Abstract
Conditioned taste aversions (CTAs) may be acquired when an animal consumes a novel taste (CS) and then experiences the symptoms of poisoning (US). This aversion may be extinguished by repeated exposure to the CS alone. However, following a latency period in which the CS is not presented, the CTA will spontaneously recover (SR). In the current study we employed an explicitly unpaired extinction procedure (EU-EXT) to determine if it could thwart SR of a CTA. Sprague-Dawley rats acquired a strong CTA after 3 pairings of saccharin (SAC the CS) and Lithium Chloride (LiCl the US). CTA acquisition was followed by extinction (EXT) training consisting of either (a) CS-only exposure (CSO) or, (b) exposure to saccharin and Lithium Chloride on alternate days (i.e., explicitly unpaired: EU). Both extinction procedures resulted in ≥ 90% reacceptance of SAC, although the EU extinction procedure (EU-EXT) significantly decreased the time necessary for rats to reach this criterion (compared to CSO controls). Rats were subsequently tested for SR of the CTA upon re-exposure to SAC following a 30-day latency period of water drinking. Rats that acquired a CTA and then underwent the CSO extinction procedure exhibited a significant suppression of SAC drinking during the SR test (as compared to their SAC drinking at the end of extinction). However, animals in the EU-EXT group did not show such suppression in drinking compared to CSO controls. These data suggest that the EU-EXT procedure may be useful in reducing both time to extinction and the spontaneous recovery of fears.
There are clear adaptive advantages to establishing and retaining fears. Fears evoke defensive reactions that protect individuals against future threats and help ensure survival. However, fears become maladaptive when they persist in contexts where threats are no longer present. Crippling clinical conditions such as panic attacks, phobias and the anxiety-laden flashbacks of Post Traumatic Stress Disorder (PTSD) are examples of pathological fear (for review, see Maren, 2005). The symptomatology of PTSD includes unsuccessful termination of fear responses (Yehuda, 2001) and resistance to extinction (EXT; Chorot & Sandin, 1993; Van der Kolk, 1994) as evidenced by spontaneous recovery (SR) of the fear (Rescorla, 2004). Thus, learning plays an important part in the expression of psychiatric symptoms that follow trauma, and the study of EXT and SR has important implications for mental illness, therapy and relapse (for review, see Bouton & Swartzentruber, 1991).
A growing literature is now addressing not only how fears are acquired, but also how they may be reduced (extinguished) and how SR of these fears may be attenuated or eliminated (Maren, 2005; Quirk, 2006; Quirk, Martinez, & Nazario-Rodríguez, 2007; Myers & Davis, 2002; Mickley et al., 2007). Much of the pre-clinical literature has employed Pavlovian fear conditioning paradigms such as the conditioned emotional response (CER). Here, a conditioned stimulus (CS), such as a tone, is paired with an aversive unconditioned stimulus (US), such as electric shock. After several such pairings, the tone elicits autonomic and behavioral fear responses, such as freezing, in anticipation of the shock (Blanchard and Blanchard, 1972; Fanselow, 1980; Quirk & Mueller, 2008; Quirk, 2006; Thomas, Longo, & Ayres, 2005).
In most studies aimed at reducing fearful responding, reduction of defensive behaviors is accomplished through the use of simple EXT - a form of learning that disassociates the CS and US by repeatedly presenting only the CS without the US (CS-only extinction: CSO) (Quirk & Mueller, 2008; Thomas, Longo & Ayres, 2005). Using these methods, rats that once froze at the sound of a tone paired with shock now move more freely in the presence of the tone.
Because EXT reduces or eliminates the avoidance behavior, it is tempting to assume that it erases the learned fear itself. However, allowing time to pass following EXT frequently evokes the re-emergence, or SR, of the conditioned response (Pavlov, 1927). The phenomenon of SR indicates that, even after many EXT trials, an animal retains a memory of conditioning that can provide a powerful basis for relapse (Quirk, 2002; Bouton, 2002).
The explicitly unpaired extinction procedure (EU-EXT) has been suggested as an alternative to simple CSO extinction methods. Essentially, following an original association between CS and US (CS+US), this procedure then extinguishes the original association by now providing a new negative CS-US contingency in which the CS never predicts the US. This methodology has been explored for over 40 years (Rescorla, 1969a,b; Baker, 1977; Kalat & Rozin, 1973) but most recently by Thomas and his associates (Rauhut, Thomas, & Ayres, 2001; Thomas & Ayres, 2004; Thomas, Longo, & Ayres, 2005). The EU procedure resulted in less fear (after 24 days) than did conventional CSO EXT (Thomas & Ayres). Moreover, the conditioned response extinguished and did not reappear between sessions (spontaneously recover) following the passage of time. Further, EU treatments thwarted both renewal and reacquisition of the CER (Rauhut, Thomas, & Ayres, 2001).
Our laboratory has extended the extinction and SR literature to the conditioned taste aversion paradigm (CTA) (Mickley et al., 2004; 2005; 2007). CTA is a defensive reaction to a learned fear (Parker, 2003) and may be acquired when an animal consumes a novel taste (CS) and then experiences the symptoms of poisoning (the US) (Garcia, Kimeldorf, & Hunt, 1961; Garcia, Kimeldorf, & Knelling, 1955). Later, the animal will avoid the taste previously associated with feelings of illness. CTA extinction results in a resumption of eating/drinking the once-avoided tastant (Rosas & Bouton, 1996; Mickley et al., 2004), but the taste aversion spontaneously recovers following a latency period of water drinking (Mickley et al., 2007).
CTA has a number of unusual properties that challenge the basic tenets of traditional learning and memory theory (Domjan, 1993; Bures, Bermudez-Rattoni, & Yamamoto, 1998). Therefore we sought to determine if the EU extinction procedure would alter a CTA in a way that is similar to that already reported in studies using the CER paradigm. Specifically, in the studies reported here, we tested the generalizability of the EU-EXT procedure by comparing its ability to reduce a conditioned taste aversion as compared to a procedure in which only the CS is presented (CSO). Further, we assessed the efficacy of the EU-EXT procedure in reducing or eliminating SR of a CTA. We hypothesized that EU-EXT would be an effective means of achieving re-acceptance of a once-avoided taste and that it would reduce the SR of a taste aversion. Our data were consistent with these predictions.
