Abstract
NMDA receptors in the spinal cord dorsal horn (SCDH) mediate some inflammatory pain behaviors. Here, we used rAAV vectors expressing an active small interfering RNA (siRNA) (vector 6) targeting the essential NR1 subunit of the NMDA receptor or a mismatch siRNA (vector MM-6) sequence to determine the consequences of RNAi-mediated knockdown of NR1 expression on NMDA receptor levels and formalin-induced pain behaviors in adult rats. Three weeks following intraparenchymal administration of the vector 6 into the right lumbar SCDH, NR1 mRNA and protein levels were significantly reduced (P<0.01) in the ipsilateral SCDH compared to the contralateral SCDH, but not in vector MM-6 or non-vector control animals. Formalin-induced phase 2 nociceptive response was significantly reduced (P<0.05) in vector 6 animals compared to controls. While neither vectors affected normal mechanical threshold, vector 6 provided protection from the mechanical allodynia seen in controls at 24 hours following intraplantar formalin. Vector 6 also prevented the increase in phosphorylated NR1 levels seen in the ipsilateral SCDH of control rats 45 minutes post-formalin. These results indicate that vector-derived siRNAs can effectively produce spatial knockdown of NR1 gene expression and this knockdown selectively attenuates in vivo NMDA receptor-mediated formalin behaviors and NR1 phosphorylation in the rat.
Perspective
This study reveals that a single administration of an siRNA-expressing viral vector produces significant knockdown of the NR1 gene in the SCDH of adult rats. This pre-clinical study demonstrates the use of RNAi to target the expression of genes mediating pain and the therapeutic potential of this approach.
Keywords: Pain, formalin, siRNA, RNA interference, NR1, NMDA receptor
Introduction
The recently discovered RNA interference (RNAi) is an evolutionarily conserved mechanism for silencing gene expression in which a targeted mRNA is degraded by a double stranded RNA sequence known as a small interfering RNA (siRNA)16. siRNAs can be derived from a longer double stranded RNA or short hairpin (sh) RNAs which can be expressed from plasmids or viral vectors31. RNAi offers several advantages over current approaches targeting genes by homologous recombination, many of which were discussed in a previous study18. Two very important advantages of this technique are, firstly, it is not limited to the mouse and secondly, siRNAs can be expressed from viral vectors to produce a long-lasting stable knockdown of target gene expression. To further probe into the first-mentioned advantage, we expanded on our previous study by investigating the applicability of RNAi in adult rats. Previously, we designed and evaluated the effects of siRNAs targeting the NR1 subunit gene in adult mice, while here we specifically investigate whether the same approach can be used to knockdown the NR1 gene in adult rats.
Functional NMDA receptors are tetramers composed of two NR1 subunits and two NR2 subunits. The NR1 subunit is required for the function of this ionotropic glutamatergic receptor 36; 26. It is well established from studies in both rats and mice that the NMDA receptor plays a very critical role in mediating injury-induced pain behaviors. This role was characterized by studies employing pharmacological antagonism (e.g. 33; 48, 4; 11, antisense oligos 39 and a conditional knockout of NR1 gene 42. In addition, we confirmed the critical role of NMDA receptors in mediating inflammatory pain by demonstrating that a viral vector expressing an siRNA that knocked down NR1 mRNA and protein expression also protected the mice from the development of CFA-induced mechanical allodynia18. Given the homology of the mouse and rat genome, siRNA sequences that are complementary to both mouse and rat NR1 gene were designed and used in the study. Having shown that viral delivery of siRNAs produces a stable long-lasting knockdown of NR1 and disruption of the NMDA receptor function with behavioral consequences in mice, we undertook this study to determine whether this technique can reliably produce similar functional outcomes in adult rats.
Therefore, we investigated the effects of intraparenchymal administration of recombinant adeno-associated viral vectors expressing an active NR1 siRNA or a scrambled siRNA sequence into the spinal cord dorsal horn (SCDH) of adult rats on the expression levels of NR1 mRNA, total and phosphorylated NR1 protein expression levels and NMDA receptor function. We focused on the effects of NR1 gene knockdown on the well-characterized formalin-induced pain response and on the development of mechanical allodynia and thermal hyperalgesia.
Materials and Methods
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee (protocol #0508-392A) of Weill Cornell Medical College.
Design of siRNAs and shDNAs
The details of the design, screening and packaging of short hairpin DNA (shDNA) into a recombinant adeno-associated virus (rAAV) serotype 2 vector are previously reported 18. Due to the very high homology between the rat (Genbank, Accession Number NM_017010) and mouse NR1 gene sequence (Genbank, Accession Number NM_008169), the siRNA sequences selected in the previous study are 100% homologous to the corresponding rat NR1 sequence: shDNA 6: GAATGTCCATCTACTCTGA TTCAAGAGA TCAGAGTAGATGGACATTC) targets the rat NR1 gene at nucleotide position 636-654. MM-6: GTATGACCATGTACTCTCA TTCAAGAGA TGAGAGTACATGGTCATAC is a randomly scrambled sequence that contained the same nucleotides as found in shDNA 6.
Injection of viral vectors
rAAV expressing green fluorescent protein (GFP), a reporter gene and either the shRNA 6 (vector 6) or MM-6 (vector MM-6) sequence was injected into adult male Sprague Dawley rat (∼250g; Taconic) SCDH as previously described for the adult mice 42; 18. The rats were briefly anesthetized with isoflurane, followed by a ketamine-xylazine mixture given intraperitoneally and the dorsal spinous processes T13 and L1 removed by laminectomy. The virus was injected intraparenchymally (IPI) into the SCDH, targeting the lumbar enlargement. Four unilateral (right side) injections of 1 μl each were administered 0.7–0.8 mm apart, at a depth of approximately 0.5 mm from the dorsal border and 0.7 mm from the midline using a glass pipette with a 40 micron diameter tip attached to a 10 μl Hamilton syringe. The syringe was mounted on a microinjector (KOPF) attached to a stereotaxic unit (KOPF). After injection, the dorsal musculature was sutured and the skin was closed. The animals were treated with the local anesthetic, bupivacaine and a triple antibiotic ointment (bacitracin-neomycin-polymixin B) topically, before being returned to their cages. Animals were carefully monitored post surgery for any weight loss, infection or signs of discomfort. Only animals with none of the above or no apparent motor deficits were used in the studies described below at least three weeks later. The three week delay between the injection of the vector and experimental measures allowed for maximal transcriptional activity of the vector in vivo (27; 42).
