Abstract
In the field, phenotypic determinants of competitive success are not always absolute. For example, contest experience may alter future competitive performance. As future contests are not determined solely on phenotypic attributes, prior experience could also potentially alter phenotype–fitness associations. In this study, we examined the influence of single and multiple experiences on contest outcomes in the jumping spider Phidippus clarus. We also examined whether phenotype–fitness associations altered as individuals gained more experience. Using both size-matched contests and a tournament design, we found that both winning and losing experience affected future contest success; males with prior winning experience were more likely to win subsequent contests. Although experience was a significant determinant of success in future contests, male weight was approximately 1.3 times more important than experience in predicting contest outcomes. Despite the importance of experience in determining contest outcomes, patterns of selection did not change between rounds. Overall, our results show that experience can be an important determinant in contest outcomes, even in short-lived invertebrates, and that experience alone is unlikely to alter phenotype–fitness associations.
Keywords: jumping spider, multiple competition, Phidippus clarus, previous experience, selection gradient, tournament design
In intrasexual competitions, phenotypic traits are often strong predictors of competitive success. For example, many studies have shown that larger males, in better condition, and with larger weaponry most often win contests (e.g. Andersson 1994; Hack 1997; Rillich et al. 2006). While the tendency in past analyses of phenotypic selection has been to investigate the predictive relationship between static morphological traits and fitness, it is also well accepted that nonstatic traits and environmental factors are important determinants of fitness/success, and failure to account for these can lead to a distorted view of phenotypic selection and the adaptive value of traits (Lande & Arnold 1983; Mitchell-Olds & Shaw 1987; Rausher 1992). One such example is past contest experience. Winning or losing experience can alter future competitive success (reviewed in: Hsu et al. 2006; Rutte et al. 2006), and in each case, prior success increases the probability of future wins, while prior failure increases the probability of future losses (Dodson & Schwaab 2001; Hsu & Wolf 2001; Stuart-Fox et al. 2006). Experience effects, however, may not be limited to the most recent contest, as individuals are likely to encounter multiple rivals throughout a breeding season, especially if individuals mate multiply and/or are long lived. Multiple encounters will probably result in multiple winning or losing experiences, and each individual experience may contribute to a cumulative effect on future contest outcomes (e.g. Hsu & Wolf 1999; Stuart-Fox et al. 2006).
In addition to the direct effect that losing and winning experience can have on contest outcomes, experience also has the potential to alter phenotype–fitness correlations, and therefore, estimates of phenotypic selection. In other words, future contest outcomes may be influenced more by experience than by phenotypic traits associated with success, resulting in a disassociation between phenotype and fitness. In this study, we quantified phenotypic selection on a suite of traits during male–male agonistic contests in a jumping spider, Phidippus clarus, while simultaneously evaluating the importance of prior contest experience. Male P. clarus engage in intense pairwise contests over access to female refuges (see below). Previous work has shown that males use a combination of self-assessment (during the assessment phase) and partial, mutual opponent assessment (during the escalated phases of contests) in determining contest outcomes, and that male weight is as a strong predictor of contest success (Elias et al. 2008). As winning males are able to maintain exclusive access to female refuges (see below), success in aggressive contests is a good indicator of male fitness. There is also clear evidence of an experience effect on male behaviour in these contests; in repeated bouts with the same opponent, winning males continue to outcompete losing males and losing males dramatically reduce behaviours associated with aggression (Elias et al. 2008). This finding suggests that experience influences subsequent contest outcomes between rivals, but whether experience also influences contest outcomes between novel individuals remains to be determined.
We had four goals in this study. To determine: (1) the effect of experience on competitive success with novel rivals in P. clarus, (2) the effect of experience on contest outcomes relative to other phenotypic traits, (3) the relative importance of the most recent experience versus past experience in determining contest outcomes, and (4) whether experience alters phenotype–fitness correlations, and therefore, selection gradients. To examine the first question, we assigned males a winning and losing experience in the first round, and then fought experienced males against naïve weight-matched opponents in a second round. Weight is a strong indicator of fighting success (Elias et al. 2008); hence, by size matching in the second interaction, we controlled for fighting ability and were thus able to isolate the effect of experience from fighting ability (Hsu et al. 2006; Stuart-Fox et al. 2006). However, this type of experimental procedure does not allow an examination of the relative effects of experience compared to other phenotypic traits (Stuart-Fox et al. 2006). Thus, to address the remaining questions, we used a tournament design where males were randomly paired against one another. A random tournament design allows for an examination of multiple phenotypic traits relative to experience while also allowing for an estimation of selection gradients in each round.
