Abstract
The gingipains of Porphyromonas gingivalis have been implicated in the virulence of this bacterium, and antibodies to the hemagglutinin/adhesin domain (HArep) of the gingipains have been shown to protect against P. gingivalis colonization. However, the cellular mechanisms involved in host responses to HArep have not been elucidated. The purpose of the present study was to determine the functional role of CD80 and CD86 in mediating systemic and mucosal immune responses to the recombinant HArep derived from the gingipain Kgp (Kgp-HArep) after intranasal (i.n.) immunization. We also investigated the effect of the mucosal adjuvants the B subunit of cholera toxin (CTB) and monophosphoryl lipid A (MPL) on the functional role of the costimulatory molecules for the induction of systemic and mucosal responses to Kgp-HArep. The in vivo functional roles of CD80 and CD86 were assessed in C57BL/6 wild-type (wt), CD80-/-, CD86-/- and CD80/CD86-/- mice following intranasal immunization with Kgp-HArep with or without adjuvant. Serum IgG and mucosal IgA antibody responses were induced following i.n. immunization of mice with Kgp-HArep, and were potentiated by CTB or MPL. A differential requirement of CD80 and/or CD86 was observed for systemic IgG anti-Kgp-HArep responses following the primary and secondary immunization with antigen alone or antigen + adjuvant. Compared to wt and CD80-/- mice, CD86-/- mice had reduced serum IgG anti-Kgp-HArep responses following the second immunization with antigen alone or antigen + CTB, whereas similar levels of serum IgG anti-Kgp-HArep antibody activity were observed in wt, CD80-/- and CD86-/- mice immunized with antigen + MPL. Analysis of the serum IgG subclass responses revealed that CD80 influenced both Th1- and Th2-like IgG subclass responses, while CD86 preferentially influenced a Th2-associated IgG subclass response to Kgp-HArep. Mucosal IgA anti-Kgp-HArep responses in saliva and vaginal washes were diminished in CD86-/- mice. In vitro stimulation of murine bone marrow-derived dendritic cells with Kgp-HArep, CTB and MPL resulted in an up-regulation of CD80 and especially CD86 expression. Taken together, our results demonstrate that CD80 and CD86 can play distinct as well as redundant roles in mediating a systemic immune response and that CD86 plays a unique role in mediating a mucosal response to Kgp-HArep following immunization via the i.n. route alone or with adjuvant.
Keywords: Costimulatory molecules, Porphyromonas gingivalis gingipain, mucosal immunization, mucosal adjuvants
1. Introduction
Porphyromonas gingivalis has been implicated as a major etiologic agent in adult periodontitis [1-3]. This bacterium expresses a variety of virulence factors, including lipopolysaccharide, hemagglutinins, fimbriae and proteases [4]. Among the proteases, the gingipains HRgpA and Kgp have been most extensively studied [5-7]. Interestingly, the hemagglutinin/adhesin domain of these gingipains contains one copy of the repeat units constituting the hemagglutinin HagA protein of P. gingivalis [8-12]. The HagA protein contains 3-4 contiguous repeats that are known as the HArep consensus [9, 10]. Studies have shown that antibodies specific for a sequence present within the HArep consensus were associated with reduced colonization of P. gingivalis in patients with periodontal disease [13], in addition to having an inhibitory effect on P. gingivalis-induced hemagglutination [14]. Our previous studies have demonstrated that the recombinant HArep antigen derived from the gingipain Kgp (Kgp-HArep) was effective in inducing humoral and mucosal immune responses, and antibodies to Kgp-HArep prevented P. gingivalis invasion of epithelial cells in vitro [15]. These findings provide evidence for the potential use of Kgp-HArep in the development of a vaccine against periodontitis.
For the development of a vaccine, it is imperative to understand not only the effectiveness of the different components for the induction of a protective response, but also the cellular mechanisms involved in mediating the response. It is well accepted that the costimulatory molecules CD80 and CD86 present on antigen-presenting cells (APC) are essential for T-cell activation and differentiation. A lack of participation of these molecules in cell signaling can result in clonal T-cell anergy, antigen-specific hyporesponsiveness or apoptosis [16-19]. Both CD80 and CD86 costimulatory molecules can be up-regulated upon cell activation; however, their receptor binding properties, kinetics and responsiveness to various stimuli may differ [20, 21], and their presence on the various APC may respond differently to the same antigen [22]. It has been shown that CD80 and CD86 can influence the immune response to immunogens by stimulating differentiation of CD4+ T cells into Th1 and Th2 lineages [23, 24]. However, it remains highly controversial whether CD80 and CD86 possess distinct roles in the differentiation and regulation of Th1 and Th2 cells [25].
The purpose of the present study was to determine the role of costimulatory molecules CD80 and CD86 in mediating the systemic and mucosal immune responses and Th cell differentiation following intranasal (i.n.) immunization with Kgp-HArep. The ability of the mucosal adjuvants the B subunit of cholera toxin (CTB) and the monophophoryl lipid A (MPL) to influence the immune response in the context of CD80/CD86 was also investigated. Furthermore, the regulation of CD80 and CD86 expression on dendritic cells was characterized after in vitro stimulation with Kgp-HArep.
2. Materials and methods
2.1. Mice
BALB/c wild-type (wt), CD80 knock-out (CD80-/-), CD86 knock-out (CD86-/-), and CD80 and CD86 double knock-out (CD80/CD86-/-) mice used in this study were bred and maintained in environment-controlled, pathogen-free conditions within the animal facility at the University of Alabama at Birmingham. The original breeding pairs of CD80-/-, CD86-/-, and CD80/CD86-/- mice were obtained from Arlene Sharpe (Brigham and Women's Hospital, Boston, MA). Female mice that were 8 to 12 weeks old were used in these studies. All experiments were done according to the National Institutes of Health guidelines, and protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
2.2. Antigen and adjuvants
Kgp-HArep was purified from the inclusion bodies of E. coli M15 [pREP4] containing the plasmid pQE-rHArep under denaturing conditions by using a His-bind resin column, according to a modification of the manufacturer's instructions (Novagen, Madison, WI), as previously described [15]. The purity of Kgp-HArep was confirmed by analysis of silver stained polyacrylamide gel and by Western blot analysis using a rabbit anti-Kgp-HArep antibody generated in this laboratory. The concentration of Kgp-HArep was estimated by the bicinchoninic acid protein determination assay (Pierce, Rockford, IL), using bovine serum albumin (BSA) as the standard.
