Abstract
A panel of 21 monoclonal antibodies (MAb) against the erythrocytic stages of Babesia bigemina was produced. The 21 MAb could be divided into four distinct immunofluorescence groups on the basis of their patterns of binding to B. bigemina-infected erythrocytes. Nine MAb positive in the indirect immunofluorescent antibody test with acetone-fixed B. bigemina-infected erythrocytes were selected for purification and further characterization. The MAb were species specific, since they did not cross-react with B. bovis, but they were not stage specific. The purpose of this study was to evaluate the biological activity of B. bigemina-specific MAb in vitro. Three MAb inhibited the multiplication of B. bigemina in vitro as assessed by a lowered percentage of parasitized erythrocytes and by a decreased incorporation of [3H]hypoxanthine. These MAb are now being utilized to purify the correspondent parasite polypeptides, either native or recombinant, in order to test them as subunit immunogens.
Full text
PDF

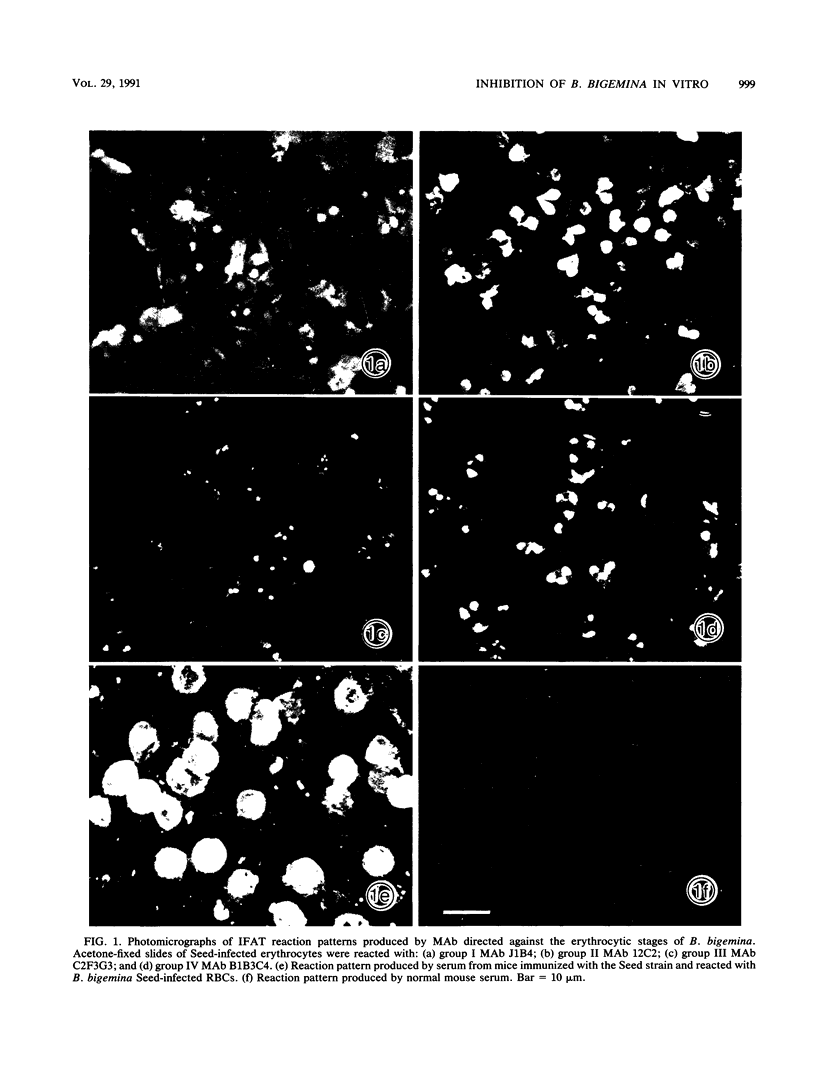



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruck C., Drebin J. A., Glineur C., Portetelle D. Purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel Blue chromatography. Methods Enzymol. 1986;121:587–596. doi: 10.1016/0076-6879(86)21058-7. [DOI] [PubMed] [Google Scholar]
- Callow L. L. Sterile immunity, coinfectious immunity and strain differences in Babesia bigemina infections. Parasitology. 1967 Aug;57(3):455–465. doi: 10.1017/s0031182000072346. [DOI] [PubMed] [Google Scholar]
- Cohen S. Monoclonal antibodies in parasitic infectious diseases. Br Med Bull. 1984 Jul;40(3):291–296. doi: 10.1093/oxfordjournals.bmb.a071991. [DOI] [PubMed] [Google Scholar]
- Curnow J. A. The use of a slide agglutination test to demonstrate antigenic differences between Babesia bigemina parasites. Aust Vet J. 1973 Jun;49(6):290–293. doi: 10.1111/j.1751-0813.1973.tb06808.x. [DOI] [PubMed] [Google Scholar]
- Figueroa J. V., Buening G. M., Kinden D. A., Green T. J. Identification of common surface antigens among Babesia bigemina isolates by using monoclonal antibodies. Parasitology. 1990 Apr;100(Pt 2):161–175. doi: 10.1017/s0031182000061163. [DOI] [PubMed] [Google Scholar]
- Fine E., Inselburg J., Obeing J., Hanson A. Plasmodium falciparum-inhibitory monoclonal antibodies produced by human hybridomas. Parasite Immunol. 1987 May;9(3):305–320. doi: 10.1111/j.1365-3024.1987.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Goff W. L., Davis W. C., Palmer G. H., McElwain T. F., Johnson W. C., Bailey J. F., McGuire T. C. Identification of Babesia bovis merozoite surface antigens by using immune bovine sera and monoclonal antibodies. Infect Immun. 1988 Sep;56(9):2363–2368. doi: 10.1128/iai.56.9.2363-2368.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff W. L., Yunker C. E. Babesia bovis: increased percentage parasitized erythrocytes in cultures and assessment of growth by incorporation of [3H]hypoxanthine. Exp Parasitol. 1986 Oct;62(2):202–210. doi: 10.1016/0014-4894(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Hall R., McBride J., Morgan G., Tait A., Zolg J. W., Walliker D., Scaife J. Antigens of the erythrocytes stages of the human malaria parasite Plasmodium falciparum detected by monoclonal antibodies. Mol Biochem Parasitol. 