Abstract
The unfavorable treatment of people with physical disfigurements is well-documented, yet little is known about basic perceptual and cognitive responses to disfigurement. Here, we identify a specialized pattern of cognitive processing consistent with the hypothesis that disfigurements act as heuristic cues to contagious disease. Disfigurements are often invariant across time and difficult to conceal, and thus observers can detect the presence of such cues without necessarily remembering the particular individuals bearing these cues. Indeed, despite the fact that disfigured faces were especially likely to hold disease-sensitive perceivers’ attention (Study 1), disfigured individuals were often confused with one another and thus not well remembered later (Study 2), revealing a disjunction of the typical relationship between elevated attention and elevated memory. We discuss the implications of our results for stigmatization of people with and without physical abnormalities and suggest the possibility that cognitive mechanisms for processing social information may be functionally tuned to the variant nature of important cues.
Keywords: threat detection, face perception, disease avoidance, evolutionary psychology, social cognition
Shakespeare’s Twelfth Night features the memorable quotation “In nature there is no blemish but the mind; none can be called deformed but the unkind” (1916, p. 68). Today, the thought of treating people with innocuous physical disfigurements negatively because of their appearance is considered more abhorrent than ever. Yet this outcome is far from unusual: People with facial and bodily abnormalities often suffer stigmatization and discrimination, from simple awkwardness in interpersonal interactions to rejection from jobs with high public visibility (e.g., Greenhouse, 2003). Researchers have recently begun to consider these negative responses to disfigurement as emerging from an evolved motivation to avoid contagious disease threats (e.g., Kurzban & Leary, 2001; Park, Faulkner, & Schaller, 2003; Zebrowitz & Collins, 1997). One of the most promising avenues for understanding the consequences of such motivations involves explicating their role in directing basic cognitive processes (e.g., Ackerman et al., 2006; Kenrick, Delton, Robertson, Becker, & Neuberg, 2007; Maner, Galliot, Rouby, & Miller, 2007). Here, we demonstrate a disjunction between visual attention and memory effects that emerges when people view facial disfigurements, and consider the implications of this disjunction for a functional approach to threat management.
Interpersonal threat processing and physical disfigurement
Inefficient threat management can result in severe harm or death; thus interpersonal threat detection and defense have historically been, and continue to be, of fundamental importance (Daly & Wilson, 1999; Green & Phillips, 2004). Many sources of threat directly signal danger, as with the facial expression of anger, but such signals are rarely perfect indicators. Other sources of threat, like disease-causing pathogens, provide even less precise cues of their presence. This threat uncertainty gives rise to a signal detection problem in which the costs of missing a real danger far outweigh the costs of mistakenly perceiving a false danger. Cognitive threat detection mechanisms may therefore exhibit a bias to minimize costs by over-inferring threat from imperfect cues (Haselton & Nettle, 2006; Nesse, 2005).
In the context of a motivation to avoid disease, this bias would have led to negative, avoidant reactions to those exhibiting a wide-range of potential disease cues. Many contagious diseases produce conspicuous physical features such as lesions (Kurzban & Leary, 2001) and, accordingly, perceivers’ reactions to these features are often strongly negative (Park et al., 2003). To the extent that there exists an overgeneralization bias (Zebrowitz, Fellous, Mignault, & Andreoletti, 2003; Zebrowitz & Rhodes, 2004), one would expect perceivers to react similarly to other physical disfigurements as well. Consistent with this bias, people appear to heuristically associate many benign physical abnormalities with contagious disease (e.g., Park, Schaller, & Crandall, 2007; Schaller, Park, & Faulkner, 2003; Zebrowitz et al., 2003), and when confronting individuals who possess such abnormalities, exhibit the kinds of avoidant behaviors that would minimize contagion risk, if such risk existed (e.g., Heinemann, Pellander, Vogelbusch, & Wojtek, 1981; Houston & Bull, 1994).
Specific processing of specific threats
Despite a relatively large body of work on the stigmatization of people with physical disfigurements, little is known about the basic cognitive processing of disfigurement. We may gain some insights by considering functional specificity, an attribute common to many psychological mechanisms (e.g., Ackerman & Kenrick, 2008; Cosmides & Tooby, 1994; Kenrick, Li, & Butner, 2003; Barrett & Kurzban, 2006). Functional specificity refers to particular forms of input criteria, processing strategies, and outputs specialized for managing evolutionarily-recurrent problems. Basic social perception mechanisms may be oriented in specialized ways as to facilitate adaptive responses to these problems (Gibson, 1979; McArthur & Baron, 1983).