METHODS
Life History
Phidippus clarus is abundant throughout North America during midsummer months. During the early season, both sexes build hibernacula (nests) in curled leaves, and return to these hibernacula each night (Hoefler 2006). Males mature before females (protandry), and mature males begin searching for and defending the hibernacula of penultimate instar females (one moult from maturity) (Hoefler 2007), preferentially choosing larger females (Hoefler 2008). Males mate with females immediately after females mature, making access to hibernacula extremely important. While defending a hibernaculum, males are likely to encounter numerous potential rivals attempting to usurp them, providing individuals with multiple competitive encounters to determine their fighting ability relative to others in the population. However, males also encounter rivals while wandering and do fight in the absence of females (Hoefler 2007).
Males perform a series of stereotyped behaviours during aggressive interactions that have been described elsewhere (Elias et al. 2008). Briefly, these behaviours can be divided into two phases: (1) a precontact phase, where males display towards one another and (2) a contact phase, where males physically interact with one another. The precontact phase begins when the two spiders orient towards one another, adopting a hunched posture. Males then approach or retreat from one another with their front legs outstretched horizontally. During these displays, males also produce a series of substrate-borne vibrations (Elias et al. 2008). The contact phase begins when the two spiders are close to each other and begin to leg-fence. Leg-fencing behaviour consists of the two males touching each other’s horizontally outstretched legs, whereby males attempt to push each other backwards with their front legs and bodies. Some of these interactions escalate further to grappling, where males lock chelicerae (jaws) and legs for relatively longer periods.
Housing and Competitions
We collected adult male P. clarus from Koffler Scientific Reserve at Joker’s Hill, King, Ontario, Canada (44°03′N, 79°29′W) for this experiment. We housed all males in individual clear plastic cages in the laboratory on a 12:12 h light:dark cycle and fed them small Acheta domesticus and Drosophila hydeii twice weekly. We placed opaque barriers between cages for at least 4 days to allow males to acclimatize to laboratory conditions, to minimize effects of prior visual interactions between caged males (Forster 1982; Land 1985; Land & Nilsson 2002) and to control for prior fighting experience in the field (unpublished data). Two days before trials, we anaesthetized males using CO2 and marked each individual with two spots of nontoxic fluorescent paint (Luminous paint, BioQuip Products, Inc., Rancho Dominguez, CA, U.S.A.) on the abdomen to allow individual identification during contests. We observed males during feeding intervals to ensure that males were not affected by the marking procedure.
We used 5×5×6 cm plastic containers as competitive arenas, which were similar in size to natural arenas (plant leaves) used by male P. clarus. We covered the walls of each arena with petroleum jelly to prevent individuals from escaping from the arena. We covered the base of each arena with a sheet of paper and changed the paper between fights with new individuals to ensure there was no webbing or pheromonal cues left by either the winner or loser. To start each contest, we placed an opaque divider in the centre of the arena and then placed one individual on either side of the divider. Individuals were allowed 1 min to acclimate to their surroundings, after which the divider was removed and the contest began. A contest lasted until an individual won two of three bouts or until 10 min had elapsed. In cases where the full time was reached, the winner was determined to be the individual that won the first bout. A male was considered to have won a bout when the rival male turned away and retreated more than two body lengths. There were no instances where each individual won only a single bout. After the outcome was decided, we removed both individuals and placed them back into their individual cages. Males were not fed between rounds.
We weighed individuals after each fight using an Ohaus electronic balance. After all fights were completed, we digitally photographed each individual (Nikon Digital Camera DXM 1200) using a Zeiss microscope (Stemi 2000C). We then used Act-1 software (Nikon Instruments, Inc., New York, NY, U.S.A.) to measure cephalothorax width (at its widest point) and the mean femur, patella–tibia and tarsus of the first legs as measurements of size.
Size-matched Contests
We collected 104 adult males for this experiment. To determine whether experience influences contest outcome in P. clarus, we (1) randomly paired males in round 1, ensuring a minimum of 10% weight difference (mean weight difference = 24%), and (2) paired each winner and loser from round 1 with a weight-matched opponent (weight difference less than 5%; mean weight difference = 4%) in round 2. There was a maximum of 60 min between the two rounds.