1983 Mar;7(3):247–265. doi: 10.1016/0166-6851(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Howard R. F., Stanley H. A., Campbell G. H., Reese R. T. Proteins responsible for a punctate fluorescence pattern in Plasmodium falciparum merozoites. Am J Trop Med Hyg. 1984 Nov;33(6):1055–1059. doi: 10.4269/ajtmh.1984.33.1055. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- McElwain T. F., Perryman L. E., Davis W. C., McGuire T. C. Antibodies define multiple proteins with epitopes exposed on the surface of live Babesia bigemina merozoites. J Immunol. 1987 Apr 1;138(7):2298–2304. [PubMed] [Google Scholar]
- Palmer D. A., Buening G. M., Carson C. A. Cryopreservation of Babesia bovis for in vitro cultivation. Parasitology. 1982 Jun;84(Pt 3):567–572. doi: 10.1017/s0031182000052835. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Ramirez E., Lambert P. H., Miescher P. A. Inhibition of P. falciparum growth in human erythrocytes by monoclonal antibodies. Nature. 1981 Jan 22;289(5795):301–303. doi: 10.1038/289301a0. [DOI] [PubMed] [Google Scholar]
- Phillips R. S., Reid G. M., McLean S. A., Pearson C. D. Antigenic diversity in Babesia divergens: preliminary results with three monoclonal antibodies to the rat-adapted strain. Res Vet Sci. 1987 Jan;42(1):96–100. [PubMed] [Google Scholar]
- Pinder M., Hewett R. S. Monoclonal antibodies detect antigenic diversity in Theileria parva parasites. J Immunol. 1980 Feb;124(2):1000–1001. [PubMed] [Google Scholar]
- Rodriguez S. D., Buening G. M., Vega C. A., Carson C. A. Babesia bovis: purification and concentration of merozoites and infected bovine erythrocytes. Exp Parasitol. 1986 Apr;61(2):236–243. doi: 10.1016/0014-4894(86)90157-8. [DOI] [PubMed] [Google Scholar]
- Taggart R. T., Samloff I. M. Stable antibody-producing murine hybridomas. Science. 1983 Mar 11;219(4589):1228–1230. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- Vega C. A., Buening G. M., Green T. J., Carson C. A. In vitro cultivation of Babesia bigemina. Am J Vet Res. 1985 Feb;46(2):416–420. [PubMed] [Google Scholar]
- Vega C. A., Buening G. M., Rodriguez S. D., Carson C. A. Concentration and enzyme content of in vitro-cultured Babesia bigemina-infected erythrocytes. J Protozool. 1986 Nov;33(4):514–518. doi: 10.1111/j.1550-7408.1986.tb05653.x. [DOI] [PubMed] [Google Scholar]
- Winger C. M., Canning E. U., Culverhouse J. D. A monoclonal antibody to Babesia divergens which inhibits merozoite invasion. Parasitology. 1987 Feb;94(Pt 1):17–27. doi: 10.1017/s0031182000053415. [DOI] [PubMed] [Google Scholar]
- Wright I. G., Mirre G. B., Rode-Bramanis K., Chamberlain M., Goodger B. V., Waltisbuhl D. J. Protective vaccination against virulent Babesia bovis with a low-molecular-weight antigen. Infect Immun. 1985 Apr;48(1):109–113. doi: 10.1128/iai.48.1.109-113.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright I. G., White M., Tracey-Patte P. D., Donaldson R. A., Goodger B. V., Waltisbuhl D. J., Mahoney D. F. Babesia bovis: isolation of a protective antigen by using monoclonal antibodies. Infect Immun. 1983 Jul;41(1):244–250. doi: 10.1128/iai.41.1.244-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]