Consider the problem of intergroup aggression: People tend to selectively allocate perceptual and cognitive resources (such as the degree of visual attention) to ingroup members more than to outgroup members (e.g., Becker, Neuberg et al., 2008; Eberhardt, Goff, Purdie, & Davies, 2004; Richeson & Trawalter, 2008; Rodin, 1987). This preferential allocation may account for the better memory typically seen for ingroup targets relative to outgroup targets. However, when displaying signals of directed threat—angry expressions—outgroup targets are remembered well, eliminating the standard ingroup memory bias (Ackerman et al., 2006). Furthermore, possibly because angry expressions hold attention (Becker, Anderson et al., 2008; Fox et al., 2000; Fox, Russo, Bowles, & Dutton, 2001) and outgroup members are seen as less constrained by the aggression-inhibiting effects of ingroup empathy and interdependence (making them especially dangerous), memory for these outgroup targets can be even better than memory for ingroup targets (Ackerman et al., 2006). These findings suggest that both memory and attention have a specialized sensitivity for the processing of aggressive threats.
Similarly, disease-causing organisms have been a recurrent problem throughout human evolutionary history (Gangestad & Buss, 1993; Low, 1994). People therefore may have acquired specific cognitive strategies for managing disease-relevant cues such as disfigurement. What might these processing strategies look like? Certainly, one must detect and encode threats in order to respond properly, and so we would expect that, as with angry expressions, disfigurement holds attention. Unlike angry expressions though, physical disfigurements are relatively stable; they often do not disappear over time and are often difficult to conceal (such features are known in the ecological literature as structural invariants; McArthur & Baron, 1983). The relatively invariant nature of disfigurement suggests that processing individuating information beyond the disfigurement itself may typically be unnecessary, and even cognitively inefficient. That is, whereas the ability to efficiently encode an individual’s angry expression will not suffice for reducing a perceiver’s later vulnerability to that individual (because the individual may continue to be threatening without displaying it), the ability to efficiently encode an individual’s disfigurement may be quite sufficient for reducing a perceiver’s later vulnerability to that individual (because the individual will continue to exhibit the contagion-implying disfigurement). One should expect, then, that memory for other information that individuates disfigured targets would be relatively poor.
Processing disjunctions
This pattern of cognitive processing represents a “processing disjunction”—a violation of the expected relationship between early and later information processing, one that may serve as a functional solution to the problem of threat management (see Kenrick et al., 2007). Standard cognitive models typically presume a monotonically increasing relationship between attention and memory (e.g., Atkinson & Shiffrin, 1968; Craik & Lockhart, 1972): The more a target is looked at, the better that target will be remembered. We see evidence for this relationship in the processing of ingroup and outgroup faces as described above. In contrast, we are predicting that although disfigurement-based threat cues may lead to elevated attention, these cues will not lead to a corresponding elevation in memory for individuating features over and above the threat cues themselves. Thus, relatively transient threat cues (e.g., angry expressions) and relatively invariant threat cues (e.g., physical disfigurements) may have similar, adaptive effects at one stage of processing (e.g., attention), but much different effects at another stage (e.g., memory).
Current research
Do attention and memory exhibit specialized strategies for the processing of disease-relevant threats? We conducted two studies to explore how disfigurement influences visual attention (important for immediate threat processing) and sociospatial memory (important for longer-term threat management).
Study 1: Attentional Adhesion
Study 1 used a dot-probe task to compare the extent to which normal and disfigured faces capture visual attention. To directly investigate the role of contagion concerns in the processing of disfigurement, we also included a condition in which participants were primed to be disease-sensitive before participating in the dot-probe task. We expected that this manipulation would differentially increase attention to disfigured faces, consistent with other research demonstrating a selective effect of primes on relevant targets (e.g., Eberhardt et al., 2004; Faulkner, Schaller, Park, & Duncan, 2004; Ferguson & Bargh, 2004; Maner et al., 2005, 2007; Neuberg, Kenrick, Maner, & Schaller, 2005).
Method
Participants
Two hundred fifty-five undergraduates (median age = 19) participated in exchange for course credit. Four participants were excluded because of computer malfunctions. Thus, 251 participants (124 female) were included in the reported analyses.