Post-injection evaluation of GFP and NR1 expression
At least 3 weeks following vector administration, the rats (∼350-400g) were anesthetized with pentobarbital (50mg/kg) and transcardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. The spinal cord was dissected and placed in 4% PFA for 2 hours before being transferred to 30% sucrose for cryoprotection. Following at least 72 hours in sucrose, the lumbar spinal cord was cut into four 4 mm sections and frozen into a mold with optimal cutting temperature (OCT) compound. Twenty micron cryostat (Leica) sections were prepared for immunohistochemistry and in situ hybridization. In some animals, floating sections (50μm) were prepared using a freezing microtome.
Immunohistochemistry
Immunohistochemistry was used to evaluate the expression and spread of GFP and the effect of the vectors on NR1 protein levels. The spinal cord sections were incubated in rabbit polyclonal antibodies to NR1 (1:250, 07-362, #21353; Upstate, Lake Placid, NY) or with GFP (1:1000; A11122; Molecular Probes Inc, Eugene, OR). The sections were then incubated in appropriate biotinylated secondary antibodies (BA-1000 and BA-9200, 1:250; Vector Laboratories, Burlingame, CA), and immunoreactivity was detected with the avidin-biotin-peroxidase complex (ABC)-3,3-diaminobenzidinetetra-hydrochloride technique 25. As a control, some slides were exposed to diluted normal goat serum instead of the primary antibody.
NR1 in situ hybridization
Both mounted and floating sections were used for in situ hybridization. The details of the procedure using mounted sections are as reported previously 18, while the details for the floating sections in situ hybridization are described by 44. The spinal cord tissue was hybridized in Digoxigenin-labeled antisense ribroprobes. Sense ribroprobes were used for background controls. The same concentrations of probes were used for both mounted and floating sections. Quantification of NR1 mRNA for the entire SCDH was estimated using the Metamorph software program (Universal Imaging, Downingtown, PA).
Western blot analysis
Knockdown of NR1 protein was also assessed using western blot analysis. Animals were sacrificed at least three weeks following the IPI injection of the viral vector. Non-vector treated animals of similar weights were used for controls. Animals were first anesthetized with isoflurane. One centimeter of the lumbar SCDH was rapidly dissected and hemisected to produce left (contralateral) and right (ipsilateral) tissue. The tissue was immediately homogenized in ice-cold modified RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA) containing a protease inhibitor (Sigma, St. Louis, MO), 2 mM PMSF and phosphatase inhibitors (2 mM Na3VO4, 2 mM NaF). Homogenates were flash frozen in liquid nitrogen, stored briefly at -80°C until dissections were completed, then defrosted and sonicated on ice. Following the determination of protein concentrations using the DC Assay kit (BioRad, Hercules, CA), samples were diluted in Laemmli sample buffer and stored at -80°C. Equal amounts (25ug) of total protein were subjected to SDS-PAGE using 10% Tris-HCl precast gels (BioRad, Hercules, CA) and then transferred to PVDF membranes (Millipore, Bedford, MA), which were subsequently blocked in 5% blotting grade milk (BioRad, Hercules, CA). Blots were incubated in primary antibody generated in mouse for NR1 (1:500; NB300-118, #BW1006; Novus Biologicals, Littleton, CO) and mouse monoclonal primary antibody for actin (1:80,000; A5441, #055K4854; Sigma, St. Louis, MO) overnight at 4°C. HRP-conjugated goat anti-mouse secondary antibody (1:40,000 for NR1 and 1:120,000 for actin; 31460, #JF11303808; Pierce, Rockford, IL) was added for 1 hour at room temperature. The blots were developed with ECL (Pierce, Rockford, IL) and exposed to film (Kodak, Rochester, NY) for various times. Exposures yielding signal intensity in the linear range were used for densitometric analysis with Fluorchem 9900 (Alpha Innotech, San Leandro, CA). Ratios of the intensity of the NR1 signal to actin (loading control) were calculated, normalized to control (contralateral side of non-vector treated animals) and averaged for animals within each group.
Behavioral testing
Formalin test
An automated nociceptive analyzer was used to determine the effect of vector 6 and vector MM-6 on nociceptive behavior following intraplantar formalin administration 49; 32. Prior to formalin injection, a soft metal band was placed on the right hind-paw of ∼350-400g adult rats (non-vector treated or animals previously injected with a viral vector) and placed inside the testing chamber for at least 30 minutes to acclimate. Fifty μL of formalin solution (5% formaldehyde in 0.9% saline) was injected into the plantar surface of the right hind-paw. Rats were then returned to the testing chamber and the number of flinches or lifts per minute was automatically recorded for a total of 60 minutes.
Mechanical Stimulus Threshold
The effect of NR1 gene knockdown on formalin induced mechanical allodynia was studied at 1 and 24 hours post formalin. The mechanical stimulus threshold of each hind-paw of each rat was determined before and three weeks after viral vector administration. Individual rats were placed in testing chambers with metal mesh floor at least 15 minutes before testing for acclimation. Using calibrated von Frey hairs, the 50% g withdrawal threshold was determined using the up-down method of Dixon3. Starting with a von Frey filament of 5.5g, filaments were applied perpendicularly against the midplantar surface of the hind-paw in a sequential ascending or descending order until the threshold for each rat was determined. The threshold value is calculated from six determinations 3. The response to 15g was accepted as the maximum stimulus threshold. The establishment of mechanical allodynia (demonstrated as a significant decrease in paw withdrawal threshold) was assessed in both of the hind-paws of non-vector treated and vector-injected animals at 1 and 24 hours (hrs) post-formalin.
Thermal (Heat) Paw-Withdrawal latency
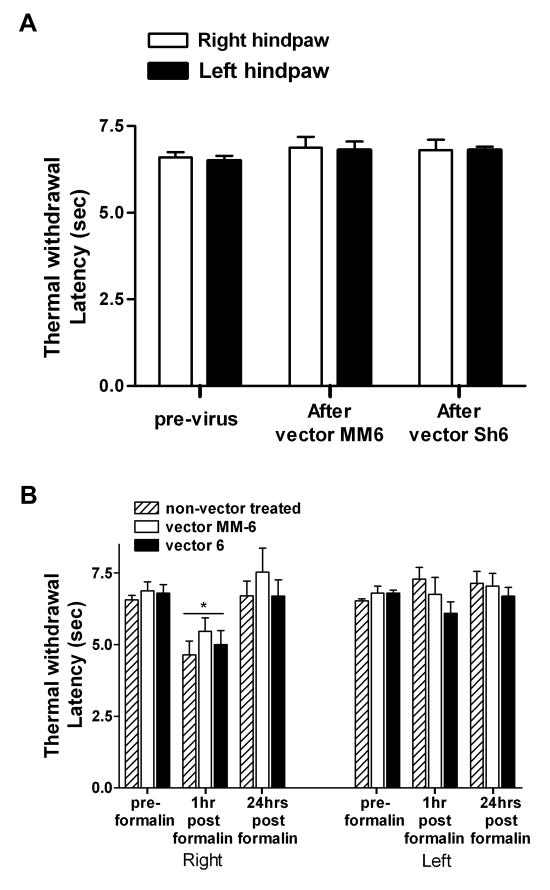
Next, we investigated the effect of NR1 gene knockdown on the establishment of thermal hyperalgesia induced by formalin. The thermal paw withdrawal latency was determined using a thermal nociceptive stimulus 22 in adult rats before and at least three weeks post vector injection. Animals were placed in a Plexiglas cage on a preheated glass plate maintained at 30°C and left to acclimate for 30 minutes. A radiant thermal stimulus was focused on the midplantar surface of the hind-paw, and the latency in seconds for the withdrawal of the paw from the heat source was determined automatically. Each paw was evaluated separately. A maximal cutoff of 20 seconds was employed. The threshold value is the average of three determinations. The development of thermal hyperalgesia (demonstrated as a significant decrease in the withdrawal latency) was also assessed in both hind-paws of non-vector treated and vector injected animals at 1 and 24hrs following formalin. Separate groups of animals were used for mechanical and thermal paw withdrawal testing.