Tournament Design
We collected 88 adult males for this experiment. Using a tournament style design, we performed three rounds of contests in a single day. In each round, males were randomly assigned opponents, with the caveat that the colour combination for the two individuals was unique, allowing individual identification during contests. All males completed contests in the current round before starting a subsequent round to ensure that all males had the same amount of experience. There was a minimum of 98 min and a maximum of 282 min between rounds (mean ± SE = 193.73±3.23 min).
Statistical Analyses
We examined experience effects using three statistical analyses. First, we compared the number of winners and losers with prior winning and losing experience using a Fisher’s exact test to determine whether experience alone affected fight outcome in size-matched and tournament design contests (e.g. Hsu & Wolf 1999). Second, we used a logistic model to determine whether the difference in size between opponents as well as prior experience of opponents affected contest outcome in size-matched contests. Third, we used a modified Bradley–Terry model (Firth 2005; e.g. Stuart-Fox et al. 2006) to examine the relative effect of the measured traits and experience in determining contest outcomes in the tournament design. The Bradley–Terry model is the appropriate method to analyse tournament data as it is explicitly aimed at partitioning the effects of past outcomes and intrinsic measures of quality in tournament designs (Firth 2005; e.g. Stuart-Fox et al. 2006). Assuming that winning a contest has a positive effect and losing a contest has a negative effect on future contests (e.g. Hsu & Wolf 1999), we quantified experience by allotting a value of 1 each time an individual won a contest and a value of -1 each time an individual lost. Since experience from immediately previous versus earlier contests can have different effects on future contest outcomes (Hsu & Wolf 1999), we coded experience in three ways: (1) most recent experience alone: we assumed that only the most recent previous experience would influence contest outcomes, and we coded experience only from the last contest; (2) cumulative experience: we assumed that each experience would have equal value in future contests, and we coded experience equally from both prior contests; (3) degrading cumulative experience: we assumed that experience only from immediately prior contests would influence contest outcomes, and we coded earlier contests with half the value of the most recent contests. We performed separate Bradley–Terry models for winning and losing and selected the most appropriate model by minimizing the Akaike Information Criterion (AIC), a measurement of a goodness of fit of the model where a lower value suggests that the model is a better fit to the data (Burnham & Anderson 2002; Akaike 1983). We also compared our best-fit model to a model that excluded experience to determine whether the model that included experience better explained our results.
Winning males are able to maintain exclusive access to female refuges (Hoefler 2007), so success in aggressive contests is a good indicator of male fitness. We performed two selection analyses. All five morphological traits examined (weight, cephalothorax width, and mean length of femur, patella–tibia and tarsus of the first legs) were highly correlated (data not shown), and selection analysis requires use of uncorrelated traits (Lande & Arnold 1983), so in the first analysis, we performed a principal component analysis (PCA using the covariance matrix; e.g. Kraft et al. 2006), which provided a new set of five uncorrelated traits suitable for selection analyses (Lande & Arnold 1983). Although the first component explained the most variance (in this case, overall size), the other components explained variation in individual ‘shapes’. Therefore, as we originally had five traits, we kept all five PC scores in our analysis. We then standardized the PC scores to allow comparison between rounds. Although this allowed us to examine how selection influences a suite of traits, it did not allow us to examine how selection influences weight, the only phenotypic predictor of success (Elias et al. 2008). Thus, in the second analysis, we examined how selection influences weight. We fitted multiple regression models to estimate standardized selection gradients of directional, quadratic and correlation selection on the principal components (Lande & Arnold 1983) separately for each round to examine whether experience altered the strength and/or direction of selection on males between rounds. We first fitted a linear regression to estimate β. We then fitted a quadratic regression on all linear, quadratic and cross-product terms to estimate the γ matrix (Lande & Arnold 1983). We doubled the values of our quadratic terms to accurately reflect how nonlinear selection functions (Stinchcombe et al. 2008).
To test for differences in selection gradients between rounds, we used a sequential model-building approach whereby the effect of (i.e. variance explained) including/excluding model terms was evaluated using partial F tests. Partial F tests are used to calculate significance based only a subset of predictor variables in a linear model (Draper & John 1988; Bowerman & O’Connell 1990). The application of this method for comparisons of nonlinear selection among different samples is outlined in Chenoweth & Blows (2005). For the Partial F test, we first fitted a model with only round as a fixed effect (model A). We then added all the linear terms as covariates (model B), and added the linear-by-round interactions (model C). To test for overall significance of linear selection, we estimated a partial F for model B against model A. To test for significance of linear selection between rounds, we estimated a partial F for model C against model B. We tested for significant variance in nonlinear selection between rounds by first adding all linear and nonlinear terms (model D) and then adding the nonlinear-by-round interaction terms (model E). To test for overall significance of nonlinear selection, we compared model D to model B, and to test for significant nonlinear selection between rounds, we compared model E to model D. We tested for significant selection in the univariate test of selection on weight in the same manner.