Cholera toxin B subunit (CTB) used in this study was purchased from List Biological Laboratory, Inc. (Campbell, CA). MPL-AF®, an aqueous formulation containing MPL at a 4:1 molar ratio with dipalmitoylphosphatidyl choline and water, was obtained from Corixa Corporation (Hamilton, MT) and is referred to as MPL in this study.
2.3. Immunizations and sample collections
Groups of mice (6 mice/group) were immunized by the intranasal (i.n.) route on days 1 and 18 with Kgp-HArep alone (25 μg), Kgp-HArep (25 μg) with CTB (20 μg), or Kgp-HArep (25 μg) with MPL (25 μg) in a volume of 20 μl (10 μl per nare). A sham-immunized, control group of mice received PBS only. The amount of antigen and adjuvant used was based on preliminary studies. Plasma, saliva and vaginal wash samples were collected, as previously described [15], prior to immunization and at 2-week intervals following the initial immunization. Mice were sacrificed 4 weeks after the second immunization, and spleens and cervical lymph nodes (CLN) were aseptically removed for in vitro assessment of antigen-specific proliferative responses.
2.4. Evaluation of antibody responses
The levels of serum IgG and IgG subclass, salivary IgA and vaginal IgA anti-Kgp-HArep antibody activity were determined by an enzyme-linked immunosorbent assay (ELISA), as previously described [15]. Data were logarithmically transformed and presented as the geometric mean x/÷ standard error of the mean (S.E.M.) for ease of interpretation.
2.5. Proliferation assay
Single-cell suspensions from spleens and CLN were prepared by mechanically dispersing the tissues through 40 μm cell strainers (Falcon, BD Labware, Franklin Lakes, NJ) into Hank's balanced salt solution (HBSS) supplemented with 10% fetal calf serum. Erythrocytes were removed by using M-lyse buffer (R&D Systems, Inc., Minneapolis, MN). Cells were then washed and suspended in RPMI 1640 supplemented with 10% fetal calf serum, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 1.5 mg/ml of sodium bicarbonate, 50 μg/ml of gentamicin sulfate, 25 mM HEPES, 50 U/ml of penicillin, and 50 μg/ml of streptomycin (complete medium). The spleen and CLN cells (2 × 105 cells/well in 96-well plates) were cultured with medium alone, 2.5 μg/ml concanavalin A (ConA), or Kgp-HArep (20 μg/ml) for 48 h (ConA) or 96 h (Kgp-HArep) in a humidified CO2 incubator at 37°C. The proliferative responses of spleen and CLN cell cultures were assessed by measuring the amount of [3H]thymidine incorporation in a liquid scintillation counter following the addition of 0.5 μCi of [3H]thymidine for the last 18 to 20 h of incubation. The results are expressed as a stimulation index (SI) calculated as the ratio of the mean counts per minute (cpm) in Kgp-HArep-stimulated cultures to the mean cpm in unstimulated control cultures.
2.6. Generation of bone marrow-derived dendritic cells
Bone marrow from femurs of wt mice were collected as previously described [26]. Dendritic cells (DC) were generated by culturing the bone marrow cells in the presence of 20 ng/ml of murine rGM-CSF (Atlanta Biologicals, Atlanta, GA) in RPMI complete medium. The resulting nonadherent DC were harvested after 10 days, and the purity of the DC population was determined by flow cytometry analysis of CD11c+ cells. This procedure resulted in > 80% CD11c+ cells.
2.7. Flow cytometry analysis
Bone marrow-derived DC (1 × 106 cells/ml) were stimulated with Kgp-HArep, CTB or MPL for 24 h. Cells were then washed and suspended in fluorescence-activated cell sorter (FACS) buffer and stained with biotin-conjugated anti-CD11c monoclonal antibody (mAb), together with FITC-labeled CD80 and PE-labeled CD86 mAbs (eBioscience, San Diego, CA) for 30 min on ice. Cells were washed three times and stained with streptavidin-PerCP (BD Biosciences, San Jose, CA) for 30 min on ice. Cells were then washed twice and suspended in 500 μl of FACS buffer and immediately analyzed using a FACSCalibur (BD Biosciences).
2.8. Statistical analysis
Statistical significance between groups was evaluated by ANOVA and the Tukey multiple-comparisons test using the InStat program (Graphpad Software, San Diego, CA). Differences between groups were considered significant at the level of P < 0.05.
3. Results
3.1. Functional role of CD80 and CD86 in the systemic antibody responses to Kgp-HArep and the adjuvanticity of CTB and MPL
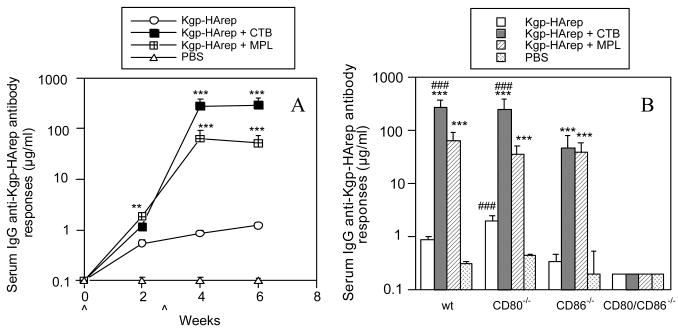
To determine the involvement of the costimulatory molecules CD80 and CD86 in host responses to Kgp-HArep and the ability of adjuvants CTB and MPL to influence such responses, wt, CD80-/-, CD86-/- and CD80/86-/- mice were immunized with antigen alone or with adjuvant by the i.n. route. An assessment of the kinetics of the systemic IgG anti-Kgp-HArep response in wt mice immunized with antigen alone revealed the induction of a serum IgG anti-Kgp-HArep antibody response that increased only slightly following the secondary immunization (Fig. 1A). Significantly higher IgG anti-Kgp-HArep antibody responses (P < 0.01 - 0.001) were seen in mice receiving antigen plus CTB or MPL compared to antigen alone. Moreover, mice immunized with Kgp-HArep + CTB had a significantly higher response (P < 0.001) than mice immunized with Kgp-HArep + MPL after the second immunization. These results suggest that CTB was more effective than MPL in potentiating a systemic IgG anti-Kgp-HArep antibody response following i.n. immunization with antigen and adjuvant.