Methods
Subjects
A total of 47 naive male Sprague-Dawley rats (Mean ± SEM weight = 420.82 ± 12.17g), supplied by Zivic Laboratories (Zelienople, PA) were used in this experiment. Animals were housed in individual plastic cages (44.45 cm long × 21.59 cm wide × 20.32 cm deep) with corncob bedding (Bed o’cobbs, Andersons Industrial Products, Maumee, OH). A 12-hr light-dark cycle (lights on at 0600 hr) was maintained, and temperature was kept within 23–26°C. Rats also had free access to Purina Rat Chow (no. 5001, PMI Nutrition International, Brentwood, MO) for the duration of the study. All animals were handled briefly during daily weighings.
Procedures were approved by the Baldwin-Wallace College Institutional Animal Care and Use Committee. Animals were procured and cared for according to the recommendations in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and in compliance with the Animal Welfare Act.
Experimental Design and Group Assignment
At the start of the study, rats were randomly assigned to one of 4 treatment groups (CTA+ CSO-EXT, CTA+EU-EXT, NO CTA+“CSO-EXT”; NO CTA+“EU-EXT” – see descriptions, below and in Table 1). CTA rats received CS+US pairings while the CS/US presentations for the NO CTA rats were available non-contingently on different days in order to avoid formation of the CTA. Once assigned, each individual NO CTA control rat was matched, as closely as possible, to an individual CTA rat of similar weight. The NO CTA rats served as yoked controls and received the same number of CS/US presentations as their experimental pairs. As part of a related study, we sacrificed about half of the rats from each of our treatment groups for immunohistochemical analysis of the brains following the extinction stage of our experiment (data to be available in a future report) (see Table 1).
Table 1.
Group Nomenclature and Treatments
| Conditioning Phase - Group Designation | Group N | CTA Conditioning | Extinction Phase – Group Designation | Group N | Extinction Training | 30-day Latency before SR test | SR Group N | SR Test Solution | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Days 1, 3, 5 | Days 2, 4, 6 | Odd Days | Even Days | |||||||
| CTA | 23 | SAC3 + LiCl4 | H2O | CTA+EU- EXT 1 | 11 | SAC | H2O+LiCl | H2O | 5 | SAC |
| CTA+CSO- EXT 2 | 12 | SAC | H2O+ SAL5 | H2O | 6 | SAC | ||||
| NO CTA | 24 | SAC | H2O + LiCl | NO CTA+ “EU-EXT” | 12 | SAC | H2O+LiCl | H2O | 6 | SAC |
| NO CTA+ “CSO- EXT” | 12 | SAC | H2O+ SAL | H2O | 6 | SAC | ||||
CTA-EU-EXT = “Explicitly Unpaired” extinction procedure in which both the CS and US are presented on alternate days
CTA-CSO-EXT = “Conditioned Stimulus Only” extinction procedure in which only the CS is presented every other day
SAC = saccharin salt dissolved in DI H2O (0.3% w/v; p.o.)
LiCl = lithium chloride injection dissolved in sterile, physiological saline (81mg/ml; 81mg/kg; i.p.)
SAL= sterile, physiological saline (0.9% w/v, NaCl; 1ml/kg; i.p.)
Materials
All chemicals were purchased from the Sigma-Aldrich Chemical Company (St. Louis, MO). Lithium chloride (LiCl) was dissolved in physiological saline to produce a final concentration of 81mg/ml and was administered at a dose of 81mg/kg. Saccharin salt was dissolved in deionized water to a final concentration of 0.3%, by mass, saccharin solution (SAC). All consummatory tests (SAC or water) involved a single bottle.
Conditioning Procedure
Rats were conditioned and tested in their home cages. All contextual cues were kept constant throughout each phase of the study. Animals were habituated to a 23 hr water deprivation schedule beginning 2 days prior to the first conditioning trial and maintained on this schedule throughout the study. This relatively brief period of water deprivation acclimation ensured that animals were motivated to drink SAC (the CS) when it was first presented. Fluid consumption was recorded daily to the nearest tenth of a gram.
On the first conditioning day, the water-deprived rats were given 30 min access to 0.3% SAC. Following SAC exposure, SAC bottles were removed and animals assigned to a CTA group (refer to Table 1 for group nomenclature) received an intraperitoneal injection of LiCl (81mg/kg; 81mg/ml; i.p.). Fifteen minutes post-injection, the animals were given 30 min access to tap water to prevent dehydration. CTA animals (Mean weight ± SEM = 429.28 ± 25.60g) received the CS-US pairings on conditioning days 1, 3, and 5. Interim days 2, 4, and 6 served as rest periods during which the CTA animals received two 30 min presentations of water separated by a 15 min interval (replacing the LiCl injection period experienced on days 1, 3, and 5). At the end of the CTA training, the 23 animals were designated as CTA and continued into the extinction phase of the experiment as either CTA+EU-EXT or CTA+CSO-EXT animals (extinction condition randomly assigned prior to the start of the experiment).
An additional control group (NO CTA; N=24; Mean weight ± SEM = 381.58 ± 6.88g) that did not receive CS-US pairings, but instead, received explicitly unpaired presentations of both the CS and US during the conditioning phase, were included in this study to account for any residual effects of LiCl and SAC exposures. These NO CTA animals received 30 min access to SAC and then, 15 min later, 30 min access to water on days 1, 3, and 5 of conditioning. On alternate days 2, 4, and 6 of conditioning, NO CTA animals were injected with LiCl during the 15 min interval between the two water presentations. This explicitly unpaired conditioning procedure allowed the NO CTA animals to receive both the CS and US throughout conditioning without forming an association or subsequent aversion to SAC (Mickley et al., 2004; 2007).
Extinction Procedure
After day 6 of the conditioning phase, the extinction phase of our study began. Beginning with day 1 of extinction training, animals received 30-min exposure to SAC every-other day (odd-numbered days). During the initial stages of extinction, CTA rats drink very little of the CS. Therefore, fifteen minutes after SAC exposure, the animals received 30 min access to water in order to prevent dehydration.