Phosphorylated NR1 subunit following intraplantar formalin
Previous studies demonstrate that increases in pNR1 occur as a function of inflammatory or nociceptive pain 50; 1. Therefore, we investigated the effects of intraplantar formalin on phosphorylation of the NR1 subunit of the NMDA receptor and the effect of vector MM-6 and vector 6 on pNR1 expression in the SCDH using western blot analysis. Non-vector treated and animals previously injected with vector MM-6 or vector 6 were sacrificed 45 minutes following intraplantar formalin in the right hind-paw. The lumbar SCDH was dissected to obtain right (ipsilateral) and left (contralateral) samples and treated as detailed above (Western Blot analysis). Blots were incubated in primary antibodies generated in rabbit for phospho-NMDA1 (pNR1) at the protein kinase C-dependent Ser896 site (1:1000; 3384, #1; Cell signaling Technology # 3384, Danvers, MA) overnight at 4°C. HRP-conjugated goat anti-rabbit secondary antibody (1:2000; 31460, #JF1144844; Pierce, Rockford, IL) was added for 1 hour at room temperature. Actin was used as a loading control. Ratios of the intensity of the pNR1 signal to actin (loading control) were calculated and normalized to control (contralateral side of non-vector treated animals).
Statistics and Graphs
A two way ANOVA with repeated measures (treatment vs time point) was followed by the Bonoferroni post hoc analysis using the Statview 4.5 software program (SAS Institute, Inc). A one way ANOVA (multiple groups), the t test (two groups) or the paired t test (before and after treatment) were used with the InStat v3 (GraphPad) program. Graphs were made with Prizm v3 (GraphPad) and imported into Adobe Illustrator v10 (Adobe) for final editing.
Results
Viral transduction in the SCDH measured by GFP labeling
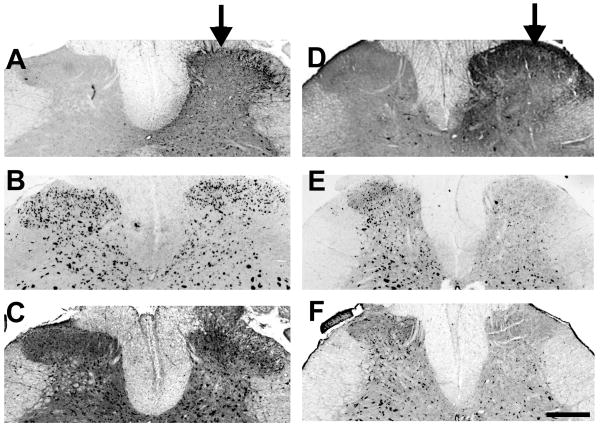
Three weeks following IPI of vector 6 or MM-6, spinal cord sections were assessed for viral transduction and NR1 knockdown. Adjacent spinal sections from an animal injected with vector MM-6 are shown in figures 1A –C, while sections from a vector 6 animal are shown in figures 1D-F. GFP expression was spatially localized to the ipsilateral SCDH in animals injected with either vector (Figure 1A and D). The rostrocaudal extent of GFP labeling was about 4 mm with most pronounced expression in the L4 and L5 segments. GFP expression extended to the caudal part of L3 and rostral part of L6 segments, although with much less prominence. GFP expression was seen in both cell bodies and neuropile.
Figure 1.
Active siRNA (vector 6) results in NR1 knockdown. Three weeks following intraparenchymal injection of an rAAV vector expressing an active siRNA (vector 6), there is a spatially localized expression of GFP (D) and knockdown of both NR1 mRNA (E) and protein expression (F) in the spinal cord dorsal horn (SCDH) of adult rats. In contrast, injection of vector MM-6 produces spatially localized GFP expression (A), but no knockdown of NR1 mRNA (B) or protein (C) in the rat SCDH. Arrows indicate the ipsilateral (injected) side; scale bar is 500 μm.
NR1 mRNA and protein expression
In adjacent sections, NR1 mRNA labeling by in situ hybridization and NR1 protein labeling by immunohistochemistry revealed an almost complete depletion of NR1 mRNA and protein in the ipsilateral dorsal horn of animals injected with vector 6 compared to the contralateral side (Figure 1E and F, respectively). This knockdown of expression at both the mRNA and protein level followed the same spatial pattern as the expression of GFP and was localized to just the ipsilateral SCDH (Figure 1). In contrast, vector MM-6 did not affect NR1 mRNA or protein labeling, although the expression of GFP is spatially localized to the ipsilateral SCDH and has the same distribution as seen after vector 6 (Figure 1B and C, respectively). Although the assessment of mRNA and protein levels and behavior were done three weeks following vector administration, observations from the mouse study showed that siRNA delivered by the rAAV used here can produce gene knockdown up to at least 6 months following a single administration 18.