We performed all statistical analyses using JMP 7.0 (2007, SAS Institute, Inc., Cary, NC, U.S.A.).
RESULTS
Size-matched Contests
There were 26 first-round contests where males were given either a winning or losing experience. Of the 26 first-round winners, 20 males won and six males lost against weight-matched opponents in round 2. Of the 26 first-round losers, six males won and 20 males lost against weight-matched opponents in round 2. First-round winners were therefore significantly more likely to win against males with similar fighting ability, while first-round losers were significantly more likely to lose against males with similar fighting ability in subsequent contests (Fisher’s exact two-tailed test: P = 0.0002). Results of the logistic model were similar, where winning experience had a significant positive effect (χ21= 9.58, P = 0.002) and weight had no effect (χ21=0.6, P = 0.69) on contest outcome.
Tournament Design
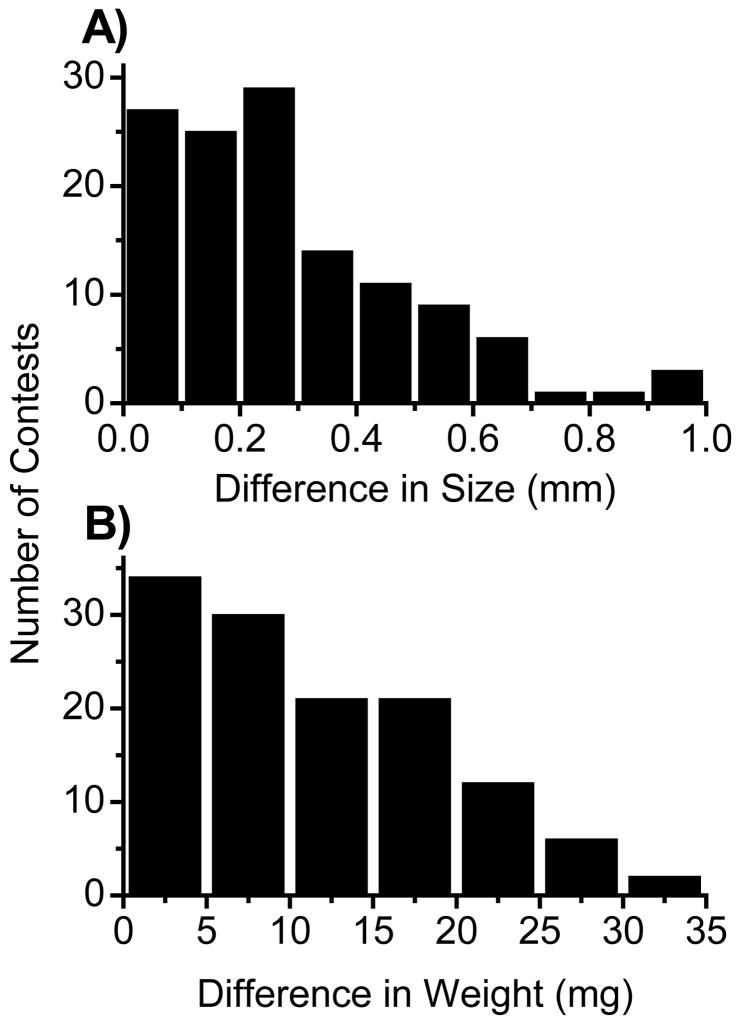
During contests, one individual died after round 1, and six individuals died during round 2. Therefore, our analysis is based on 44 first-round fights (N=88 individuals), 42 second-round fights (N=84 individuals) and 40 third-round fights (N=80 individuals) for a total of 126 contests. Of these contests, 93 were between individuals that differed in weight by at least 10%, and 43 were between individuals that differed in size by at least 10% (Fig. 1). All traits were normally distributed. There was no significant difference in weight (mean ± SE weight difference: round 1: 10.14 ± 0.86 mg; round 2: 11.47 ± 0.87 mg; round 3: 12.77 ± 0.90 mg; F2,249= 2.21, P=0.11) or body size (mean ± SE cephalothorax width difference: round 1: 0.276 ± 0.032 mm; round 2: 0.265 ± 0.0332 mm; round 3: 0.296 ± 0.0340 mm; F2,249 = 0.22, P=0.80) between contestants in each round. Male weight tended to decrease throughout the trials, but the difference in weight between trials was not significant (repeated measures ANOVA: F1,78=2.186, P=0.12).