The NO CTA control group was continued into the extinction phase of the study, but since they had no aversion to extinguish, these control animals were yoked to animals in experimental groups (CTA+CSO-EXT or CTA+EU-EXT) based on initial weights and were considered to have “extinguished” on the day that their yoked CTA counterpart reached the asymptotic extinction criterion (see below).
On days when there was no SAC exposure (even-numbered days), animals received two 30 min presentations of tap water. Within 15 min of the first water exposure, all animals designated as CTA+EU-EXT or NO CTA+“EU-EXT” were injected with LiCl (81mg/kg, i.p.) and all animals designated as CTA+CSO-EXT or NO CTA “CSO-EXT” were injected with a comparable volume of physiological saline (i.p.; refer to Table 1). Fifteen minutes post-injection, animals were given a second, 30-min opportunity to drink to tap water.
The extinction phase of the study was complete once rats reached a 90% SAC re-acceptance level (see extinction criteria discussed, below). Note: approximately half the rats were sacrificed for brain assays on the day they achieved asymptotic extinction (histology data not reported here). See Table 1 and the Experimental Design and Group Assignment section for details regarding N.
Spontaneous Recovery Test
Upon reaching the extinction criterion (90% SAC reacceptance, see below), animals were given access to water only for the next 29 days (refer to Table 1 for group Ns). During this period, rats received two 30-min presentations of water (spaced 15 min apart) each day. This procedure corresponded to the temporal characteristics of the previous CTA and EXT training regimens. Thirty days following the end of extinction training, rats were exposed to SAC for 30 min as a test of CTA SR.
Statistical Analysis
We wished to estimate levels of normal, familiar baseline SAC drinking as a means to evaluate the degree to which the rats in this study had extinguished their CTA. However, recording several days of baseline SAC pre-exposure in our animals would have impeded future CTA training, due to latent inhibition effects. Moreover, we also wished to avoid the bias associated with the rat’s initial hesitation to consume novel substances (neophobia; Domjan & Gillan, 1977). Therefore, normal, familiar SAC consumption was determined by averaging SAC consumption on the third day of exposure from a separate group (N = 10) of similarly-sized rats not used in the current study.
We adopted the naming convention originally established by Nolan et al. (1997) to describe the phases of CTA extinction. Saccharin drinking levels to enter each of the three phases of extinction were defined as a percentage of baseline saccharin consumption: Static (less than 10% of baseline), Dynamic (10%–80% of baseline) and Asymptotic (greater than 80% of baseline). In this experiment, the end point criterion for asymptotic extinction was defined as SAC consumption greater than or equal to 90% of the baseline (Mickley et al., 2004).
SPSS software (Chicago, IL) was used for all statistical analyses. A repeated measures analysis of variance (RM-ANOVA; Kirk, 1982) was used to evaluate SAC consumption within and between groups that received either NO CTA or CTA training during the conditioning phase of the experiment. An independent samples t-test was used to compare the mean total liquid consumption (SAC+H2O, i.e., total volume of these liquids consumed during their sequential, single-bottle, presentations each day) of these two groups of animals during the conditioning phase of the study. An independent samples t-test was also performed to assess: (1) differences in the mean total liquid consumption (SAC+H2O) by the CTA+CSO-EXT and CTA+EU-EXT rats during the first 15 days of EXT training, (2) between-group differences in the total days to asymptotic extinction as well as the durations of the static, dynamic and asymptotic phases, and (3) SAC drinking of these two groups on the asymptotic extinction and SR test days. Finally, paired samples t-tests evaluated group differences in SAC consumption on the day of asymptotic extinction compared to the SR test day. SR of a CTA was operationally defined as significant suppression of SAC drinking as compared to the level of SAC consumption at the point of asymptotic extinction. Statistical significance was evaluated using an α = 0.05.
Results
Conditioning
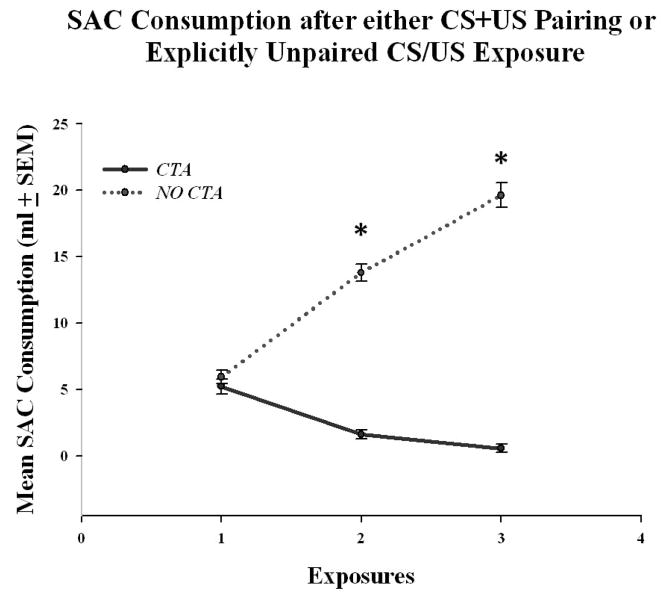
The amount of SAC consumed over the three day conditioning period indicated that all the CTA animals had acquired a strong taste aversion, whereas the NO CTA rats did not acquire a CTA (refer to Figure 1). On the first day of conditioning, the animals showed a neophobic response, indicated by the low consumption of SAC. A repeated measures ANOVA [Treatment (CTA or NO CTA) × Trial] revealed a significant treatment effect [F (1, 45) = 334.761, p < 0.001], a significant change in SAC drinking over trials [F (2, 90) = 39.055, p < 0.001], and a significant interaction [F (2, 90) = 164.834, p < 0.001]. In addition, two separate repeated measures ANOVAs were run to analyze SAC consumption over the three days of conditioning for the CTA and NO CTA groups. This analysis showed that the CTA groups had a significant decline in SAC drinking over the three days [F (1, 23) = 56.894, p < 0.001], and also that the NO CTA groups had a steady rise in SAC consumption [F (1, 22) = 161.234, p < 0.001].
Figure 1.
