explanation for chronotype. Evening types had a greater ratio of phase-advancing to phase-delaying light that presumably offset their greater tendency to phase delay, and phase occurred later relative to the habitual timing of sleep, reflecting the longer (more negative) phase angle of entrainment expected with a longer circadian period (Goulet et al., 2007; Mongrain et al., 2004; Moore-Ede et al., 1982). In another subset of chronotypes (that is, those with intermediate phases, as in Figure 1b), they demonstrated differences consistent with a homeostatic explanation. Morning types had higher decay rates in slow wave activity and awoke at an earlier circadian phase, presumably reflecting a more rapid dissipation of the homeostatic sleep-drive (Mongrain et al., 2004, 2006).
The different findings may also be due to the different patterns of light exposure in the laboratory versus the field. Duffy and colleagues (1999) studied subjects after three days of L:D 16:8 at 150 lux:<0.03 lux. Under these conditions, the timing of the sleep/wake and light/dark schedules is the same, and light intensity is uniform across the day. Although this provides the most valid measure of phase angle of entrainment to the light-dark cycle, it has been shown to alter the timing of phase relative to sleep. Wright and colleagues (2005) demonstrated the interval between the melatonin onset and habitual sleep time/darkness onset changed after subjects were subjected to two-five days of uniform light exposure of 150 or 450 lux during the day. Circadian phase shifted later despite the fact the timing of light as well as ratio of the duration of light to darkness was the same as it had been in the outside world. This may have been due to a relative increase in evening (phase-delaying) light exposure and/or a relative decrease in morning (phase-advancing) light exposure under the uniform light conditions of the laboratory (150 or 450 lux). Therefore, the timing of sleep relative to phase measured following uniform light conditions is primarily a measure of the phase angle of entrainment to the unique light/dark cycle of the laboratory and might differ from the timing of sleep relative to phase in the natural environment.
Finally, differences in the timing of sleep relative to phase between chronotypes may also be due to complex age, sex, and imposed schedule effects. Although an association between age and chronotype has long been known to exist (see, e.g., Tonetti et al., 2008), the circadian component of chronotype has been found to be muted with age. Circadian period correlates with diurnal preference and phase in young subjects but not older ones (Duffy & Czeisler, 2002). Although Duffy et al. (1999) found the habitual timing of sleep occurred later relative to phase in young male morning types, it was earlier relative to phase in older male and female morning types—a pattern more consistent with the homeostatic relationship described by Mongrain et al. (2004, 2006). We included age in all of our models, but we did not find a significant correlation between age and PAD. Sex should also be considered, as several studies have found the habitual timing of sleep occurs later relative to phase in women (Baehr et al., 2000; Campbell et al., 1989; Mongrain et al., 2004). Although there was a tendency for sleep to occur later relative to phase in women (longer PAD), this was not significant when we controlled for multiple covariates (age, daylength, overlap status, and employment status). If sex differences do exist in the timing of sleep relative to circadian phase, they could be due to differences in either homeostatic sleep-drive or circadian parameters (i.e., period or responsiveness to zeitgebers). No sex differences have been found in homeostatic sleep-drive (Armitage et al., 2000; Mongrain et al., 2005), suggesting the sex differences that have been found may be circadian in etiology; females may have a shorter τ and/or may be more responsive to environmental time cues. The effects of schedule constraints may also be a factor (Leonhard & Randler, 2009; Roenneberg et al., 2003b). To roughly control for photoperiod, we required subjects to keep a regular sleep/wake schedule of their choosing for a week (Goulet et al., 2007). To accomplish this, it is likely that both types kept more of a “work day” schedule on their “free days,” and because evening types tend to shift the timing of sleep later on “free days” more than morning types, this probably resulted in a greater change in sleep timing from baseline for the evening types (Roenneberg et al., 2003b). Furthermore, the majority of our subjects had some constraints on their schedule either because they were employed or were students.
LIMITATIONS
We did not measure ocular light exposure directly. Although we attempted to account for obvious instances where the light sensor was covered, more subtle instances might persist in the data set. Moreover, our results do not adequately take into account the shape of the light PRC or dose-response curve. We did not measure the spectral composition of the light, and it is likely that the type of light exposure (artificial versus natural) varied across the day. Although no difference was found in the timing of sleep relative to phase, polysomnography was not obtained, and, therefore, no conclusions can be made about differences in homeostatic sleep-drive between the two groups. Furthermore, our subdivision of subjects into overlapping and non-overlapping categories is somewhat arbitrary, as it was based solely on the clock hour of the DLMO, and it is conceivable that subdivision of the dataset using different criteria would yield different results.