Quantification of NR1 mRNA and protein expression
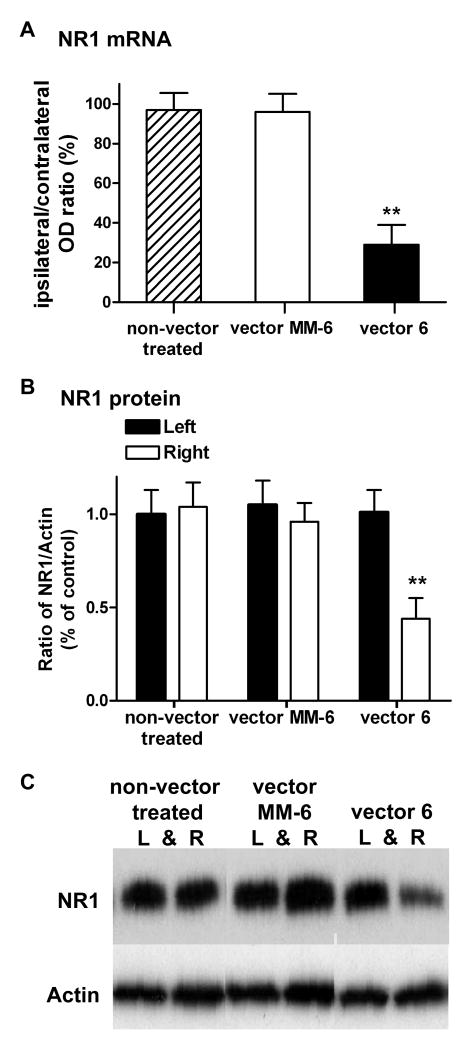
As mentioned previously 18, to determine the extent of knockdown of NR1 mRNA levels, we analyzed the in situ hybridization images using Metamorph software. Measuring the integrated gray level per unit area (integrated optical density; OD) in the whole SCDH region of the ipsilateral side and comparing to the corresponding contralateral side, estimated the changes in the NR1 mRNA. Four spinal cord sections approximately 600 μm apart were averaged per animal. Figure 2A shows that in the active vector 6 animals (n=6), there was a significant reduction in NR1 mRNA (71%; P<0.01) in the ipsilateral SCDH compared to the contralateral SCDH. In contrast, there was no significant change in the ipsilateral/contralateral OD ratio of non-vector control (n=3) or vector MM-6 animals (n=6). Consistent with the observation of a knockdown of both NR1 mRNA and protein expression by immunohistochemical labeling, western blot analysis also revealed a reduction of NR1 protein in the ipsilateral (right) SCDH of vector 6 injected animals compared to the contralateral (left) side. The level of NR1, expressed as a ratio to actin, was compared in a total of six groups. As shown in Figure 2B, the contralateral SCDH of non-vector treated animals (n = 8) was normalized to 1 and served as the control group. In a total of 8 animals, there was a significant reduction (56%) in the amount of NR1 protein in the ipsilateral SCDH of vector 6 injected animals, compared to the control group (P<0.01). The magnitude of knockdown of NR1 protein levels using western blot analysis may be somewhat less than the actual knockdown for the following reasons. First, it may include NR1 subunits found on primary afferents and other pre-synaptic terminals, which are not affected by the rAAV virus since the virus does not transduce retrogradely 42. Second, the viral spread covered a much shorter rostrocaudal distance than the 1 cm of SCDH tissue used for western blot analysis. In contrast, there was no significant difference (P>0.10) in the expression level of NR1 in the contralateral SCDH of vector 6 injected animals, nor was there any significant change in the NR1/actin ratio for the right SCDH of non-vector treated animals or the ipsilateral (right) and contralateral (left) SCDH of vector MM-6 injected animals (n=8). An example of the western blot is shown in Figure 2C.
Figure 2.
Active siRNA vector 6 reduces NR1 mRNA and protein expression. (A) Vector 6 significantly (**P<0.01) reduces the integrated optical density (OD) value of NR1 mRNA (by in situ hybridization) in the ipsilateral spinal cord dorsal horn (SCDH) compared to the contralateral side (expressed as a ratio) (n=6). In contrast, in both non-vector control and vector MM-6 animals, there is no significant reduction in the ratio of ipsilateral/contralateral OD, signifying no reduction in NR1 mRNA levels in the ipsilateral SCDH of these two groups. B) Western blot analysis measuring the NR1/actin ratio shows that injection of vector 6 results in a significant reduction (**P<0.01) in NR1 protein in the SCDH of adult rats (n=8) compared to controls (left SCDH of non-vector treated animals; n =8). The level of NR1 protein was also significantly different compared to the contralateral side of vector 6 injected animals. In contrast, there were no significant differences in the expression level of NR1 protein in left or right side of MM-6 injected animals (n = 8) compared to the control group. C) An example of the western blot is shown for NR1 and actin expression in left and right SCDH for a non-vector treated animal, a vector MM-6 injected and a vector 6 injected animal.
Knockdown of NR1 decreased phase 2 of the formalin response
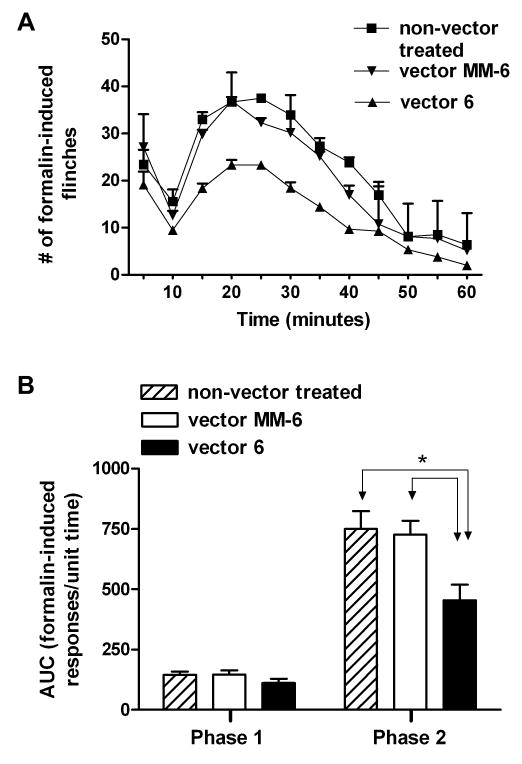
Twenty non-vector treated adult rats received an intraplantar injection of formalin into the right hind-paw. These rats displayed the normal biphasic formalin response, with phase 1 characterized as 0-9 minute response and phase 2 as 10-60 minute post-injection response. Between phase 1 and 2 was an interphase of decreased activity 9; 24. Twenty-six animals were also injected with formalin (vector 6; n=12 and vector MM-6; n =14) at least three weeks following viral injection. Both groups of animals displayed the biphasic formalin responses (Figure 3A). Measurement of the area under the curve (AUC; Figure 3B) showed that the phase 1 formalin response was not significantly altered in both groups of injected animals (vector 6: 112 ±18 & vector MM-6: 147 ±17) compared to non-vector treated animals (145 ± 13). However, the phase 2 formalin response was significantly reduced in vector 6 injected animals (455 ± 65) compared to both non-vector treated (750 ± 74; P<0.05) and vector MM-6 injected (725 ± 57; P<0.05) animals. There was no difference in the phase 2 response of non-vector treated and vector MM-6 groups.
Figure 3.