Fig. 1.
Time course of the serum IgG anti-Kgp-HArep antibody responses in wt mice (A) and serum IgG anti-Kgp-HArep antibody responses in wt, CD80-/-, CD86-/- and CD80/CD86-/- mice 4 weeks post the initial immunization (B). Mice were immunized (ˆ) with Kgp-HArep, Kgp-HArep + CTB, Kgp-HArep + MPL or PBS by the i.n. route on days 0 and 18. “ˆ” indicates day of immunization. Values are expressed as the geometric mean ×/÷ S.E.M. (n = 6). Values are significantly different from the Kgp-HArep group at P < 0.001 (***) or P < 0.01 (**), or from the CD86-/- mice receiving the same immunization at P < 0.001 (###).
We next compared the serum IgG anti-Kgp-HArep responses induced in wt, CD80-/-, CD86-/- and CD80/CD86-/- mice. The time course of the antibody response in CD80-/- and CD86-/- mice was similar to that observed in wt mice (data not shown); however, no responses were induced in the CD80/CD86-/- mice (Fig. 1B). At week 4 after the initial immunization, the response to Kgp-HArep alone in CD80-/- mice was slightly higher, but not significant different from that seen in wt mice (Fig. 1B). However, the response in CD80-/- mice was significantly higher (P < 0.001) than that seen in CD86-/- mice. These results suggest a greater requirement for the costimulatory molecule CD86 than CD80 for a serum IgG antibody response to Kgp-HArep following i.n. immunization with antigen alone. Thus, CD86 appears to play a nonredundant role for the induction of a serum IgG antibody response to Kgp-HArep. The responses seen in wt, CD80-/- and CD86-/- mice immunized with antigen and CTB or MPL were significantly higher (P < 0.001) than those seen in mice immunized with antigen alone (Fig. 1B). The magnitude of the serum IgG anti-Kgp-HArep response following the second immunization with Kgp-HArep + MPL was similar in wt, CD80-/- and CD86-/- mice (Fig. 1B). However, mice deficient in CD86 had significantly lower (P < 0.001) levels of serum IgG anti-Kgp-HArep antibody activity than wt and CD80-/- mice immunized with Kgp-HArep + CTB. These results suggest a greater dependency for CD86 than CD80 for the adjuvant effect of CTB on responses to Kgp-HArep. The results also suggest that CD80 and CD86 can compensate for a serum IgG antibody response to Kgp-HArep following i.n. immunization with antigen + MPL.
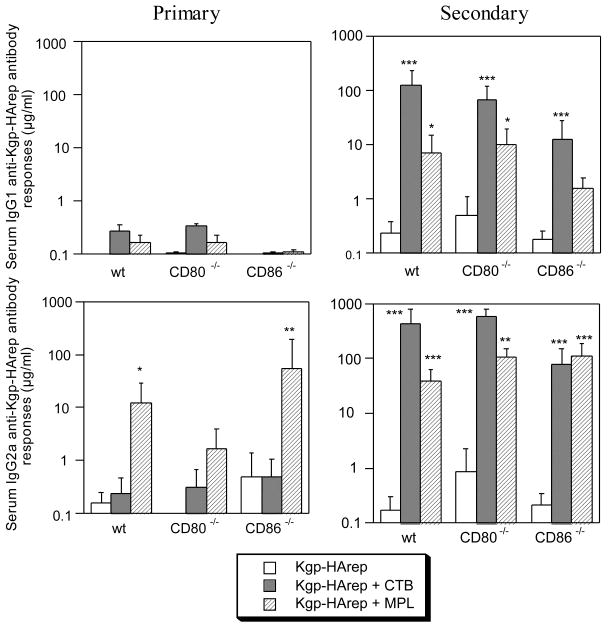
To learn more about the functional role of CD80 and CD86 in modulating the nature of the serum response to Kgp-HArep, and to gain insight into the Th cell subsets involved, serum samples were assessed for the IgG subclasses of the anti-Kgp-HArep antibody response. Two weeks following the initial i.n. immunization with Kgp-HArep alone, a low, preferential serum IgG2a antibody response was seen in wt and CD86-/-, but not CD80-/- mice (Fig. 2), suggesting that CD80 influences the induction of the Th1-type IgG subclass response to Kgp-HArep following the initial immunization. Immunization of mice with antigen + CTB resulted in an IgG1 anti-Kgp-HArep antibody response in wt and CD80-/-, but not CD86-/- mice, and an IgG2a anti-Kgp-HArep antibody response in wt, CD80-/- and CD86-/- mice. A preferential IgG2a anti-Kgp-HArep response was induced in mice immunized with antigen + MPL. In this case, the magnitude of the IgG2a responses in wt and CD86-/-, but not CD80-/- mice were significantly higher (P < 0.05 - 0.01) than those seen in mice immunized with antigen alone. Finally, MPL also potentiated a slight IgG1 antibody response in wt and CD80-/-, but not CD86-/- mice. These results indicate that MPL was effective in potentiating an IgG2a anti-Kgp-HArep response following a primary immunization, and suggest that CD80 influences this Th1-type response. Taken together, these results also suggest that CD86 influences the induction of a Th2-type IgG subclass response following a primary immunization with Kgp-HArep and adjuvant.
Fig. 2.
