Abstract
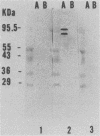
Eighty strains of pathogenic Escherichia coli, representing each of the major diarrheal disease-causing groups, were examined by direct enzyme-linked immunosorbent assay (ELISA) for the presence of proteins associated with a 60-MDa plasmid from E. coli serotype O157:H7. Antiserum specific for plasmid-encoded proteins was prepared by immunizing a rabbit with a wild-type E. coli O157:H7 strain (strain 7785) and absorbing the serum with a plasmid-cured derivative (strain 2-45). Use of this antiserum in Western immunoblot analysis detected two proteins of 82 and 92 kDa in strain 7785 but not in strain 2-45. All 16 wild-type E. coli O157:H7 strains and all 10 Shiga-like toxin (SLT)-producing E. coli strains of serotypes other than O157 were ELISA positive. Thirteen of 14 enterotoxigenic and all of 24 enteroinvasive E. coli strains were ELISA negative, as were all of 16 E. coli strains isolated from healthy persons. Of 16 traditional enteropathogenic E. coli (EPEC) serotypes, 10 were ELISA positive, including 10 of 12 strains carrying the EPEC adherence factor gene. Absorption of the serum with an EPEC adherence factor-positive EPEC eliminated EPEC reactivity. This study demonstrates that two plasmid-mediated proteins are common to E. coli O157:H7 strains and to SLT-producing strains of other serotypes. Detection of these proteins by ELISA provides a sensitive and specific screening test for identifying SLT-producing E. coli of both O157 and non-O157 serotypes. Identification of the cross-reactive proteins found in EPEC could provide the basis for a single assay to detect both EPEC and SLT-producing E. coli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldini M. M., Nataro J. P., Kaper J. B. Localization of a determinant for HEp-2 adherence by enteropathogenic Escherichia coli. Infect Immun. 1986 Apr;52(1):334–336. doi: 10.1128/iai.52.1.334-336.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp C. A., Greene K. D., Downes F. P., Sowers E. G., Wells J. G., Wachsmuth I. K. Unusual verotoxin-producing Escherichia coli associated with hemorrhagic colitis. J Clin Microbiol. 1987 Aug;25(8):1486–1489. doi: 10.1128/jcm.25.8.1486-1489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Jerse A. E., Yu J., Tall B. D., Kaper J. B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Heesemann J., Laufs R., O'Brien A. D., Tacket C. O., Levine M. M. A plasmid of enterohemorrhagic Escherichia coli O157:H7 is required for expression of a new fimbrial antigen and for adhesion to epithelial cells. Infect Immun. 1987 Feb;55(2):455–461. doi: 10.1128/iai.55.2.455-461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna J. G., Major G. N., Williams R. S. Methods for reducing non-specific antibody binding in enzyme-linked immunosorbent assays. J Immunol Methods. 1985 Dec 27;85(2):409–419. doi: 10.1016/0022-1759(85)90150-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Xu J. G., Kaper J. B., Lior H., Prado V., Tall B., Nataro J., Karch H., Wachsmuth K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987 Jul;156(1):175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Pál T., Pácsa A. S., Emödy L., Vörös S., Sélley E. Modified enzyme-linked immunosorbent assay for detecting enteroinvasive Escherichia coli and virulent Shigella strains. J Clin Microbiol. 1985 Mar;21(3):415–418. doi: 10.1128/jcm.21.3.415-418.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley L. W., Remis R. S., Helgerson S. D., McGee H. B., Wells J. G., Davis B. R., Hebert R. J., Olcott E. S., Johnson L. M., Hargrett N. T. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983 Mar 24;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- Riley L. W. The epidemiologic, clinical, and microbiologic features of hemorrhagic colitis. Annu Rev Microbiol. 1987;41:383–407. doi: 10.1146/annurev.mi.41.100187.002123. [DOI] [PubMed] [Google Scholar]
- Rumschlag H. S., Shinnick T. M., Cohen M. L. Serological responses of patients with lepromatous and tuberculoid leprosy to 30-, 31-, and 32-kilodalton antigens of Mycobacterium tuberculosis. J Clin Microbiol. 1988 Oct;26(10):2200–2202. doi: 10.1128/jcm.26.10.2200-2202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Marques L. R., Newland J. W., Smith H. W., Holmes R. K., O'Brien A. D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986 Jul;53(1):135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth I., Cohen M. L., Rumschlag H. S., Riley L. W., White E. H., Carr J. H., Bond W. W., Wachsmuth I. K. Influence of the 60-megadalton plasmid on adherence of Escherichia coli O157:H7 and genetic derivatives. Infect Immun. 1990 May;58(5):1223–1231. doi: 10.1128/iai.58.5.1223-1231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S., Karch H., Wachsmuth K. I., Robins-Browne R. M., O'Brien A. D., Lior H., Cohen M. L., Smithers J., Levine M. M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987 Dec;55(12):3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]