Mean volume of SAC consumption (± SEM) after either three CS+US pairings (CTA) or three explicitly unpaired CS/US exposures (NO CTA). The CTA group showed a significant decrease in the amount of SAC consumed over the three exposures. The NO CTA group showed a significant increase in SAC consumption over the same three periods. This indicates that the CTA groups had acquired the CTA, whereas the NO CTA group did not. * = significantly different from the CTA animals (α = 0.05).
In order to confirm that the thirst and general consummatory behaviors were not different between the rats that formed a CTA and those that did not, we compared the mean total liquid consumption (SAC+H2O; presented sequentially, as described above, in single-bottle tests) of the two groups of animals. The mean daily total liquid consumption of rats in the CTA group [Mean volume (ml) consumed ± SEM = 21.34 ± 0.74] was not significantly different from rats in the NO CTA group [Mean volume (ml) consumed ± SEM = 21.54 ± 0.95].
Lastly, to verify that the CTA+CSO-EXT and CTA+EU-EXT animals had acquired the same level of aversion to SAC, the animals’ SAC consumption levels on the first day of extinction (after all conditioning trials) were compared. The mean SAC consumption of the CTA+CSO-EXT group [Mean volume (ml) consumed ± SEM = 0.08 ± 0.027] was not significantly different from that of the CTA+EU-EXT group [Mean volume (ml) consumed ± SEM =.01 ± .06)].
Extinction
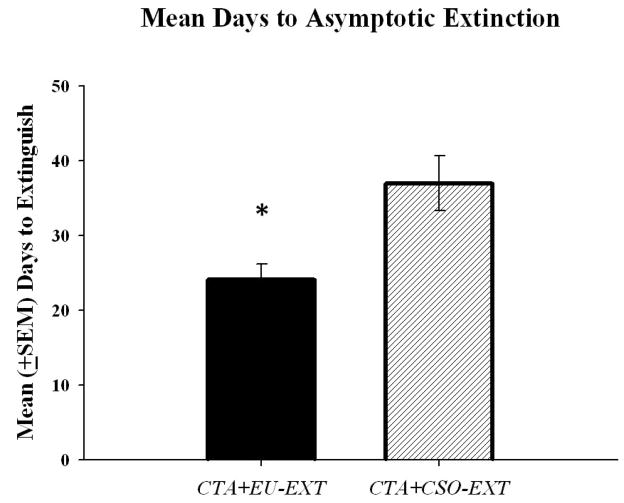
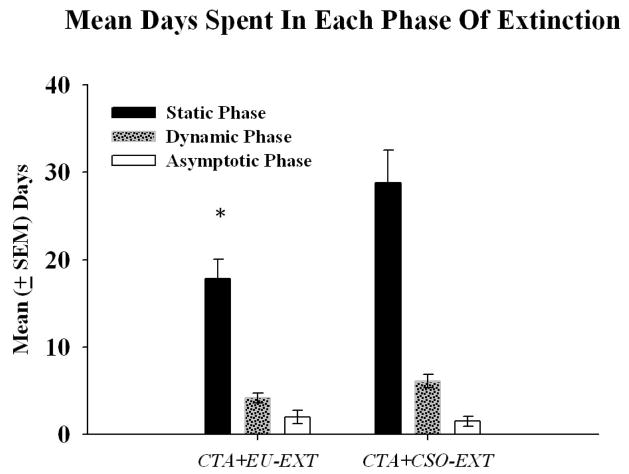
Rats in the CTA+CSO-EXT and CTA+EU-EXT groups achieved the same levels of asymptotic extinction (% of baseline). Rats in the CTA+EU-EXT group drank amounts of SAC on the day of asymptotic extinction that were not reliably different [t (15.91) = 1.74, p = 0.10; unequal variances] from the volumes consumed by rats assigned to the CTA+CSO-EXT group. However, the mean times to reach asymptotic extinction for these groups were significantly different (see Figure 2). The CTA+EU-EXT animals extinguished the CTA more rapidly than did the CTA+CSO-EXT group [t (21) = 3.00, p = 0.007]. To further explore this difference, the lengths of each of the phases of extinction (as described by Nolan et al., 1997) were determined and compared. The data revealed a significant CTA+CSO-EXT vs. CTA+EU-EXT group difference in the days to complete the static phase of extinction (i.e., to return to 10% of baseline SAC drinking) (see Figure 3) [t (21) = 2.52, p = 0.02]. However there were no differences between these groups in the length of time to reach the dynamic and asymptotic phases of extinction.
Figure 2.
Mean days (± SEM) for animals to reach asymptotic extinction. Animals either underwent the CTA+EU-EXT or CTA+CSO-EXT procedure. The CTA+EU-EXT group took significantly fewer days to extinguish the learned fear than the CTA+CSO-EXT group. * = significantly different from the CTA+CSO-EXT animals (α = 0.05).
Figure 3.
Mean days (± SEM) spent in each phase of extinction (Nolan et al., 1997) for animals that underwent either CTA+EU-EXT or CTA+CSO-EXT procedures. Rats that experienced the CTA+EU-EXT procedure spent significantly fewer days in the static phase (SAC reacceptance of less than 10% of baseline) than the CTA+CSO-EXT group [* = significantly different from the CTA+CSO-EXT animals (α = 0.05)]. However, the CTA+EU-EXT and CTA+CSO-EXT groups spent comparable number of days in both the dynamic (SAC reacceptance greater than 10 % but less than 80% from baseline) and asymptotic phases (SAC reacceptance of greater than 80% from baseline).
An additional analysis was performed to determine if the CTA+EU-EXT animals had altered hydration during the extinction phase. The total daily liquid consumed (SAC + H2O) during the first 15 days of the extinction process was compared between the CTA+EU-EXT and CTA+CSO-EXT groups [Mean volume (ml) consumed/day ± SEM = 20.71 ± 1.06 and 21.97± 1.06, respectively]. An independent samples t-test revealed that there was not a significant difference in liquid consumption between these groups.