Serum IgG subclass responses to Kgp-HArep in wt, CD80-/- and CD86-/- mice 2 (primary) and 4 (secondary) weeks post the initial immunizaion. Mice were immunized by the i.n. route with Kgp-HArep, Kgp-HArep + CTB or Kgp-HArep + MPL on days 0 and 18. Values are expressed as the geometric mean ×/÷ S.E.M (n = 6). Values are significantly different from the Kgp-HArep group at P < 0.001 (***), P < 0.01 (**) or P < 0.05 (*).
Following the second i.n. immunization, antigen alone induced an increase in the levels of the serum IgG1 and IgG2a responses in wt, CD80-/- and CD86-/- mice. The response in CD80-/- mice was higher than seen in CD86-/- mice, suggesting an influence of CD86 for the Th1- and Th2-type responses to Kgp-HArep following the second immunization. The IgG1 and IgG2a responses induced in mice following the second immunization with antigen and adjuvant were in general significantly higher (P < 0.05 - 0.001) than those induced with antigen alone (Fig. 2). CTB potentiated both the IgG1 and IgG2a anti-Kgp-HArep antibody responses in wt, CD80-/- and CD86-/- mice following a second immunization. The IgG1 and IgG2a anti-Kgp-HArep responses induced in CD86-/- mice were lower than those induced in wt and CD80-/- mice, suggesting a role for CD86 in CTB potentiation of Th1- and Th2-type responses to Kgp-HArep following a second i.n. immunization.
The preferential induction of a serum IgG2a anti-Kgp-HArep responses seen in mice following an initial i.n. immunization with antigen + MPL was also seen following the second immunization, and the magnitude of the IgG2a responses induced in wt, CD80-/- and CD86-/- mice were similar (Fig. 2). MPL also potentiated a serum IgG1 anti-Kgp-HArep response following the second i.n. immunization. In this case, the IgG1 response in CD86-/- mice was lower than that seen in wt and CD80-/- mice. These results demonstrate that MPL potentiates a preferential Th1-associated IgG subclass response, and suggest that CD80 and CD86 compensate for the adjuvant activity of MPL to promote the Th1-type response to Kgp-HArep following the secondary immunization. The results also suggest that CD86 influences the induction of an IgG1 antibody response (Th2-type) following the second immunization with Kgp-HArep + MPL.
3.2. Functional role of CD80 and CD86 in the mucosal antibody responses to Kgp-HArep with CTB or MPL as adjuvant
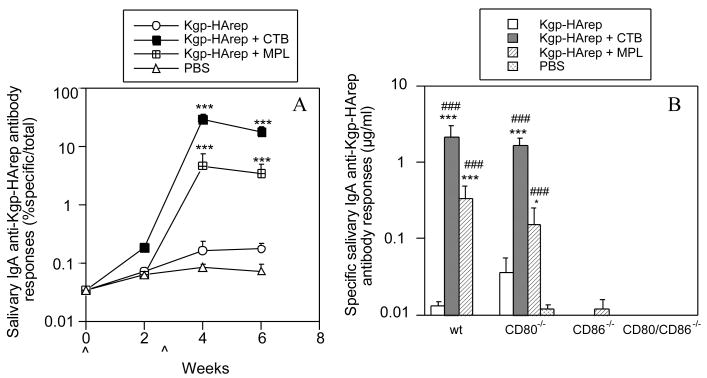
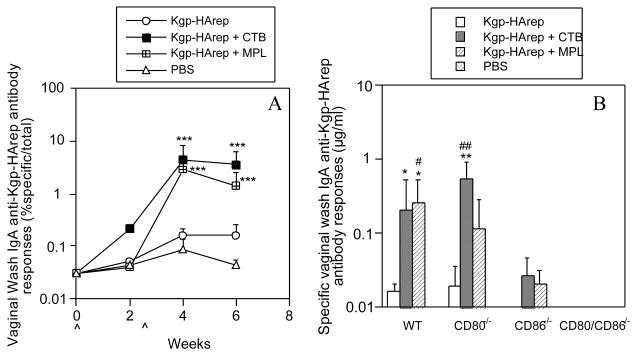
In order to determine the involvement of CD80 and CD86 in the mucosal immune responses following i.n. immunization with Kgp-HArep alone or in combination with an adjuvant, individual saliva and vaginal samples were assessed for the level of total IgA and specific IgA anti-Kgp-HArep activity. Immunization of wt mice with Kgp-HArep resulted in a slight increase in the salivary and vaginal IgA anti-Kgp-HArep responses following the second immunization (Fig. 3A and 4A). These responses were significantly enhanced (P < 0.001) when CTB or MPL was given with the antigen. Furthermore, Kgp-HArep + CTB induced significantly higher (P < 0.01) salivary IgA anti-Kgp-HArep antibody responses than Kgp-HArep + MPL following the second i.n. immunization (Fig. 3A and 4A).
Fig. 3.
Time course of the salivary IgA anti-Kgp-HArep antibody responses in wt mice (A) and specific salivary IgA anti-Kgp-HArep antibody responses in wt, CD80-/-, CD86-/- and CD80/CD86-/- mice 4 weeks post the initial immunization (B). Mice were immunized (ˆ) with Kgp-HArep, Kgp-HArep + CTB, Kgp-HArep + MPL or PBS by the i.n. route on days 0 and 18. “ˆ” indicates day of immunization. Values are expressed as the geometric mean ×/÷ S.E.M. (n = 6). Values are significantly different from the Kgp-HArep group at P < 0.001 (***) or P < 0.05 (*), or from the CD86-/- mice receiving the same immunization at P < 0.001 (###).
Fig. 4.
Time course of the vaginal wash IgA anti- Kgp-HArep antibody responses in wt mice (A) and specific vaginal wash IgA anti- Kgp-HArep antibody responses in wt, CD80-/-, CD86-/- and CD80/CD86-/- mice 4 weeks post the initial immunization (B). Mice were immunized (ˆ) with Kgp-HArep, Kgp-HArep + CTB, Kgp-HArep + MPL or PBS by the i.n. route on days 0 and 18. “ˆ” indicates day of immunization. Values are expressed as the geometric mean ×/÷ S.E.M. (n = 6). Values are significantly different from the Kgp-HArep group at P < 0.01 (**) or P < 0.05 (*), or from the CD86-/- mice receiving the same immunization at P < 0.01 (##) or P < 0.05 (#).