Figure 1.
The distribution of the difference in (a) size (cephalothorax width) and (b) weight between competing males.
Of the 44 males that won in round 1, 43 survived and fought in round 2. Of these, 26 males won and 17 males lost in round 2. Of the 44 males that lost in round 1, 14 males won and 29 males lost in round 2. Thus, first-round winners had greater success than first-round losers in the subsequent round (Fisher’s exact two-tailed test: P = 0.006). We examined the third-round results in the same manner. There were 39 winning males in round 2; 27 of these won and 12 lost in round 3. Of the 40 losers from round 2, 12 males won and 28 males lost in round 3. Second-round winners also won more contests in round 3, while second-round losers lost significantly more contests in round 3 (Fisher’s exact two-tailed test: P<0.0001).
We analysed the tournament results using a Bradley–Terry model. Of the three candidate models for predicting fight outcomes, Model 1 (incorporating only most recent experience) was the best fit (AIC: Model 1: −216.30; Model 2: −214.571; Model 3: −215.65). Model 1 also explained the greatest proportion of the variance in contest outcomes (χ2121=49.86, P<0.0001, R2=0.2879) even compared to the model excluding experience (AIC = −212.18; χ2121=44.95, P<0.0001, R2=0.2595). In Model 1, both weight and previous experience significantly predicted contest outcomes (Table 1). Weight was approximately 1.3 times more important than previous experience in determining contest outcomes (standardized coefficients, Table 1).
Table 1.
Coefficients from the Bradley–Terry model, with experience coded by previous experience
| β | γ2 | P | Standardized β* | |
|---|---|---|---|---|
| Weight | −0.09±0.04 | 4.589 | 0.03 | −0.93±0.68 |
| Experience | −0.42±0.19 | 4.94 | 0.03 | −0.71±0.29 |
| Cephalothorax width | −1.70±1.30 | 1.71 | 0.19 | −0.89±0.57 |
| Femur length | −0.79±2.41 | 0.11 | 0.74 | −1.12±0.31 |
| Patella–tibia length | 1.29±1.95 | 0.44 | 0.50 | 0.40±0.55 |
| Tarsus length | −0.21±2.06 | 0.01 | 0.91 | −0.02±0.61 |
Standardized coefficients allow comparison of the relative strength of the various factors in the model.
Estimates of Phenotypic Selection
Table 2 shows how each of the original traits contributed to the new PC scores. All traits loaded positively on PC1, and thus, PC1 can be considered a measurement of morphological size and condition. The other principal component scores (PC 2–5) reflect variation in morphological shape. Linear selection gradients are shown for each round in Table 3. There was significant positive selection on PC1 in each round, with relatively stronger selection evident in round 3. Results from partial F tests showed significant overall linear selection (F5, 243=7.46, P<0.001), but no difference in selection gradients between rounds (F10, 233=0.87, P=0.56). Nonlinear selection gradients are shown in Table 4. There was significant positive quadratic selection on PC4, however, overall nonlinear selection was not significant (partial F test: F15, 228=1.36, P=0.17), and there was no difference in nonlinear selection between rounds (partial F test: F45,188=0.52, P=0.99).
Table 2.
Principal component analysis on the five original traits
| Eigenvalues | PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|---|
| Weight | 4.538 | 0.99826 | −0.05634 | −0.01721 | −0.00253 | 0.00027 |
| Cephalothorax | 0.239 | 0.02533 | 0.14493 | 0.95903 | 0.23278 | −0.06655 |
| Femur | 0.082 | 0.02522 | 0.44429 | 0.07089 | −0.33478 | 0.82757 |
| Patella–-tibia | 0.053 | 0.03991 | 0.73930 | −0.05760 | −0.38416 | −0.54859 |
| Tarsus | 0.023 | 0.02464 | 0.48153 | −0.26763 | 0.82834 | 0.09875 |
Table 3.