NR1 knockdown reduces phase 2 formalin response. Intraplantar administration of formalin induces a biphasic formalin response (phase 1; 0-9 minutes and phase 2; 10-60 minutes) in all three groups of animals (non-vector treated; n = 20, vector MM-6; n = 14 and vector 6 injected; n = 12). However, in the active siRNA group (vector 6) the phase 2 response is reduced compared to the other two groups. B) Quantitative measurement of the formalin response (area under the curve; AUC) reveals that the phase 2 formalin response is significantly reduced in the vector 6 injected animals compared to the MM-6 injected (*P<0.05) and non-vector treated animals (*P<0.05). There was no difference in the MM-6 group compared to the non-vector treated animals nor was there any difference in the phase 1 formalin response in any of the three groups.
Mechanical allodynia following formalin
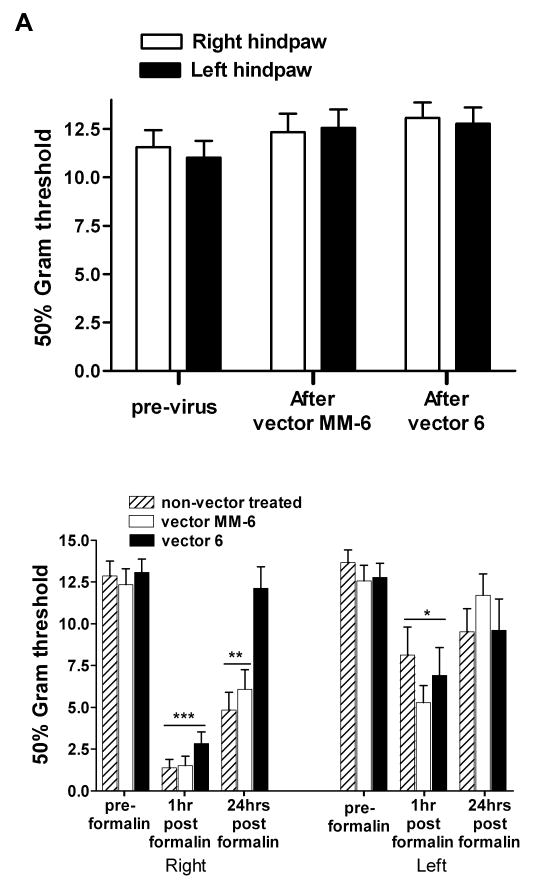
IPI injection of vector 6 (n=10) or vector MM-6 (n=9) into the SCDH did not affect the mechanical threshold response to von Frey filaments (P = 0.8; Figure 4A), in agreement with previous studies suggesting that the NMDA receptor does not mediate responses to innocuous stimuli, consistent with observations in adult mice18. One hour following injection of the inflammatory agent formalin into the right hind-paw, vector 6, vector MM-6 as well the non-vector treated (n=10) animals showed a significant reduction in mechanical threshold. A two way ANOVA with repeated measures (treatment vs time point) indicated that, for the right paw, a main effect of treatment F2,52 = 9.454; P<0.001, a main effect of time point F2,52 = 94.946; P<0.0001 and an interaction between the two, F4,52 = 4.057; P<0.01. The post hoc analysis (Bonoferroni) revealed that the 1 hr time points for all 3 treatments were significantly different than the corresponding groups at either the pre-formalin or 24 hr time points (P<0.001). At 24 hrs the vector 6 group was not different than the corresponding vector 6 pre-formalin group but was different from the each of the groups at 1 hr and from the non-vector treated and vector MM-6 groups at the 24 hr time point (P<0.01). The decrease in mechanical threshold response is consistent with the development of allodynia at 1hr post formalin administration. Therefore, the vector 6 treatment protected the animals from mechanical allodynia at 24 hrs after formalin. Although the reduction of mechanical threshold was most significant in the right paw of all three groups, formalin's effect was bilateral. A two way ANOVA with repeated measures (treatment vs time point) indicated that, for the left paw a main effect of time point only F2,52 = 14.530; P<0,001. The post hoc analysis (Bonoferroni) revealed that the 1 hr time points for all 3 treatments were significantly different from the corresponding groups at the pre-formalin time point only (P<0.001) (Figure 4B).
Figure 4.
Spatial knockdown of NR1 in the spinal cord dorsal horn (SCDH) provides protection from the establishment of mechanical allodynia 24hrs post formalin. A) Three weeks after the intraparenchymal injection of vector 6 (n =10) or MM-6 (n =9) into the right SCDH, the baseline mechanical responses to von Frey filaments are not altered. B) One hour post intraplantar injection of formalin into the right hind-paw, all three treatments were significantly different than the corresponding groups at either the preformalin or 24 hr time points (***P<0.001). At 24 hrs the vector 6 group was not different than the corresponding vector 6 pre-formalin group but was different from the both the non-vector treated and vector MM-6 groups at the 1 hr time point (**P<0.01). In all three groups of animals, the effect of formalin was bilateral causing a significant lowering of mechanical response in the left hind-paw at 1hr post formalin (*P<0.05) compared to the pre-formalin threshold.
Thermal hyperalgesia following formalin
We also investigated the development of thermal hyperalgesia post-formalin in non-vector treated, vector 6 injected and vector MM-6 injected animals. As was observed with mechanical threshold, injection of vector 6 (n=6) or vector MM-6 (n=8) had no effect on acute thermal responses measured three weeks following injection (Figure 5A), again suggesting that knockdown of NR1 has no effect on baseline thermal responses. A two way ANOVA with repeated measures (treatment vs time point) indicated a main effect of treatment only for the right paw responses, F2,38 = 24.971; p<0.001. The post hoc analysis (Bonoferroni) revealed that the 1 hr time points for all 3 treatments were significantly different from the corresponding groups at the pre-formalin and 24 hr time points (p<0.05). Unlike mechanical allodynia, where bilateral effects are observed at 1hr following formalin, there was no evidence of thermal hyperalgesia in the left hind-paw in any of the three groups of animals. This observation suggests that formalin, but not the siRNA, exerts a differential effect on mechanoreceptors compared to thermoreceptors.
Figure 5.
Spatial knockdown of NR1 does not affect the thermal withdrawal latency in adult rats. A) Intraparenchymal injection of vector MM-6 (n =8) or vector 6 (n = 6) does not affect baseline thermal withdrawal latencies measured three or four weeks following vector administration. B) However, one hour post intraplantar formalin, for the right paw, all 3 treatments were significantly different from the corresponding groups at the preformalin and 24 hr time points (*P<0.05).
We assessed the peripheral inflammatory response caused by formalin by measuring paw diameter before, 1hr and 24hrs post formalin in 8 non-vector treated control animals. There was a significant increase in the right hind-paw 1hr post formalin (from 6.2 ± 0.1 to 7.7 ± 0.2 mm; P<0.01), although by 24hrs the paw sizes had returned to pre-formalin values (6.5 ± 0.04 mm). There was no change in the diameter of the left hind-paw at any of the post formalin time-points compared to before formalin (6.2 ± 0.05mm).