We next compared the magnitude of the IgA anti-Kgp-HArep antibody response in wt, CD80-/-, CD86-/- and CD80/CD86-/- mice following the second i.n. immunization. Since the levels of total salivary and vaginal IgA in CD86-/- and CD80/CD86-/- mice were significantly lower (P < 0.001) than those in wt mice (data not shown), the results are expressed as μg/ml of IgA anti-Kgp-HArep antibody activity instead of % specific IgA antibody/total IgA activity. Immunization of mice with antigen alone resulted in the induction of a salivary IgA anti-Kgp-HArep antibody response in CD80-/- mice that was higher, but not significantly different from that seen in wt mice (Fig. 3B). No salivary IgA response to Kgp-HArep was induced in CD86-/- mice. Immunization with antigen and CTB or MPL resulted in the induction of a salivary IgA anti-Kgp-HArep response in wt and CD80-/- mice that was significantly higher (P < 0.001) than that seen in mice immunized with antigen alone. The responses in wt and CD80-/- mice were also significantly higher (P < 0.001) compared to those in similarly immunized CD86-/- mice. Interestingly, although MPL potentiated the salivary IgA anti-Kgp-HArep response in CD80-/- mice (P < 0.05), it was not to the extent as seen in wt mice (P < 0.001). A slight response was induced in CD86-/- mice following immunization with antigen + MPL, but not with antigen alone or antigen + CTB. A similar response pattern was seen regarding vaginal IgA anti-Kgp-HArep antibody responses (Fig. 4B). Immunization with antigen alone resulted in the induction of a response in CD80-/-, but not CD86-/- mice. Immunization with antigen + CTB resulted in the induction of vaginal IgA anti-Kgp-HArep responses in wt and CD80-/- mice that were significantly higher (P < 0.05 and 0.01, respectively) than that seen in mice immunized with antigen alone. The response in CD80-/- mice was also significantly higher (P < 0.01) than that seen in similarly immunized CD86-/- mice. The IgA anti-Kgp-HArep response in CD80-/- mice immunized with antigen + MPL was higher, but not significantly different from that seen in CD80-/- mice immunized with antigen alone or in similarly immunized CD86-/- mice (Fig.4B). A slight IgA specific response was detected in the vaginal wash samples of CD86-/- mice immunized with antigen + CTB or MPL. Essentially no specific salivary and vaginal wash IgA response was detected in CD80/CD86-/- mice (Figs. 3B and 4B). These results demonstrate the critical role of CD86 for the induction of mucosal IgA anti-Kgp-HArep immune responses.
3.3. Functional role of CD80 and CD86 in KgpHA-specific proliferative responses
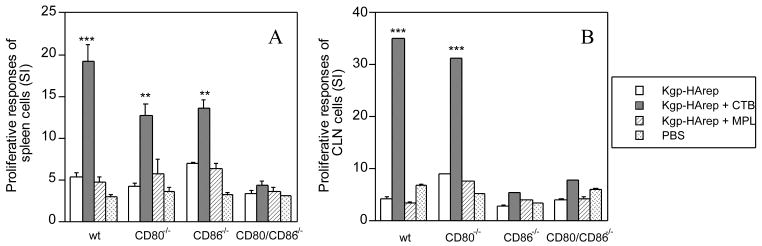
In order to determine the contribution of CD80 and CD86 to adjuvant-dependent and adjuvant-independent immune responses to Kgp-HArep at systemic and mucosal sites, we next assessed antigen-specific proliferative responses of spleen and CLN cell cultures from wt, CD80-/-, CD86-/- and CD80/CD86-/- mice following i.n. immunization with antigen with and without adjuvant. Significantly higher proliferative responses were observed in spleen cell cultures derived from wt, CD80-/- and CD86-/- mice immunized with Kgp-HArep + CTB compared to cultures derived from mice immunized with antigen alone or antigen + MPL (P < 0.01 or P < 0.001) (Fig. 5A). Furthermore, the proliferative response of spleen cell cultures derived from wt mice immunized with Kgp-HArep + CTB was significantly higher (P < 0.05) than that seen with cultures derived from similarly immunized CD80-/- and CD86-/- mice. No difference was observed in the response of spleen cell cultures derived from CD80-/- and CD86-/- mice immunized with antigen + CTB (Fig. 5A). These latter results indicate that CD80 and CD86 compensate for the antigen specific cellular response of spleen cells from mice immunized by a mucosal route with Kgp-HArep and the adjuvant CTB. However, this was not the case for the antigen-specific cellular response of CLN cells from these mice, since significantly higher (P < 0.001) proliferative responses were observed with CLN derived cells from wt and CD80-/-, but not CD86-/- mice immunized with Kgp-HArep + CTB compared to responses of cells from the other groups of mice (Fig. 5B). The critical role of CD86 for antigen-specific cellular responses of CLN cells from mice immunized by a mucosal route with Kgp-HArep + CTB is evident from the results. Spleen and CLN cell cultures from wt, and CD80-/- and CD86-/- mice immunized with antigen alone or antigen + MPL showed essentially comparable proliferative responses, which were higher but not significantly different from the responses of cells derived from mice given PBS only (Figs. 5A & 5B).
Fig. 5.
Proliferative responses to Kgp-HArep of spleen (A) and CLN (B) cells from wt, CD80-/-, CD86-/- and CD80/CD86-/- mice 4 weeks after the 2nd immunization. Results are presented as the stimulation index calculated as the ratio of the mean counts in stimulated cultures to the mean counts in the control cultures. Values are expressed as the arithmetic mean ± S.E.M. (n = 6) for spleen cells, and as the mean of the triplicate wells for CLN cells. Values are significantly different from the Kgp-HArep group in the same kind of mice at P < 0.001 (***) or P < 0.01 (**).