Spontaneous Recovery (SR) Test
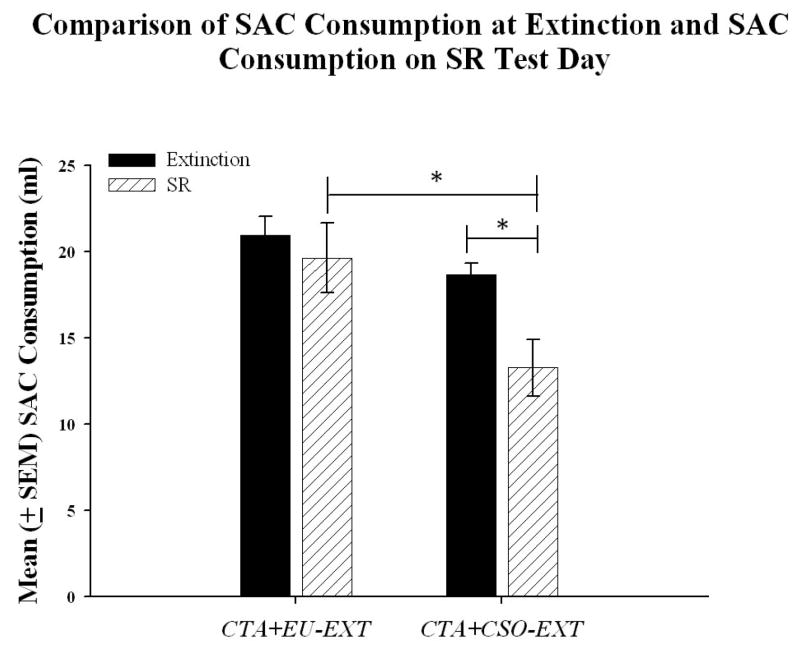
The SR test data revealed that the CTA+EU-EXT group drank significantly more SAC than the CTA+CSO-EXT group [t (9) = 2.47, p = 0.04] when rats had an opportunity to drink the sweet liquid following a 29-day water-only latency period. A paired sample t-test determined that the volumes of SAC consumed by the rats in the CTA+EU-EXT group on the day of asymptotic extinction and on the SR test day were not significantly different, indicating that the animals did not experience SR of the CTA [t (4) = −0.55, p = 0.66]. However, the CTA+CSO-EXT group drank significantly less SAC on the SR test day than on the day of asymptotic extinction [t (5) = 2.72, p = 0.04], which indicates SR of the CTA (see Figure 4).
Figure 4.
Mean volume of SAC consumption (± SEM) on the day of asymptotic extinction and on the subsequent SR test day for both the CTA+EU-EXT and CTA+CSO-EXT animals. On the day of the final extinction test, the CTA+EU-EXT and CTA+CSO-EXT groups drank comparable amounts of SAC (p > 0.05; see text). The CTA+CSO-EXT group drank significantly more SAC on the last day of extinction than on the day of the SR test, indicating a spontaneous recovery of the CTA. However, the CTA+EU-EXT animals drank nearly the same amount of SAC on the day of extinction as they did on the SR test day, suggesting that the EU-EXT procedure may be effective in blocking SR. * = groups indicated are significantly different, α= 0.05.
As expected, the NO CTA animals drank large amounts of SAC throughout the study once the animals overcame the initial neophobia. The average volume of SAC consumed at a time parallel to when their yoked CTA pairs were reaching asymptotic extinction was 28.92 ± 2.65 ml [Mean SAC consumption ± SEM]. This average daily consumption did not change significantly (following 30 days of water drinking) during the final “SR” test: Mean SAC consumption (ml) ± SEM= 27.620 ± 1.27. Additionally, the NO CTA+“EU-EXT” [Mean SAC consumption (ml) ± SEM= 28.42 ± 1.82] and the NO CTA+“CSO-EXT” [Mean SAC consumption (ml) ± SEM= 27.420 ± 1.67] did not show any differences in SAC consumption at the time of the “SR” test. In these non-conditioned animals, repeated non-contingent exposures to the CS and/or US did not lead to a suppression of SAC drinking over the course of this study.
Discussion
In this study, we compared the effectiveness of two CTA extinction procedures: (1) presentation of the CS only (CSO-EXT), and (2) presentation of the CS explicitly unpaired with the US (EU-EXT). Our data suggest that both methods of extinction produced the same level of asymptotic reacceptance of the once-avoided taste. However, the CTA+EU-EXT procedure produced more rapid extinction of a CTA than did exposure to the CS only. Further, CTA+EU-EXT significantly inhibited spontaneous recovery of the CTA when the CS was re-introduced 30-days after asymptotic extinction was achieved.
These findings are, in many ways, consistent with those of other laboratories that have used CER paradigms to assess the effectiveness of EU-EXT to thwart renewal/relapse of conditioned fears (Rauhut, Thomas, & Ayres, 2001; Thomas, Longo, & Ayers, 2005). Although these investigators reported that the EU procedure retarded the rate of extinction in the classical fear conditioning paradigm, our data indicate that the EU-EXT procedure allows more-rapid extinction of a CTA. A comparison of the methodologies of the CER and CTA may provide some hints about why this is the case. The time courses of extinction are clearly very different in the 2 paradigms. Progress on CER extinction may be observed in a matter of minutes whereas movement into the dynamic phase of CTA extinction may take a month, or longer. This is due, in part, to the schedule of CS and US exposures necessary for each paradigm. Within the classical fear conditioning paradigm, multiple presentations of the CS and US are made available within an hour (Thomas, Longo, & Ayers). But the CSs and USs of CTA extinction are presented once every 24 or 48 hours (Mickley et al., 2004; 2005; 2007). These different time periods may very well affect the ability of the animal to consolidate, retrieve and/or reconsolidate contingencies associated with the CS or US. The duration of CS and US exposures during each presentation also differs between CERs and CTAs. Tone and shock presentations characteristic of CER studies are under precise control of the experimenter and are typically of short duration. In our studies, however, taste exposures were voluntary and therefore determined by our subjects. Gustatory sensations were of unknown duration, and the malaise associated with LiCl may have lasted an hour or more (Meachum & Bernstein, 1990). Perhaps these longer exposures to the CS and US in the CTA paradigm are differentiating factors that affect the relative rates of CTA vs. CER extinction. Finally, animals may perceive different saliencies in the two types of extinction learning discussed here. Rejecting potential food sources can have devastating consequences for an animal (perhaps more so than the discomfort of transient foot pain) and engenders additional risk-taking that may facilitate the speed of CTA extinction learning.