Phospho-NR1 expression following intraplantar formalin
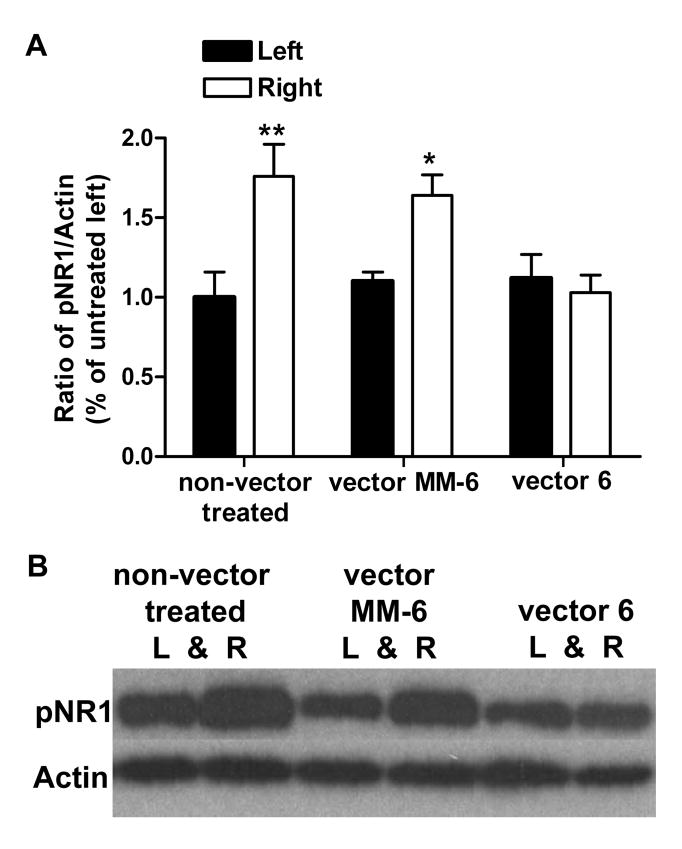
Forty-five minutes following intraplantar formalin, there was a significant increase in the level of phosphorylated (p)NR1 at the PKC dependent Ser896 site in the right SCDH of control animals (n=5) by 76% (P<0.01) compared to the left side. A significant increase was also seen in the right SCDH of vector MM-6 animals (n=5; 64%) following formalin (P<0.05), but not the left SCDH (10%; P> 0.05). There was no significant increase in pNR1 in either ipsilateral or contralateral SCDH of vector 6 treated animals (n=5; P> 0.05) despite displaying biphasic nociceptive responses following formalin (Figure 6A). An example of the western blot is shown in Figure 6B.
Figure 6.
Formalin induces NR1 phosphorylation. A) Forty-five minutes following intraplantar formalin in the right hind-paw, there is a significant increase in pNR1 levels in the ipsilateral spinal cord dorsal horn (SCDH) of non-vector treated (n=5; 76%, **P<0.01) and vector MM-6 (n=5; 64%, *P<0.05) animals compared to the contralateral sides. pNR1 levels are not increased in the either sides of the SCDH of vector 6 treated animals (n=5). B) An example of the western blot is shown for pNR1 and actin expression in left and right SCDH for a non-vector treated animal, a vector MM-6 injected and a vector 6 injected animal.
Discussion
This study investigates the utility of a vector-based approach of RNAi to knockdown the NR1 subunit of the NMDA receptor in the SCDH of adult rats. Because of the homology in the rat and mouse NR1 gene, we used siRNA sequences previously successful in adult mice 18. The NR1 gene was selected because firstly, it is essential for NMDA receptor functions and secondly, the NMDA receptor plays very critical roles in injury-induced pain and plasticity, which makes it feasible to assess behaviorally the consequences of a decrease in NMDA receptor function. An online paradigm from Whitehead Institute of Biomedical Research, MIT was used to design potential siRNA sequences, which were subsequently screened using the cell-based PsiCheck assay 18. This facile approach differentiates active and inactive siRNA sequences in vitro before proceeding to in vivo applications.
Studies examining the consequences of NR1 knockdown on NMDA receptor functions used antisense oligonucleotide 39 and a Cre-lox P conditional knockout technique 42 in mice. Although these techniques produced significant NR1 knockdown (30-50% and 80-90%, respectively), there are several advantages of RNAi. Firstly, a single administration of a vector expressing an siRNA produces prolonged, spatially localized NR1 gene knockdown in adult rats, as previously demonstrated in adult mice 18. Quantification of the in situ hybridization images revealed about 70% knockdown in the ipsilateral SCDH mRNA levels of vector 6 animals. This magnitude of mRNA knockdown and the pattern of GFP transduction and spread were very similar to that observed in mice 18. Similarly, western blot analysis revealed nearly 60% knockdown in NR1 protein levels in the ipsilateral SCDH of vector 6 animals compared to the contralateral SCDH and to vector MM-6 and non-vector treated animals. This is unlike antisense oligonucleotide, which requires repeated administration 39 or acute siRNA approaches which yield transient gene knockdown 43. Secondly, unlike homologous recombination, RNAi can be easily applied to the rat to target any gene. This is especially advantageous since the rat has been widely used in many preclinical pain studies. Traditionally, the more direct approach to studying receptor functions has been mainly confined to the use of pharmacological antagonists, which can be limited both by selectivity and spatial distribution. Thirdly, although RNAi produces incomplete gene knockdown, the behavioral consequences are often comparable to complete or conditional knockout. However, it is less expensive and can be used to study genes that are critical during development such as the NMDA receptor, in which case, complete NR1 knockout produces neonatal lethality 17. Overall, RNAi is a facile approach to selectively target gene expression in adults while eliminating problems associated with developmental aberrations, viability or pharmacological cross-reactivity.
Potential disadvantages of RNAi include off-target effects, where non-targeted genes can be knocked down and activation of the interferon system, which can ultimately result in cell death. In a previous study, nuclear staining with TO-PRO3 demonstrated normal number and morphology of nuclei when vector-injected and non-injected SCDH were compared. We did not observe any significant local cell loss or systemic toxicity up to 6 months following the injection of vector 6 18. In that study and the present study, no differences were observed in the levels of NR1 protein when the ipsilateral and contralateral SCDH of mice injected with the control vector were compared. These observations, together with the direct demonstration of a substantial loss of NR1 mRNA indicate that the decrease in NR1 expression resulted from an RNAi-induced cleavage of NR1 mRNA and not as a result of non-specific secondary changes in cellular processes.