3.4. Regulation of CD80 and CD86 expression on dendritc cells by KgpHA
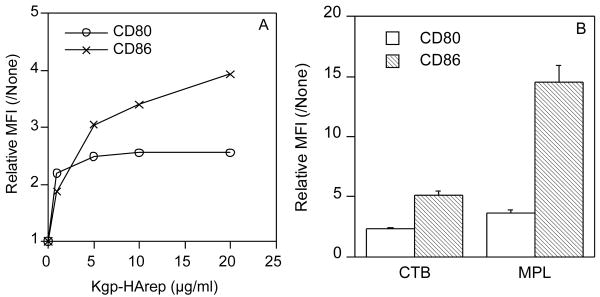
Past studies assessing the functional role of CD80 and CD86 in mediating the immunogenic properties of mucosally applied antigens have shown a strong correlation between the ability of the antigen to selectively up-regulate CD80 or CD86 on APC and the preferential role the up-regulated costimulatory molecule plays in mediating systemic and mucosal antibody responses to the antigen [27, 28]. Since DC are the most potent APC [29], we next assessed the ability of Kgp-HArep to up-regulate CD80 and CD86 expression on murine bone marrow-derived DC. Stimulation of DC with Kgp-HArep induced an up-regulation of CD86 in a dose-dependent manner (Fig. 6A). The expression of CD80 was also up-regulated but not to the extent of CD86. CTB and especially MPL induced a preferential up-regulation of CD86 compared to CD80 (Fig. 6B).
Fig. 6.
Effect of Kgp-HArep (A), CTB and MPL (B) on CD80 and CD86 expression on murine bone marrow-derived DC. DC were cultured with Kgp-HArep (1 – 20 μg/ml), CTB (10 μg/ml) or MPL (10 μg/ml) for 24 h. Results are shown as relative MFI, which is the ratio of stimulated to non-stimulated MFI.
4. Discussion
Previous in vitro and in vivo studies have reported marked differences in the individual roles of CD80 and CD86 for responses to various antigens [30-32], indicating the importance of defining the function of these costimulatory molecules for responses to specific immunogens. In the present study, we investigated the functional role of the costimulatory molecules CD80 and CD86 for the induction of systemic and mucosal immune responses to the virulence antigen Kgp-HArep of P. gingivalis following i.n. immunization of antigen alone or antigen in combination with the mucosal adjuvants CTB or MPL.
Our results with Kgp-HArep indicate a primary role of CD86 for both systemic IgG and mucosal IgA antibody responses following i.n. immunization with antigen alone. Furthermore, a more pronounced up-regulation in the expression of CD86 compared to CD80 was seen following activation of bone marrow-derived DC with Kgp-HArep. These findings suggest that the ability of Kgp-HArep to preferentially increase the expression of CD86 on DC could in part affect the magnitude of the immune response to this antigen in a CD86-dependent manner. This would be in agreement with findings of others assessing the functional roles of CD80 and CD86 in mediating immunogenic properties of mucosally administered antigens and demonstrating a strong correlation between the ability of an antigen to selectively up-regulate CD80 or CD86 on antigen-presenting cells and the preferential role the up-regulated costimulatory molecules play in mediating systemic and mucosal responses to the antigen [27, 28, 33].
Since adjuvants are commonly used in mucosal vaccines to enhance the magnitude and quality of immune responses [34], we also investigated how the mucosal adjuvants CTB and MPL affected the functional role of the costimulatory molecules for the induction of systemic and mucosal immune responses to Kgp-HArep. Both adjuvants augmented immune responses to Kgp-HArep following mucosal immunization, which was in agreement with our previous findings [15]. However, in the present study we also delineated differential roles for CD80 and CD86 in the adjuvant activities of CTB and MPL. When CTB was used as an adjuvant, a lower serum IgG specific antibody response was seen in CD86-/- mice compared to CD80-/- or wt mice. Furthermore, immunization of CD80-/- mice resulted in a mixed IgG1 and IgG2a response following the initial immunization, which shifted towards a preferential Th1-type (IgG2a) response after the second immunization. Conversely, when MPL was used as an adjuvant, both costimulatory molecules contributed to the induction of a specific serum IgG antibody response, which was preferentially of the IgG2a subclass following both the first and second i.n. immunizations. These differences in responses may relate to the efficiency and threshold of inducing and up regulating the expression of CD80 and CD86, respectively. In this regard, CTB induced a slight up-regulation of CD86 expression on DC, whereas MPL induced a higher up-regulation of CD80 and especially CD86 expression than CTB. These findings on the up-regulation of CD86 by CTB and of CD80 and/or CD86 by MPL were in agreement with previous results of Park et al. [35] and of Ismaili and co-workers [29] and De Becker et al. [36], respectively. The difference in the ability of MPL compared to CTB to up-regulate the expression of costimulatory molecules could account for the ability of MPL to potentiate a significant serum IgG anti-Kgp-HArep antibody response following the initial immunization.
There is growing evidence that both the route of immunization and the type of adjuvant used play critical roles in influencing the nature of antigen-specific humoral immune responses [27, 37, 38]. However, the nature of the response can also be influenced by the antigen. In this regard, studies using MPL as adjuvant have reported shifts to either Th1 or Th2-type responses contingent on the antigen used [36, 39-42]. The ability of CD80 and CD86 to influence the nature of immune responses has been controversial [24, 37, 43, 44]. Some studies have reported that CD80 and CD86 have differential effects on T-cell differentiation [24, 44], whereas others do not support these findings [37, 43]. In the present study, the lack of a serum IgG and mucosal IgA anti-Kgp-HArep antibody response in CD80/CD86-/- mice supports the critical role of these costimulatory molecules for antigen-specific antibody responses. Furthermore, we observed a dependence of CD86 for Th2-type serum IgG subclass responses, but no strict dependency on CD80 for selectively dictating Th1- or Th2-type antigen-specific serum IgG subclass responses. In the context of the mucosal IgA anti-Kgp-HArep antibody response, we have shown that CD86 was critical for the induction of a mucosal IgA antibody response to Kgp-HArep following i.n. immunization. Since Th2-derived cytokines are important for IgA specific responses [45], our results further support a dependency of the CD86 costimulatory molecule for Th2-type responses.