Linear selection gradients on the five principal component scores
| Round 1 | Round 2 | Round 3 | |
|---|---|---|---|
| PC1 | 0.16±0.05** | 0.12±0.05* | 0.25±0.05*** |
| PC2 | 0.0001±0.05 | −0.04±0.05 | 0.003±0.05 |
| PC3 | 0.03±0.05 | −0.05±0.05 | −0.05±0.05 |
| PC4 | 0.007±0.05 | −0.02±0.05 | −0.09±0.05 |
| PC5 | −0.03±0.05 | −0.01±0.05 | 0.05±0.05 |
P<0.05;
P<0.01;
P<0.001.
Table 4.
Nonlinear selection gradients on the five principal component scores
| Round | PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|---|
| 1 | PC1 | −0.04±0.12 | ||||
| PC2 | −0.004±0.07 | −0.04±0.06 | ||||
| PC3 | 0.09±0.07 | 0.04±0.08 | −0.002±0.06 | |||
| PC4 | −0.05±0.06 | −0.09±0.09 | 0.01±0.06 | 0.20±0.08* | ||
| PC5 | −0.07±0.06 | −0.02±0.08 | −0.01±0.07 | 0.001±0.053 | 0.04±0.07 | |
| 2 | PC1 | −0.08±0.14 | ||||
| PC2 | 0.02±0.06 | −0.04±0.06 | ||||
| PC3 | 0.07±0.08 | 0.08±0.09 | 0.01±0.06 | |||
| PC4 | 0.08±0.07 | −0.03±0.10 | 0.03±0.06 | 0.06±0.08 | ||
| PC5 | 0.007±0.061 | 0.10±0.08 | 0.05±0.08 | 0.01±0.06 | 0.04±0.08 | |
| 3 | PC1 | 0.16±0.12 | ||||
| PC2 | 0.05±0.06 | −0.04±0.06 | ||||
| PC3 | 0.02±0.07 | 0.03±0.08 | −0.06±0.06 | |||
| PC4 | 0.11±0.07 | −0.09±0.09 | 0.09±0.06 | 0.06±0.08 | ||
| PC5 | 0.06±0.06 | 0.01±0.08 | 0.04±0.07 | −0.02±0.05 | −0.02±0.06 |
Values across the diagonal are quadratic selection gradients, and values below the diagonal are correlational selection gradients.
P<0.05.
Our estimate of univariate selection gradients on male weight in each round showed significant positive selection on weight in each round (round 1: β= 0.15±0.05, F1,86=2.96, P=0.004; round 2: β = 0.12±0.05, F1,82=2.38, P=0.02; round 3: β = 0.22±0.05, F1,78=4.43, P<0.0001). Using a partial F test, overall linear selection on male weight was significant (F1,248=31.00, P<0.0001). There was no significant difference in the pattern of selection between rounds (partial F test: F2,246=0.90, P=0.41).
DISCUSSION
As in a previous study (Elias et al. 2008), we found that weight was the only morphological trait that strongly predicted contest outcome in male P. clarus. There was also strong selection, overall, and in each round, on male size (multivariate analysis) and male weight (univariate analysis). Male P. clarus mainly use self-assessment to determine the outcome of contests (Elias et al. 2008), so an individual’s weight (or condition in the multivariate analysis) probably determines an individual’s fighting threshold/ability. Thus, even though males undergo a lengthy signalling period, weight is the only reliable cue for determining how long a male will persist in physical contests. Although weight was the most important determinant of contest outcomes in P. clarus in our study, all males tended to lose weight between bouts. An individual’s weight probably varies not only throughout the breeding season, but also across days or hours, as it did in our study, where males fought three opponents within 8 h. Thus, it may be difficult for an individual to ascertain their own fighting ability relative to others in the population based on weight alone. This may help explain why individual-based thresholds (self-/cumulative assessment) best explain contest dynamics in P. clarus (Elias et al. 2008).
We also found that both winning and losing experience greatly contributed to future contest outcomes in P. clarus. Winners were more likely to win subsequent contests, while losers were more likely to lose subsequent contests. This result occurred in both the size-matched trials, and in the tournament design where opponents were chosen randomly to simulate natural contests. Furthermore, an individual’s most recent prior experience explained most of the variation in male fight outcome. Although experience had a relatively strong effect on contest outcomes in P. clarus, there was no significant difference between estimated selection gradients between rounds in either the univariate or the multiavariate analysis. Thus, we found no evidence that experience had either a reinforcing effect or a weakening effect on the strength of selection. This may be because the importance of weight in determining contest outcomes is relatively more important (1.3 times greater) in this species. Thus, unless individuals are relatively similar in size, it is unlikely that experience alone will alter phenotype–fitness associations. In the tournament design, weight may have had a greater influence on contest outcomes since only 19.5% of trials in second and third rounds were between individuals that differed in size by less than 10%.