It should be acknowledged that the timing of light exposure is gated by the timing of sleep and a host of demographic variables (e.g., employment status), and that non-circadian differences between chronotypes could have caused the different patterns of light exposure across the 24 h day (see Figure 2) (Monk et al., 2004; Roenneberg et al., 2003b). However, we do not think this invalidates our conclusions about the phase angle of entrainment, as our primary aim was to determine the steady-state relationship between ECP and the timing of light exposure across the circadian day (Figure 2), regardless of the reasons for a particular pattern of light exposure. According to fundamental circadian theory, any differences in the timing or intensity of light exposure, for whatever reason, would result in a different phase angle of entrainment in such a way that entrainment was maintained (Roenneberg et al., 2003a). This difference in phase angle of entrainment should be solely dependent upon differences in τ and coupling strength between the pacemaker and the relevant zeitgeber(s).
CONCLUSIONS
We have demonstrated that evening compared to morning types have a higher ratio of phase-advancing to phase-delaying light, indicative of a difference in phase angle of entrainment between the two groups. We interpret this as indicating a longer circadian τ in evening types. Further work determining whether chronotypes are differentially sensitive to the resetting effects of light is indicated.
ACKNOWLEDGMENTS
The research was supported by grants from the Public Health Service: K23RR017636 to JSE; R01 EY018312-09A1, R01 HD42125, and R01 AG21826 to AJL; and MO1 RR000334 and UL1 RR024120 to Oregon Health & Science University and the Oregon Clinical and Translational Research Institute, respectively. Dr. Emens is also supported by the Sleep Research Society Foundation Gillin Award and a NARSAD Young Investigator Award.
Footnotes
Publisher's Disclaimer: The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Armitage R, Hoffmann R, Trivedi M, Rush AJ. Slow-wave activity in NREM sleep: Sex and age effects in depressed outpatients and healthy controls. Psychiatry Res. 2000;95:201–213. doi: 10.1016/s0165-1781(00)00178-5. [DOI] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm with an emphasis on morningness-eveningness. J. Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Brown H, Prescott R. Applied mixed models in medicine. John Wiley & Sons; West Sussex, UK: 2003. pp. 33–101. [Google Scholar]
- Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe A, Herzel H, Kramer A. Molecular insights into human daily behavior. Proc. Nat. Acad. Sci. 2008;105:1602–1607. doi: 10.1073/pnas.0707772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J. Biol. Rhythms. 2008;23:374–376. doi: 10.1177/0748730408318592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Jewett ME, Dijk DJ. Human circadian melatonin rhythm phase delay during a fixed sleep-wake schedule interspersed with nights of sleep deprivation. J. Pineal Res. 2003;35:149–157. doi: 10.1034/j.1600-079x.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Gillin JC, Kripke DF, Erikson P, Clopton P. Gender differences in the circadian temperature rhythms of healthy elderly subjects: Relationships to sleep quality. Sleep. 1989;12:529–536. [PubMed] [Google Scholar]
- Chang A, Scheer FA, Czeisler CA. Adaptation of the human circadian system by prior light history. Sleep (Abstract Suppl.) 2008;31:A45–A46. [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type O) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk D-J, Kronauer RE. Stability, precision and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circa-dian phase, and diurnal preference in humans. Neurosci. Lett. 2002;318:117–120. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Wright KP., Jr Entrainment of the human circadian system by light. J. Biol. Rhythms. 2005;20:326–338. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk D, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young or older people. J. Invest. Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningnesseveningness, usual wake time, and circadian phase. Behav. Neurosci. 2001;115:895–899. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- Goulet G, Mongrain V, Desrosiers C, Paquet J, Dumont M. Daily light exposure in morning-type and evening-type individuals. J. Biol. Rhythms. 2007;22:151–158. doi: 10.1177/0748730406297780. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Wright KP, Kronauer RE, Czeisler CA. Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc. Nat. Acad. Sci. USA. 2007;104:9081–9086. doi: 10.1073/pnas.0702835104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J. Pineal Res. 2002;33:198–203. doi: 10.1034/j.1600-079x.2002.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasser SA, Hanifin JP, Rollag MD, Brainard GC. Dim light adaptation attenuates acute melatonin suppression in humans. J. Biol. Rhythms. 2006;21:394–404. doi: 10.1177/0748730406292391. [DOI] [PubMed] [Google Scholar]
- Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase-response curve to single bright light pulses in human subjects. J. Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhard C, Randler C. In sync with the family: Children and partners influence the sleep-wake circadian rhythm and social habits of women. Chronobiol. Int. 2009;26 doi: 10.1080/07420520902821101. in press. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL. The dim light melatonin onset (DLMO) as a marker for circadian phase position. Chronobiol. Int. 1989;6:93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Boney RS, Clemons AA, Anderson NR, Pen SD, Bauer VK, Cutler NL, Harker CT. Assays for measuring the dim light melatonin onset (DLMO) in human plasma. Sleep Res. 1997;26:733. [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc. Nat. Acad. Sci. USA. 2006;103:7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Uchiyama M, Shibui K, Kim K, Kudo Y, Hirokuni T, Suzuki H, Okawa M. Diurnal preference, sleep habits, circadian sleep propensity and melatonin rhythm in healthy human subjects. Neurosci. Lett. 2000;280:199–202. doi: 10.1016/s0304-3940(00)00793-x. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Lavoie S, Selmaoui B, Paquet J, Dumont M. Phase relationships between sleep-wake cycle and underlying circadian rhythms in morningness-eveningness. J. Biol. Rhythms. 2004;19:248–257. doi: 10.1177/0748730404264365. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Carrier J, Dumont M. Chronotype and sex effects on sleep architecture and quantitative sleep EEG in healthy young adults. Sleep. 2005;28:819–827. doi: 10.1093/sleep/28.7.819. [DOI] [PubMed] [Google Scholar]
- Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. J. Sleep Res. 2006;15:162–166. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Potts JM, DeGrazia JM, Kupfer DJ. Morningness-eveningness and life-style regularity. Chronobiol. Int. 2004;21:435–443. doi: 10.1081/cbi-120038614. [DOI] [PubMed] [Google Scholar]
- Moore-Ede M, Sulzman FM, Fuller CA. The clocks that time us. Harvard University Press; Cambridge, Mass.: 1982. pp. 74–77. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: Pacemaker as clock. J. Comp. Physiol. 1976;106:291–331. [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky MH. Ethical and methodological standards for laboratory and medical biological rhythm research. Chronobiol. Int. 2008;25:999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Randler C. Differences in sleep and circadian preference between Eastern and Western German adolescents. Chronobiol. Int. 2008;25:565–575. doi: 10.1080/07420520802257794. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Daan S, Merrow M. The art of entrainment. J. Biol. Rhythms. 2003a;18:183–194. doi: 10.1177/0748730403018003001. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J. Biol. Rhythms. 2003b;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kumar CJ, Merrow M. The human circadian clock entrains to sun time. Curr. Biol. 2007;17:R44–R45. doi: 10.1016/j.cub.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Shanahan TL, Kronauer RE, Duffy JF, Williams GH, Czeisler CA. Melatonin rhythm observed throughout a three-cycle bright-light stimulus designed to reset the human circadian pacemaker. J. Biol. Rhythms. 1999;14:237–253. doi: 10.1177/074873099129000560. [DOI] [PubMed] [Google Scholar]
- Smith KA, Schoen MW, Czeisler CA. Adaptation of human pineal melatonin suppression by recent photic history. J. Clin. Endocrinol. Metabol. 2004;89:3610–3614. doi: 10.1210/jc.2003-032100. [DOI] [PubMed] [Google Scholar]
- Taillard J, Philip P, Coste O, Sagaspe P, Bioulac B. The circadian and homeostatic modulation of sleep pressure during wakefulness differs between morning and evening chronotypes. J. Sleep Res. 2003;12:275–282. doi: 10.1046/j.0962-1105.2003.00369.x. [DOI] [PubMed] [Google Scholar]
- Tonetti L, Fabbri M, Natale V. Sex difference in sleep-time preference and sleep need: A cross-sectional survey among Italian pre-adolescents, adolescents, and adults. Chronobiol. Int. 2008;25:745–759. doi: 10.1080/07420520802394191. [DOI] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J. Biol. Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Wright SP. Adjusted P-values for simultaneous inference. Biometrics. 1992;48:1005–1013. [Google Scholar]
- Wright KP, Jr, Hughes RJ, Kronauer RE, Dijk DJ, Czeisler CA. Intrinsic near-24-h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans. Proc. Nat. Acad. Sci. USA. 2001;98:14027–14032. doi: 10.1073/pnas.201530198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, Jr, Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J. Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]