The costimulatory molecules CD80 and CD86 have been shown to compensate for each other for the generation of a systemic antibody response [37, 43], as was also seen in the present study for the induction of serum IgG anti-Kgp-HArep antibody responses, e.g., following immunization with antigen + MPL. However, this was not the case for the induction of a mucosal response to this antigen. In the present study, no specific salivary or vaginal IgA antibody response was induced in the absence of the costimulatory molecule CD86. This finding indicates that the compensatory mechanism involved in the systemic response did not apply for a mucosal response following i.n. immunization with Kgp-HArep. Perhaps this effect has to do with an inherent characteristic of the mucosal system in CD86-/- mice, i.e., low total IgA levels, or a strict requirement for the CD86 costimulatory molecule. Or perhaps this effect is due to the adjuvant, the antigen, the route of immunization, or a combination of these factors. It is most likely the latter possibility, since previous studies have reported a nonredundant role of CD80, as well as a compensatory role of CD80 and CD86 for inducing IgA antibody response following mucosal immunization with different antigens [27].
In summary, we have shown that the costimulatory molecules CD80 and CD86 play differential roles in the adjuvant activities of CTB and MPL. The differences in the responses induced following immunization with Kgp-HArep and CTB or MPL appear to relate to the efficiency and threshold of inducing and upregulating the expression of CD80 and CD86. We have also demonstrated that while CD80 and CD86 compensate for the induction of a systemic response, this was not the case for the induction of a mucosal response. In this regard, we have shown that CD86 plays a unique role in mediating a mucosal IgA response to Kgp-HArep following i.n. immunization with Kgp-HArep alone or with adjuvant. Thus, this study provides new insight on the role of the costimulatory molecules CD80 and CD86 in mediating immune responses to microbial antigens derived from P. gingivalis following mucosal immunization.
Acknowledgments
We thank Arlene Sharpe of Brigham and Women's Hospital (Boston, MA) for providing our breeding pairs of B7 knockout mice. We also acknowledge Corixa Corporation (Hamilton, MT) for the MPL used in this study.
This work was supported by U.S. Public Health Science grants DE14215 and DE09081 from the National Institute of Dental and Craniofacial Research and AI56460 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dzink JL, Socransky SS, Haffajee AD. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988;15:316–23. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 2.Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–63. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RLJ. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 4.Holt SC, Kesavalu L, Walker S, Genco CA. Virulence factors of Porphyromonas gingivalis. Periodontology 2000. 1999;20:168–238. doi: 10.1111/j.1600-0757.1999.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 5.Imamura T. The role of gingipains in the pathogenesis of periodontal disease. J Periodontol. 2003;74:111–18. doi: 10.1902/jop.2003.74.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Kuramitsu HK. Proteases of Porphyromonas gingivalis: what don't they do? Oral Microbiol Immunol. 1998;13:263–70. doi: 10.1111/j.1399-302x.1998.tb00706.x. [DOI] [PubMed] [Google Scholar]
- 7.Potempa J, Banbula A, Travis J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontology 2000. 2000;24:153–92. doi: 10.1034/j.1600-0757.2000.2240108.x. [DOI] [PubMed] [Google Scholar]
- 8.Barkocy-Gallagher GA, Han N, Patti JM, Whitlock J, Progulske-Fox A, Lantz M. Analysis of the prtP gene encoding porphypain, a cysteine proteinase of Porphyromonas gingivalis. J Bacteriol. 1996;178:2734–41. doi: 10.1128/jb.178.10.2734-2741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han N, Whitlock J, Progulske-Fox A. The hemagglutinin gene A (hagA) of Porphyromonas gingivalis 381 contains four large, contiguous, direct repeats. Infect Immun. 1996;64:4000–07. doi: 10.1128/iai.64.10.4000-4007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozarov E, Whitlock J, Dong H, Carrasco E, Progulske-Fox A. The number of direct repeats of hagA is variable among Porphyromonas gingivalis strains. Infect Immun. 1998;66:4721–25. doi: 10.1128/iai.66.10.4721-4725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pike RN, McGraw W, Potempa J, Travis J. Lysine- and arginine specific proteinases from Porphyromonas gingivalisIsolation, characterization and evidence for the existance of complexes with hemagglutinins. J Biol Chem. 1994;269:406–11. [PubMed] [Google Scholar]
- 12.Pike RN, Potempa J, McGraw W, Coetzer THT, Travis J. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J Bacteriol. 1996;178:2876–82. doi: 10.1128/jb.178.10.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Booth V, Ashley FP, Lehner T. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect Immun. 1996;64:422–27. doi: 10.1128/iai.64.2.422-427.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis MA, Aduse-Opoku J, Slaney JM, Rangarajan M, Booth V, Cridland J, et al. Characterization of an adherence and antigenic determinant of the ArgI protease of Porphyromonas gingivalis which is present in multiple gene products. Infect Immun. 1996;64:2532–39. doi: 10.1128/iai.64.7.2532-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang P, Yang QB, Balkovetz DF, Lewis JP, Clements JD, Michalek SM, et al. Effectiveness of the B subunit of cholera toxin in potentiating immune responses to the recombinant hemagglutinin/adhesin domain of the gingipain Kgp from Porphyromonas gingivalis. Vaccine. 2005;23:4734–44. doi: 10.1016/j.vaccine.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Azuma M, Yssel H, Phillips JH, Spits H, Lanier LL. Functional expression of B7/BB1 on activated lymphocytes. J Exp Med. 1993;177:845–50. doi: 10.1084/jem.177.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers CA, Allison JP. Costimulation in T cell responses. Curr Opin Immunol. 1997;9:396–404. doi: 10.1016/s0952-7915(97)80087-8. [DOI] [PubMed] [Google Scholar]