Although experience influences contest outcomes, it is not a heritable trait itself. However, there may be a heritable basis to how an individual responds physiologically and behaviourally to positive or negative contest experience as well as how long memories last, and these traits may be heritable (Hsu et al. 2006). For example, if hormone titres change either during or after contests (e.g. Earley & Hsu 2008), behavioural changes may result that allow individuals to decrease the costs associated with fights, resulting in potential fitness increases (Rutte et al. 2006). This can occur if individuals that have recently lost a contest are less likely to initiate or escalate future contests, and/or if individuals that have recently won contests are bolder (e.g. Frost et al. 2007). Additionally, if information from multiple experiences is reliable, selection may act upon the mechanisms associated with long-term memory formation (Kandel et al. 2000). It is therefore important to begin examining whether experience alters phenotype–fitness associations under different competitive circumstances and its potential effect on the evolution of learning and memory.
However, experience is not the only factor that is likely to affect patterns of selection in P. clarus. As male P. clarus defend female’s hibernacula from other males, ownership is likely to influence contest outcomes, as shown in other species (Hoefler 2002; Olsson & Shine 2000). Males also show variation in maturation rates, which results in a significant increase in male size as the season progresses (M. M. Kasumovic & D. O. Elias, unpublished data). Although later-maturing, larger males are likely to outcompete smaller protandrous males, these smaller protandrous males would gain access to female’s hibernacula before larger males, and would thus gain experience before larger males mature. Together, ownership and previous winning experience of smaller protandrous males may outweigh any size benefits (e.g. Hoefler 2006). Further studies examining multiple factors and the effect that such factors can have on selection in concert may clarify how selection functions in contests and whether patterns of selection can change within a single breeding season (e.g. Kasumovic et al. 2008).
Acknowledgments
We thank J. M. Brandt, T. Peckmezian, K. Permapaladas and S. Sivilinghem for field and laboratory assistance. We also thank S. Lailvaux, M. Hall, and the Integrative Behaviour & Neuroscience Group (University of Toronto, Scarborough) for useful discussions and comments on the manuscript. This project was funded by a grants from Natural Sciences and Engineering Research Council of Canada (NSERC) Postgraduate Scholarship B, Ontario Graduate Student Fellowship, and an Animal Behavior Society Student Grant to M.M.K., National Science Foundation International Research Fellowship Program (0502239) and National Institutes of Health National Research Service Award (1F32GM076091-01A1) to D.O.E., NSERC Discovery Grants (229029-2004 to M.C.B.A. and 238882 241419 to A.C.M.), and grants from the Canadian Foundation for Innovation and Ontario Innovation Trust (M.C.B.A. and A.C.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike H. Information measures and model selection. Bulletin of the International. Statistical Institute. 1983;44:277–291. [Google Scholar]
- Andersson M. Sexual Selection. Princeton, New Jersey: Princeton University Press.; 1994. [Google Scholar]
- Bowerman BL, O’Connell RT. Linear Statistical Models: an Applied Approach. Belmont, California: Duxbury Press.; 1990. [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multi-model Inference. New York: Springer; 2002. [Google Scholar]
- Chenoweth SF, Blows MW. Contrasting mutual sexual selection on homologous signal traits in Drosophila serrata. American Naturalist. 2005;165:281–289. doi: 10.1086/427271. [DOI] [PubMed] [Google Scholar]
- Dodson GN, Schwaab AT. Body size, leg autonomy, and prior experience as factors in the fighting success of male crab spiders, Misumenoides formosipes. Journal of Insect Behavior. 2001;14:841–855. [Google Scholar]
- Draper NR, John JA. Response-surface designs for quantitative and qualitative variables. Technometrics. 1988;30:423–428. [Google Scholar]
- Earley RL, Hsu Y. Reciprocity between endocrine state and contest behavior in the killifish, Kryptolebias marmoratus. Hormones and Behavior. 2008;53:442–451. doi: 10.1016/j.yhbeh.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Elias DO, Kasumovic MM, Punzalan D, Andrade MCB, Mason AC. Male assessment during aggressive contests in jumping spiders. Animal Behaviour. 2008;76:901–910. doi: 10.1016/j.anbehav.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth D. Bradley-Terry models in R. Journal of Statistical Software. 2005;12:1–12. [Google Scholar]