The differences between the behavioral results produced by the EU-EXT and CSO-EXT procedures raise some important theoretical questions. Is it possible that fear of the CS has been somehow intensified by the CSO-EXT procedure but not the EU-EXT methods? Eysenck (1968; 1979) argued that, in patients with high anxiety, the CR (e.g., an internal state of fear) is uncomfortable and may serve as an aversive US substitute. Consistent with this proposition, “incubation of fear” would increase over successive non-reinforced presentations of the CS. Thus, reinforcement of the CS may continue during extinction if the CR is elicited by the CS during this process. The theory has been tested (although never in the context of CTA) and experimental findings have not always supported Eysenck’s original proposition (Richards & Martin, 1990). However, “incubation of fear” has been demonstrated under certain, well-defined but limited, experimental conditions in which CS presentations are spaced and the level of physiological arousal is high (Cain, Blouin, & Barad, 2004). In our study, the CS was presented at the same frequency (once, every-other day) in both the EU-EXT and CSO-EXT conditions. Therefore the frequency of CS exposure does not allow us to differentiate the two extinction methods or explain the dissimilar behavioral results they produce. The interleaving of the US presentations every-other day during extinction training may have provided a heightened sense of arousal in the EU-EXT animals as compared with the CSO-EXT rats. If this is the case, then we would expect a greater “incubation of fear” in the rats undergoing the EU procedure when, in fact, their SAC avoidance reactions (as measured by a reduced SR of the CTA) were attenuated by this extinction method. Therefore, our data seem more consistent with the hypothesis that greater excitation during extinction leads to greater extinction (Rescorla, 2000) – a finding harmonious with the clinical observation that effective treatment depends on generating a sufficient level of anxiety and sympathetic activation to induce effective behavioral extinction (Stampfl & Levis, 1967; Holmes, Moulds, & Kavanaugh, 2007).
Could our EU-EXT procedure have caused habituation to the US or turned the CS into a conditioned inhibitor? According to Thomas, Longo and Ayres (2005), the EU procedure does neither of these things in the context of a CER paradigm. It should be noted, however, that our findings differ from those of Thomas, Longo and Ayres (2005), because rats exposed to the EU-EXT condition extinguished their CTAs more rapidly than the rats that went through the CSO-EXT treatments. It may be the case that the EU procedure converted SAC into a conditioned inhibitor (CI) and thus prevented the appearance of spontaneous recovery. It should be noted, however, that our paradigm was not a typical CI paradigm where a CTA is created and then later a “safe” solution is paired with the CS without consequential illness. This procedure creates a clear preference for the safe solution (Best, 1975). However, Calton, Mitchell and Schachtman (1996) showed that a CI can also be produced through a CSO CTA extinction process. Their CIs passed retardation and summation tests normally applied to evaluate CIs. In a retardation test, the putative CI should be slow to acquire (or re-acquire) excitatory responding relative to a novel stimulus. In a summation test, the CI is presented in compound with a known excitor and the conditioned responding to the stimulus compound is expected to be low relative to the excitor alone. Calton, Mitchell and Schachtman’s (1996) findings contrast with the results from other laboratories which failed to demonstrate that an extinguished CS acts as a CI when tested in compound with an excitatory CS (LoLordo & Rescorla, 1966; Rescorla, 1967; Reberg, 1972; Hendry, 1982). However, the authors attribute this to the earlier studies’ failure to provide sufficient extinction to produce an inhibitor and suggest that, in order for extinction to become a “net” CI, the level of inhibition must exceed that of excitation to the CS. Both the EU-EXT and CSO-EXT procedures employed in the current study produced the same levels of asymptotic extinction – suggesting equal levels of inhibition were achieved. However, it was only the EU-EXT procedure that suppressed SR of the CTA. Further, data from conditioned fear paradigms indicate that it takes substantially longer to produce CI than extinction (B.L. Thomas, personal communication, 2009), while our data indicate that the EU-EXT rats extinguished their CTA more rapidly than did rats that underwent the CSO procedure. While our data provide a necessary first step in describing the behavioral sequelae following the EU-EXT- vs. CSO-mediated extinction of a CTA, we did not employ summation or retardation tests to determine the extent to which our findings are attributable to conditioned inhibition. This is a logical and important next step that must be taken as we assess the underlying mechanisms that support the behavioral results we report here.
Although the current experiments do not speak directly to the underlying reasons why the EU-EXT process shortens extinction as compared to the CSO-EXT procedure, the shapes of the extinction curves may offer some clues (see Figure 3 to get a sense of the time rats from our two EXT groups spent in each phase of extinction). Rat CTA extinction curves often resemble a probit function (Mickley et al., 2004), with relatively long static phases followed by rapid re-acceptance of the once-poisoned CS as the animals quickly move to asymptotic levels of consumption. In the current study, once rats start to re-accept the once-avoided SAC, the EU and CSO extinction curves are quite similar in their time course. However, the EU procedure seems to make the rats more likely to re-initiate CS sampling. Since animals in the CTA+CSO-EXT and CTA+EU-EXT groups drank liquids (SAC + water) in about the same volumes/day, this readiness to initiate CS exposure cannot be attributed to greater motivation (thirst). Future studies may shed more light on the neuro-behavioral mechanisms that mediate this propensity of rats to more quickly challenge their “bait shyness” during the course of the EU-EXT procedure.
Rats in the CTA+EU-EXT group moved through the static phase of extinction more rapidly than did rats in the CTA+CSO-EXT group and thereby also achieved asymptotic extinction more quickly. Thus the CTA+EU-EXT animals experienced comparatively few extinction trials. Given this information, one might expect that residual effects of the CTA would be more prominent in this group. However, if this was the case, there would have been some bias towards enhanced SR of the CTA. Instead, our CTA+EU-EXT animals exhibited a reduced SR, and their SAC drinking was not significantly less than their drinking at the end of the extinction procedure. Thus, the EU-EXT procedure was sufficiently powerful to overcome the potential tendency to retain aspects of the CTA memory in those animals that experience fewer extinction trials.