- 18.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 19.Martin M, Michalek SM, Katz J. Role of innate immune factors in the adjuvant activity of monophosphoryl lipid A. Infect Immun. 2003;71:2498–507. doi: 10.1128/IAI.71.5.2498-2507.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kariv I, Truneh A, Sweet RW. Analysis of the site of interaction of CD28 with its counter-receptors CD80 and CD86 and correlation with function. J Immunol. 1996;157:29–38. [PubMed] [Google Scholar]
- 21.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 22.Yang Q-B, Martin M, Michalek SM, Katz J. Mechanisms of monophosphoryl lipid A augmentation of host responses to recombinant HagB from Porphyromonas gingivalis. Infect Immun. 2002;70:3557–65. doi: 10.1128/IAI.70.7.3557-3565.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman GJ, Boussiotis VA, Anumanthan A, Bernstein GM, Ke XY, Rennert PD, et al. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 24.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, et al. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–18. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 25.Bagenstose LM, Class R, Salgame P, Monestier M. B7-1 and B7-2 co-stimulatory molecules are required for mercury-induced autoimmunity. Clin Exp Immunol. 2002;127:12–19. doi: 10.1046/j.1365-2249.2002.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz MB, Kukutsch N, Ogilvie ALJ, Robner S, Koch F, Romani N, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 27.Garcia CA, Martin M, Michalek SM. Role of B7 costimulatory molecules in mediating systemic and mucosal antibody responses to attenuated Salmonella enterica serovar Typhimurium and its cloned antigen. Infect Immun. 2004;72:5824–31. doi: 10.1128/IAI.72.10.5824-5831.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin M, Sharpe A, Clements JD, Michalek SM. Role of B7 costimulatory molecules in the adjuvant activity of the heat-labile enterotoxin of Escherichia coli. J Immunol. 2002;169:1744–52. doi: 10.4049/jimmunol.169.4.1744. [DOI] [PubMed] [Google Scholar]
- 29.Ismaili J, Rennesson J, Aksoy E, Vekemans J, Vincart B, Amraoui Z, et al. Monophosphoryl lipid A activates both human dendrictic cells and T cells. J Immunol. 2002;168:926–32. doi: 10.4049/jimmunol.168.2.926. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z-Q, Khan AQ, Shen Y, Schartman J, Peach R, Lees A, et al. B7 requirments for primary and secondary protein-and polysaccharide-specific Ig isotype responses to Streptococcus pneumoniae. J Immunol. 2000;165:6849–48. doi: 10.4049/jimmunol.165.12.6840. [DOI] [PubMed] [Google Scholar]
- 31.Thebeau LG, Morrison LA. Mechanism of reduced T-cell effector functions and class-switched antibody responses to herpes simplex virus type 2 in the absence of B7 costimulation. J Virol. 2003;77:2426–35. doi: 10.1128/JVI.77.4.2426-2435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasilevko V, Ghochikyan A, Holterman MJ, Agadjanyan MG. CD80(B7-1) and CD86(B7-2) are functionally equivalent in the initiation and maintenance of CD4+ T cell proliferation after activation with suboptimal doses of PHA. DNA Cell Biol. 2002;21:137–49. doi: 10.1089/10445490252925404. [DOI] [PubMed] [Google Scholar]
- 33.Cong Y, Weaver CT, Elson CO. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J Immunol. 1997;159:5301–08. [PubMed] [Google Scholar]
- 34.Boyaka PN, McGhee JR, Czerkinsky C, Mestecky J. Mucosal vaccines: an overview. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. San Diego: Elsevier Academic Press; 2005. pp. 855–74. [Google Scholar]
- 35.Park S, Chun S, Kim P. Intraperitoneal delivery of cholera toxin B subunit enhances systemic and mucosal antibody responses. Mol Cells. 2003;16:106–12. [PubMed] [Google Scholar]
- 36.De Becker G, Moulin V, Pajak B, Bruck C, Francotte M, Thiriart C, et al. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int Immunol. 2000;12:807–15. doi: 10.1093/intimm/12.6.807. [DOI] [PubMed] [Google Scholar]
- 37.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–13. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 38.Zhang P, Yang Q-B, Marciani DJ, Martin M, Clements JD, Michalek SM, et al. Effectiveness of the quijalla saponin semi-synthetic analog GPI-0100 in potentiating mucosal and systemic responses to recombinant HagB from Porphyromonas gingivalis. Vaccine. 2003;21:4459–71. doi: 10.1016/s0264-410x(03)00438-9. [DOI] [PubMed] [Google Scholar]
- 39.Baldridge JR, Yorgensen Y, Ward JR, Ulrich JT. Monophosphoryl lipid A enhances mucosal and systemic immunity to vaccine antigens following intranasal administration. Vaccine. 2000;18:2416–25. doi: 10.1016/s0264-410x(99)00572-1. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki S, Hamajima K, Fukushima J, Ihata A, Ishii N, Gorai I, et al. Comparison of intranasal and intramuscular immunization against human immunodeficiency virus type 1 with a DNA-monophosphoryl lipid A adjuvant vaccine. Infect Immun. 1998;66:823–26. doi: 10.1128/iai.66.2.823-826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers K, Beining P, Betts M, Snippe H, Inman J, Golding B. Monophosphoryl lipid A behaves as a T-cell-independent type 1 carrier for hapten-specific antibody responses in mice. Infect Immun. 1995;63:168–74. doi: 10.1128/iai.63.1.168-174.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moore A, McCarthy L, Mills KH. The adjuvant combination of monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from TH2 to Th1. Vaccine. 1999;17:2517–27. doi: 10.1016/s0264-410x(99)00062-6. [DOI] [PubMed] [Google Scholar]
- 43.Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, et al. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97–105. [PubMed] [Google Scholar]
- 44.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 45.Strober W, Fagarasan S, Lycke N. IgA B cell development. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. Third. San Diego: Elsevier Academic Press; 2005. pp. 583–616. [Google Scholar]
- 46.Gardby E, Wrammert J, Schon K, Ekman L, Leanderson T, Lycke N. Strong differential regulation of serum and mucosal IgA responses as revealed in CD28-deficient mice using cholera toxin adjuvant. J Immunol. 2003;170:55–63. doi: 10.4049/jimmunol.170.1.55. [DOI] [PubMed] [Google Scholar]