- Forster L. Visual communication in jumping spiders (Salticidae) In: Witt PN, Rovner JS, editors. Spider Communication: Mechanisms and Ecological Significance. Princeton, New Jersey: Princeton University Press.; 1982. pp. 161–212. [Google Scholar]
- Frost AJ, Winrow-Giffen A, Ashley PJ. Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proceedings of the Royal Society of London, Series B. 2007;274:333–339. doi: 10.1098/rspb.2006.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack MA. The energetic costs of fighting in the house cricket, Acheta domesticus L. Behavioral Ecology. 1997;8:28–36. [Google Scholar]
- Hoefler CD. Is contest experience a trump card? The interaction of residency status, experience, and body size on fighting success in Misumenoides formosipes (Araneae: Thomisidae) Journal of Insect Behavior. 2002;15:779–790. [Google Scholar]
- Hoefler CD. Jumping spiders in space: movement patterns, nest site fidelity and the use of beacons. Animal Behaviour. 2006;71:109–116. [Google Scholar]
- Hoefler CD. Male mate choice and size-assortative pairing in a jumping spider, Phidippus clarus. Animal Behaviour. 2007;73:943–954. [Google Scholar]
- Hoefler CD. The costs of male courtship and potential benefits of male choice for large mates in Phidippus clarus (Aranea, Salticidae) Journal of Arachnology. 2008;36:210–212. [Google Scholar]
- Hsu Y, Earley RL, Wolf LL. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biological Reviews. 2006;81:33–74. doi: 10.1017/S146479310500686X. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Wolf LL. The winner and loser effect: integrating multiple experiences. Animal Behaviour. 1999;57:903–910. doi: 10.1006/anbe.1998.1049. [DOI] [PubMed] [Google Scholar]
- Hsu Y, Wolf LL. The winner and loser effect: what fighting behaviours are influenced? Animal Behaviour. 2001;61:777–786. [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York: McGraw-Hill.; 2000. [Google Scholar]
- Kasumovic MM, Bruce MJ, Andrade MCB, Herberstein ME. Spatial and temporal demographic variation drives within-season fluctuations in sexual selection. Evolution. 2008;62:2316–2325. doi: 10.1111/j.1558-5646.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- Kraft PG, Franklin CE, Blows MW. Predator induced phenotypic plasticity in tadpoles: extension or innovation. Journal of Evolutionary Biology. 2006;19:450–458. doi: 10.1111/j.1420-9101.2005.01015.x. [DOI] [PubMed] [Google Scholar]
- Land MF. The morphology and optics of spider eyes. In: Barth FG, editor. Neurobiology of Arachnids. New York: Springer-Verlag; 1985. pp. 53–78. [Google Scholar]
- Land MF, Nilsson DE. Animal Eyes. Oxford: Oxford University Press; 2002. [Google Scholar]
- Lande R, Arnold SJ. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Mitchell-Olds T, Shaw RG. Regression analysis of natural selection: statistical inference and biological interpretation. Evolution. 1987;41:1149–1161. doi: 10.1111/j.1558-5646.1987.tb02457.x. [DOI] [PubMed] [Google Scholar]
- Olsson M, Shine R. Ownership influences the outcome of male-male contests in the scincid lizard, Niveoscincus microlepidotus. Behavioral Ecology. 2000;11:587–590. [Google Scholar]
- Rausher MD. The measurement of selection on quantitative traits: biases due to environmental covariances between traits and fitness. Evolution. 1992;46:616–626. doi: 10.1111/j.1558-5646.1992.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Rillich J, Schilderberger K, Stevenson PA. Assessment strategy of fighting crickets revealed by manipulating information exchange. Animal Behaviour. 2006;74:823–836. [Google Scholar]
- Rutte C, Taborsky M, Brinkhof MWG. What sets the odds of winning and losing? Trends in Ecology & Evolution. 2006;21:16–21. doi: 10.1016/j.tree.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Stinchcombe J, Agrawal AF, Hohenlohe PA, Arnold SJ, Blows MW. Estimating non-linear selection gradients using quadratic regression coefficients: double or nothing? Evolution. 2008;62:2435–2440. doi: 10.1111/j.1558-5646.2008.00449.x. [DOI] [PubMed] [Google Scholar]
- Stuart-Fox DM, Firth D, Moussalli A, Whiting MJ. Multiple signals in chameleon contests: designing and analysing animal contests as a tournament. Animal Behaviour. 2006;71:1263–1271. [Google Scholar]