Neither vector 6 (NR1 knockdown) nor vector MM-6 affected normal thermal and mechanical thresholds. This is because these responses are not NMDA receptor-dependent, but involve fast AMPA receptor-mediated transmission 46. This implies and validates previous work from this laboratory that the rAAV vector is safe and by itself does not affect acute mechanical or thermal responses 42; 18. Although not measured in the present study, we found that a conditional knockout of NR1 using the same vector does not affect the expression of the NR2A or NR2B subunits 42.
In contrast, nociceptive behavior induced by intraplantar formalin 14 was altered in vector 6 animals, indicating that knockdown of NR1 gene disrupts NMDA receptor-mediated functions. Specifically, the phase 2 formalin response, which may be attributed to spinal cord sensitization, a phenomenon characterized by increased excitability of spinal neurons 12; 37, was significantly reduced. Since the NMDA receptor is implicated in mediating central sensitization 48; 42, a disruption in NMDA receptor function will affect this nociceptive response. Phase 1 response which results from the initial activation of nociceptive afferents 34; 37, was not affected by NR1 knockdown. Phosphorylation of NR1 at a PKC dependent site appears to be a critical requirement for phase 2 response 28; 50. In non-vector and vector MM-6 animals, formalin robustly increased pNR1 levels in the ipsilateral SCDH but failed to in vector 6 animals (see Figure 6), despite evoking a reduced biphasic nociceptive response. It is understandable why PKC dependent phosphorylation of NR1 is vital to phase 2 response i.e. central sensitization, since PKC activity increases the trafficking of NR1 subunits to the synapse 29; 38 and reduces the Mg2+ block 8, rendering the NMDA receptor more accessible and responsive. The NMDA receptor involvement in formalin-induced responses was previously shown using antagonists 20; 10; 4; 15; 40, antisense 30; 39, the conditional knockout 42 and siRNAs targeting the NR2B subunit 43. It is evident that RNAi-mediated knockdown of NR1 does not completely block phase 2 formalin response, a finding similar to that observed with antisense 39 and conditional knockout 42. While the magnitude of knockdown may somewhat parallel the reduction in phase 2 response, it is more likely that other systems such as the non-NMDA glutamatergic receptors 6 and pro-nociceptive neuropeptides 5; 23 make significant contributions to this nociceptive response. In contrast to the phase 2 formalin response, mechanical allodynia occurring at 24hrs following intraplantar formalin is completely blocked in vector 6 animals, suggesting that the maintenance of mechanical allodynia is entirely NMDA-receptor dependent and as previously reported, centrally mediated 41; 47; 45. The involvement of the NMDA receptor in the establishment of inflammation-induced mechanical allodynia is also observed with other inflammatory pain models. For example, CFA-induced mechanical allodynia is maintained beyond 24hrs, but is prevented with NR1 receptor knockdown 18 or antagonism 13; 21. An apparent discrepancy is the fact that protection is seen only at 24hrs and not 1hr post-formalin. Furthermore, at 1hr post-formalin, a lesser degree of mechanical allodynia is observed contralaterally. Several possibilities may account for these observations. First, this immediate response results primarily from peripheral, but not central, sensitization. At 1hr post-formalin, the injected paws are still significantly swollen indicative of peripheral sensitization. Blocking peripheral sensitization with topical lidocaine prevented CFA-induced phosphorylation of both NR1 and GluR1 subunits in adult rats 19, suggesting that peripheral sensitization may initiate nociceptive behaviors such as mechanical allodynia. Conversely, phosphorylated NMDA receptors may be required to maintain mechanical allodynia at 24hrs when the inflammation has subsided. Second, cytokines released from non-neuronal cells may be responsible for the mechanical allodynia occurring at 1hr post-formalin and moreover may account for the bilateral effects seen in all three groups of animals. In previous reports, bilateral mechanical allodynia induced by peri-sciatic administration of a pro-inflammatory cytokine or zymosan 2; 35 was blocked by pretreatment with fluorocitrate, a glial metabolic inhibitor or CNI-1493, a global inhibitor of pro-inflammatory cytokines. In both cases, the unilateral peripheral injury led to bilateral activation of glia and release of cytokines within the SCDH. In a similar manner, formalin may activate SCDH glia to release cytokines thus mediating the short-lasting bilateral mechanical allodynia in all three groups of animals. A greater release of cytokines ipsilaterally may account for the more pronounced mechanical allodynia in the injected hind-paw.
Formalin-induced thermal hyperalgesia observed only at 1hr in all three groups of animals was unaffected by NR1 knockdown. It is unlikely that the siRNA had a differential effect on thermoreceptors or mechanoreceptors. Instead, formalin may be less potent than other inflammatory agents such as CFA 21 or bee-venom 7, which reliably induces maintained thermal hyperalgesia in adult rats.
In this study, we show that a single administration of vector-based siRNAs can effectively target gene expression in the adult rat, as was previously demonstrated in adult mice. The active vector 6 was equally effective in attenuating CFA evoked mechanical allodynia in mice (Garraway et al, 2007) as well as both the spontaneous phase 2 behaviors and the 24 hr evoked allodynia after formalin in rats. Thus both spontaneous and evoked inflammatory pain, require a functional NMDA receptor.
The potential of viral-based gene targeting is enormous and provides an essential alternative to study injury-induced pain and other behaviors in mammalian systems where conventional approaches such as constitutive gene deletion or pharmacology are not feasible. This pre-clinical study demonstrates the use of RNAi to target the expression of genes mediating pain and the therapeutic potential of this approach.