Our data provide additional information regarding the ongoing debate about whether extinction produces “new learning” (i.e., that the CTA memory is retained but there is a new understanding that it no longer applies in this new temporal context) (Bouton & Bolles, 1979; Bouton & Swartzentruber, 1991; Rescorla, 2001; Baeyens, Eelen, & Crombez, 1995; and review in Mickley et al., 2007) or “unlearning” (i.e., that the CTA memory is erased) (Bouton & Swartzentruber, 1991; Richards, Farley, & Alkon, 1984). Previous data from our laboratory suggest that the patterns of c-Fos expression (an indicator of neural activity) in the brains of rats that have undergone the CSO-EXT process following acquisition of a CTA do not resemble the brains of rats that have been exposed to the same CS and US but did not acquire the CTA (NO CTA; Mickley et al., 2004; Mickley et al., 2007). Thus, our CTA+CSO-EXT data have been consistent with a variety of other studies indicating that extinction induces new learning (for reviews, see Myers, Ressler, & Davis, 2008; Quirk & Mueller, 2008).
However, since the CTA+EU-EXT procedure seems to attenuate SR of a CTA, this raises the possibility that, under some circumstances, elimination or unlearning of the fear itself may be achieved. This implies that the CS re-enters a state that is functionally identical to the state of a neutral stimulus that was never involved in a CS+US contingency (Richards, Farley, & Alkon, 1984; Barad, 2006). At this stage, it is uncertain the extent to which EU-EXT procedures might be effective in producing a reversal of neurophysiological markers indicative of a CTA memory. However, our laboratory is currently studying patterns of c-Fos protein expression in the brains of rats exposed to CTA+CSO-EXT vs. CTA+EU-EXT procedures (Mickley et al., 2008).
In conclusion, our data indicate that the EU-EXT procedure is an effective way to attenuate SR of a CTA. These finding parallel similar results reported by other laboratories using the CER paradigm (Rauhut, Thomas, & Ayres, 2001; Thomas & Ayres, 2004; Thomas, Longo, & Ayres, 2005). Following additional pre-clinical testing, health care providers treating disorders where fear is prominent may wish to consider how the EU-EXT procedures described here may best be adapted in order to facilitate a variety of therapeutic approaches (Basoglu, Salcioglu, & Livanou, 2007).
Acknowledgments
These studies were supported by NIMH Award 1-R15-MH63720-03. The authors wish to acknowledge the following students and technicians for their excellent contributions to this research: Orion Biesan, Sarah Clark, Gary Coleman, Jennifer Dunger, Sarah Frischmann, Jennifer Graebert, Nick Grisak, Natalie Hogan, Ivan Islamaj, Kyle Ketchesin, Bruce Kinley, Daniel Petersen, Douglas Placko & Dave Revta. Some of these findings, in preliminary form, were presented at the 2008, International Behavioral Neuroscience Society Meeting, St. Thomas, VI. The animals involved in this experiment were procured, maintained and used in accordance with the Animal Welfare Act and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources National Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baeyens F, Eelen P, Crombez G. Pavlovian associations are forever: On classical conditioning and extinction. Journal of Psychophysiology. 1995;9:127–141. [Google Scholar]
- Baker AG. Conditioned inhibition arising from a between-sessions negative correlation. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:144–155. [Google Scholar]
- Barad M. Is extinction of fear erasure or inhibition? Why both, of course. Learning and Memory. 2006;13:108–109. doi: 10.1101/lm.211306. [DOI] [PubMed] [Google Scholar]
- Basoglu M, Salcioglu E, Livanou M. A randomized controlled study of single-session behavioral treatment of earthquake-related post-traumatic stress disorder using and earthquake simulator. Psychological Medicine. 2007;37:203–213. doi: 10.1017/S0033291706009123. [DOI] [PubMed] [Google Scholar]
- Best MB. Conditioned and latent inhibition in taste-aversion learning: Clarifying the role of learned safety. Journal of Experimental Psychology, Animal Behavioral Processes. 1975;104:97–113. doi: 10.1037//0097-7403.1.2.97. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. Journal of Comparative and Physiological Psychology. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;15:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10:445–466. [Google Scholar]
- Bouton ME, Swartzentruber D. Sources of relapse after extinction in Pavlovian and instrumental learning. Clinical Psychology Review. 1991;11:123–140. [Google Scholar]
- Bures J, Bermudez-Rattoni F, Yamamoto T. Conditioned taste aversion: Memory of a special kind. Oxford: Oxford University Press; 1998. [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learning and Memory. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calton JL, Mitchell KG, Schachtman TR. Conditioned inhibition produced by extinction of a conditioned stimulus. Learning and Motivation. 1996;27:335–361. doi: 10.1006/lmot.1996.0020. [DOI] [PubMed] [Google Scholar]
- Chorot P, Sandin B. Effects of UCS intensity and duration of exposure of nonreinforced CS on conditioned electrodermal responses: An experimental analysis of the incubation theory of anxiety. Psychological Reports. 1993;73:931–941. doi: 10.2466/pr0.1993.73.3.931. [DOI] [PubMed] [Google Scholar]
- Domjan M. The principles of learning and behavior. 3. Brooks/Cole Publishing Company; 1993. [Google Scholar]
- Domjan M, Gillan DJ. Taste-aversion conditioning with expected versus unexpected drug treatment. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:297–309. doi: 10.1037//0097-7403.3.4.297. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. A theory of the incubation of anxiety/fear responses. Behavioral Research Therapy. 1968;6:309–321. doi: 10.1016/0005-7967(68)90064-8. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The conditioning model of neurosis. Behavioral Brain Science. 1979;2:155–199. [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. The Pavlovian Journal of Biological Science. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Hunt EL. The use of ionizing radiation as a motivating stimulus. Radiation Research. 1961;12:719–727. doi: 10.1037/h0038361. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Knelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Hendry JS. Summation of undetected excitation following extinction of the CER. Animal Learning and Behavior. 1982;10:476–482. [Google Scholar]