Acknowledgments
We thank Amanda Weyerbacher and Ann Marie Gregus for assistance with western blot analysis and Paigee Chou for assistance with in situ hybridization. This work is supported in part, by NIDA grants, DA001457 and DA000198 (CEI), NIDA training grant DA007274 and a minority supplement to DA001457 (SG) and NIDA center grant DA005130.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- 2.Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- 3.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 4.Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- 5.Chapman V, Buritova J, Honore P, Besson JM. Physiological contributions of neurokinin 1 receptor activation, and interactions with NMDA receptors, to inflammatory-evoked spinal c-Fos expression. J Neurophysiol. 1996;76:1817–1827. doi: 10.1152/jn.1996.76.3.1817. [DOI] [PubMed] [Google Scholar]
- 6.Chapman V, Dickenson AH. Time-related roles of excitatory amino acid receptors during persistent noxiously evoked responses of rat dorsal horn neurones. Brain Res. 1995;703:45–50. doi: 10.1016/0006-8993(95)01063-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Luo C, Li H, Chen H. Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: a comparative study with the formalin test. Pain. 1999;83:67–76. doi: 10.1016/s0304-3959(99)00075-5. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 9.Coderre TJ, Fundytus ME, McKenna JE, Dalal S, Melzack R. The formalin test: a validation of the weighted-scores method of behavioural pain rating. Pain. 1993;54:43–50. doi: 10.1016/0304-3959(93)90098-A. [DOI] [PubMed] [Google Scholar]
- 10.Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis AM, Inturrisi CE. Attenuation of hyperalgesia by LY235959, a competitive N-methyl-D-aspartate receptor antagonist. Brain Res. 2001;894:150–153. doi: 10.1016/s0006-8993(00)03325-4. [DOI] [PubMed] [Google Scholar]
- 12.Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurones. Neurosci Lett. 1987;83:207–211. doi: 10.1016/0304-3940(87)90242-4. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 14.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 15.Elliott K, Hynansky A, Inturrisi CE. Dextromethorphan attenuates and reverses analgesic tolerance to morphine. Pain. 1994;59:361–368. doi: 10.1016/0304-3959(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 16.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 17.Forrest D, Yuzaki M, Soares HD, Ng L, Luk DC, Sheng M, Stewart CL, Morgan JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 18.Garraway SM, Xu Q, Inturrisi CE. Design and evaluation of small interfering RNAs that target expression of the N-methyl-D-aspartate receptor NR1 subunit gene in the spinal cord dorsal horn. J Pharmacol Exp Ther. 2007;322:982–988. doi: 10.1124/jpet.107.123125. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, Zou SP, Ikeda T, Dubner R, Ren K. Rapid and lasting increase in serine phosphorylation of rat spinal cord NMDAR1 and GluR1 subunits after peripheral inflammation. Thalamus & Related Systems. 2005;3:9–18. [Google Scholar]
- 20.Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990;518:218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- 21.Hama A, Woon Lee J, Sagen J. Differential efficacy of intrathecal NMDA receptor antagonists on inflammatory mechanical and thermal hyperalgesia in rats. Eur J Pharmacol. 2003;459:49–58. doi: 10.1016/s0014-2999(02)02828-5. [DOI] [PubMed] [Google Scholar]
- 22.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 23.Henry JL, Yashpal K, Pitcher GM, Chabot J, Coderre TJ. Evidence for tonic activation of NK-1 receptors during the second phase of the formalin test in the Rat. J Neurosci. 1999;19:6588–6598. doi: 10.1523/JNEUROSCI.19-15-06588.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henry JL, Yashpal K, Pitcher GM, Coderre TJ. Physiological evidence that the ‘interphase’ in the formalin test is due to active inhibition. Pain. 1999;82:57–63. doi: 10.1016/S0304-3959(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 25.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 26.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishie S. Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 27.Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, Brandon EP, Schaffer D, Verma IM, Lee KF, Heinemann SF, Gage FH. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc Natl Acad Sci USA. 2002;99:2320–2325. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HW, Kwon YB, Roh DH, Yoon SY, Han HJ, Kim KW, Beitz AJ, Lee JH. Intrathecal treatment with sigma1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br J Pharmacol. 2006;148:490–498. doi: 10.1038/sj.bjp.0706764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- 30.Lee IO, Yukhananov R, Standaert DG, Crosby G. NMDA-R1 antisense oligodeoxynucleotides modify formalin-induced nociception and spinal c-Fos expression in rat spinal cord. Pharmacol Biochem Behav. 2004;79:183–188. doi: 10.1016/j.pbb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman J, Song E, Lee SK, Shankar P. Interfering with disease: opportunities and roadblocks to harnessing RNA interference. Trends Mol Med. 2003;9:397–403. doi: 10.1016/S1471-4914(03)00143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannino CA, South SM, Quinones-Jenab V, Inturrisi CE. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formalin test. J Pain. 2007;8:334–342. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Mao J, Price DD, Hayes RL, Lu J, Mayer DJ. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain Res. 1992;598:271–278. doi: 10.1016/0006-8993(92)90193-d. [DOI] [PubMed] [Google Scholar]
- 34.McCall WD, Tanner KD, Levine JD. Formalin induces biphasic activity in C-fibers in the rat. Neurosci Lett. 1996;208:45–48. doi: 10.1016/0304-3940(96)12552-0. [DOI] [PubMed] [Google Scholar]
- 35.Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 37.Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 38.Scott DB, Blanpied TA, Ehlers MD. Coordinated PKA and PKC phosphorylation suppresses RXR-mediated ER retention and regulates the surface delivery of NMDA receptors. Neuropharmacology. 2003;45:755–767. doi: 10.1016/s0028-3908(03)00250-8. [DOI] [PubMed] [Google Scholar]
- 39.Shimoyama N, Shimoyama M, Davis AM, Monaghan DT, Inturrisi CE. An antisense oligonucleotide to the N-methyl-D-aspartate (NMDA) subunit NMDAR1 attenuates NMDA-induced nociception, hyperalgesia, and morphine tolerance. J Pharmacol Exp Ther. 2005;312:834–840. doi: 10.1124/jpet.104.074856. [DOI] [PubMed] [Google Scholar]
- 40.Shimoyama N, Shimoyama M, Elliott KJ, Inturrisi CE. d-Methadone is antinociceptive in the rat formalin test. J Pharmacol Exp Ther. 1997;283:648–652. [PubMed] [Google Scholar]
- 41.Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. J Neurophysiol. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- 42.South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulavsky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan PH, Yang LC, Shih HC, Lan KC, Cheng JT. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther. 2005;12:59–66. doi: 10.1038/sj.gt.3302376. [DOI] [PubMed] [Google Scholar]
- 44.Watakabe A, Ichinohe N, Ohsawa S, Hashikawa T, Komatsu Y, Rockland KS, Yamamori T. Comparative analysis of layer-specific genes in Mammalian neocortex. Cereb Cortex. 2007;17:1918–1933. doi: 10.1093/cercor/bhl102. [DOI] [PubMed] [Google Scholar]
- 45.Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology. 2007;106:864–867. doi: 10.1097/01.anes.0000264769.87038.55. [DOI] [PubMed] [Google Scholar]
- 46.Woolf CJ, Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. Proc Natl Acad Sci U S A. 1999;96:7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolf CJ, Shortland P, Sivilotti LG. Sensitization of high mechanothreshold superficial dorsal horn and flexor motor neurones following chemosensitive primary afferent activation. Pain. 1994;58:141–155. doi: 10.1016/0304-3959(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 48.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 49.Yaksh TL, Ozaki G, McCumber D, Rathbun M, Svensson C, Malkmus S, Yaksh MC. An automated flinch detecting system for use in the formalin nociceptive bioassay. J Appl Physiol. 2001;90:2386–2402. doi: 10.1152/jappl.2001.90.6.2386. [DOI] [PubMed] [Google Scholar]
- 50.Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci. 2006;31:539–548. doi: 10.1016/j.mcn.2005.11.008. [DOI] [PubMed] [Google Scholar]