- Holmes EA, Moulds ML, Kavanagh D. Memory suppression in PTSD treatment? Science. 2007;318:1722. doi: 10.1126/science.318.5857.1722a. [Letter to Editor] [DOI] [PubMed] [Google Scholar]
- Kalat JW, Rozin P. “Learned safety” as a mechanism in long-delay taste aversion learning in rats. Journal of Comparative and Physiological Psychology. 1973;83:198–207. doi: 10.1037/h0034424. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. 2. Monterey, CA: Brooks/Cole; 1982. [Google Scholar]
- LoLordo VM, Rescorla RA. Protection of the fear-eliciting capacity of a stimulus from extinction. Acta Biologiae Experimentalis. 1966;26:251–258. [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–97. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Meachum CL, Bernstein IL. Conditioned Responses to a taste conditioned stimulus paired with lithium chloride administration. Behavioral Neuroscience. 1990;104:711–715. doi: 10.1037//0735-7044.104.5.711. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, McMullen CA, Yocom AM, Valentine EL, Dengler-Crish CM, et al. Dynamic processing of taste aversion extinction in the brain. Brain Research. 2004;1061:79–89. doi: 10.1016/j.brainres.2004.04.071. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, Yocom AM, Wellman JA, Biada JM. A Role for prefrontal cortex in extinction of a conditioned taste aversion. Brain Research. 2005;105:176–182. doi: 10.1016/j.brainres.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Hoxha Z, Bacik S, Kenmuir CL, Wellman J, Biada JM, et al. Spontaneous recovery of a conditioned taste aversion differentially alters extinction-induced changes in c-Fos protein expression in rat amygdala and neocortex. Brain Research. 2007;1152:139–157. doi: 10.1016/j.brainres.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Mickley GA, DiSorbo A, Wilson GN, Huffman J, Bacik S, Hoxha Z, et al. 2008 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2008. Spontaneous recovery of fear may be attenuated without a corresponding change in c-Fos expression in the medial prefrontal cortex, gustatory neocortex, or amygdala Program No. 487.20. http://www.abstractsonline.com/plan/ViewAbstract.aspx?sKey=44c2a76d-e93b-482f-946a-96e75f837e5e&cKey=790bb241-7556-4e03-a962-ca394e52f633. [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learning and Memory. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington (DC): National Academy Press; 1996. [Google Scholar]
- Nolan LJ, McCaughey SA, Giza BK, Rhinehart-Doty JA, Smith JC, Scott TR. Extinction of a conditioned taste aversion in rats: I. Behavioral effects. Physiology and Behavior. 1997;61:319–323. doi: 10.1016/s0031-9384(96)00411-8. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: Evidence for two different processes. Learning and Behavior. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, translator. London: Oxford University Press; 1927. [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learning and Memory. 2002;9:402–7. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ. Extinction: New excitement for an old phenomenon. Biological Psychiatry. 2006;60:317–18. doi: 10.1016/j.biopsych.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Martinez KG, Nazario Rodríguez LL. Translating findings from basic fear research to clinical psychiatry in Puerto Rico. Puerto Rico Health Sciences Journal. 2007;26:321–328. [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Thomas BL, Ayres JJB. Treatments that weaken Pavlovian conditioned fear and thwart its renewal in rats: Implications for treating human phobias. Journal of Experimental Psychology: Animal Behavior Processes. 2001;27:99–114. [PubMed] [Google Scholar]
- Reberg D. Compound tests for excitation in early acquisition and after prolonged extinction of conditioned suppression. Learning and Motivation. 1972;3:246–258. [Google Scholar]
- Rescorla RA. Inhibition of delay in Pavlovian fear conditioning. Journal of Comparative and Physiological Psychology. 1967;64:114–120. doi: 10.1037/h0024810. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Conditioned inhibition of fear resulting from negative CS-US contingencies. Journal of Comparative and Physiological Psychology. 1969a;67:504–509. doi: 10.1037/h0027313. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Establishment of a positive reinforcer through contrast with shock. Journal of Comparative and Physiological Psychology. 1969b;67:260–263. doi: 10.1037/h0026789. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Extinction can be enhanced by a concurrent excitor. Journal of Experimental Psychology, Animal Behavior Processes. 2000;26:251–260. doi: 10.1037//0097-7403.26.3.251. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Experimental Extinction. In: Mowrer RR, Klein SB, editors. Handbook of Contemporary Learning Theories. Mahwah NJ: Lawrence Erlbaum; 2001. pp. 119–154. [Google Scholar]
- Rescorla RA. Spontaneous Recovery. Learning and Memory. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Richards WG, Farley J, Alkon DL. Extinction of associative learning in Hermissenda: Behavior and neural correlates. Behavioral Brain Research. 1984;14:161–170. doi: 10.1016/0166-4328(84)90185-2. [DOI] [PubMed] [Google Scholar]
- Richards M, Martin I. Eysenck’s incubation of fear hypothesis: An experimental test. Behavioral Research Therapy. 1990;28:373–384. doi: 10.1016/0005-7967(90)90156-d. [DOI] [PubMed] [Google Scholar]
- Rosas JM, Bouton ME. Spontaneous recovery after extinction of a conditioned taste aversion. Animal Learning and Behavior. 1996;24:341–348. [Google Scholar]
- Stampfl TG, Levis DJ. Essentials of implosive therapy: A learning-theory-based psychodynamic behavioral therapy. Journal of Abnormal Psychology. 1967;72:496–503. doi: 10.1037/h0025238. [DOI] [PubMed] [Google Scholar]
- Thomas BL, Ayres JJB. Use of the ABA fear renewal paradigm to assess the effects of extinction with co-present fear inhibitors or excitors: Implications for theories of extinction and for treating human fears and phobias. Learning and Motivation. 2004;35:22–52. [Google Scholar]
- Thomas BL, Longo CL, Ayres JJB. Thwarting the renewal (relapse) of conditioned fear with the explicitly unpaired procedure: Possible interpretations and implications for treating human fears and phobias. Learning and Motivation. 2005;36:374–407. [Google Scholar]
- Van der Kolk BA. The body keeps the score: Memory and the evolving psychobiology of posttraumatic stress. Harvard Reviewof Psychiatry. 1994;5:253–265. doi: 10.3109/10673229409017088. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. Journal of Clinical Psychiatry. 2001;61(Suppl 17):41–46. [PubMed] [Google Scholar]