Materials
Two slideshows were used, purportedly as part of an unrelated experiment, to prime either disease-sensitivity or a control state (between-participants). These slideshows featured 10 slides depicting either images and text related to contagion (e.g., a dirty sponge, a sneezing person) or architecture (e.g., public buildings). The disease-sensitivity slideshow has been effectively used in previous research (e.g., Faulkner et al., 2004).
Stimulus photographs included 64 color front-oriented faces, sized to 150×200 pixels. Target faces were both male and female and of a similar age to our participant sample. For one version of these stimuli, computer software was used to add flat pink coloration to a random area of the face (simulating a port-wine stain) or to adjust the location of one pupil (simulating strabismus), features that are both salient and yet not symptomatic of contagious disease. Which faces bore disfigurements and which did not was counterbalanced between participants.
The dot-probe task required participants to view faces appearing in one quadrant of a computer screen and then quickly identify shapes (circle, square) appearing in distinct quadrants, thus providing a measure of the speed with which perceivers disengaged visual attention from target faces (Maner et al., 2007). Each face was viewed twice, for a total of 128 trials. On each trial, a blank screen appeared for 2000ms, followed by a central fixation symbol (+) for 1000ms. Next, this symbol was replaced by a color facial photograph in one of four screen quadrants. Faces varied by sex and by disfiguring cue (none or port wine stain/strabismus) within-participants; because these cues do not logically imply contagious disease, any biases in their processing implicate the presence of heuristic threat management processes. After 500ms, the photo was replaced by a shape which appeared either in the same quadrant as the photograph (filler trials; 25% of total) or in a different quadrant (disengagement trials; 75% of total) and remained until a response was given. To do this, participants used two keyboard keys (A and L) labeled with stickers featuring a circle or a square. A sample trial from the task appears in Figure 1. Photographs and trial type (filler or disengagement) were randomly presented within-participants.
Figure 1.
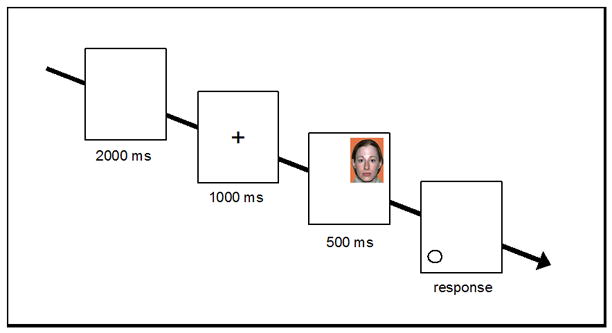
A single disengagement trial in the dot-probe task (Study 1).
Procedure
Participants received initial instructions in small groups of 1–3 and then proceeded to participate using individual computers separated by dividers. Participants were told that the main experimental task (a shape identification game) did not require the entire session time, so they would first evaluate a slideshow being constructed for use in a different study. After viewing the slideshow on their individual computers, participants responded to several filler questions (e.g., “How many slides did you see?”).
Instructions for the dot-probe task described above were then given on the screen: Participants were told to look at both the central fixation symbol and the photographs when they appeared, and to accurately categorize the shapes. Speed of response on the disengagement trials was the primary dependent variable. All participants were then probed for suspicion, fully debriefed and released.
Results
Following standard approaches to such attention-probe tasks (e.g., Fox et al., 2001), response times less than 200ms and greater than 2.5 standard deviations from the mean were excluded as outliers (0.6% of trials). Response times for correct trials were averaged for each of the four target photo types, and log-transformed to correct for skew (data presented in milliseconds for readability). Trials with incorrect responses (3%) were excluded from analysis. This number is comparable to those obtained in other dot-probe studies (e.g., Maner et al., 2007). We also collapsed across both types of target disfigurements, as no significant response time differences emerged between the two in the analyses (ps>.21).
Primary analyses
A Disease Prime × Target Disfigurement × Target Sex mixed ANOVA revealed the expected main effect of disfigurement: Disfigured faces held attention longer than normal faces, F(1, 249) = 16.17, p<.001, ηp2 = .06. This was qualified by a Disease Prime × Disfigurement interaction, F(1, 249) = 4.86, p<.03, ηp2 = .02, indicating that the disease-sensitivity prime made it harder to disengage from disfigured targets than from normal targets (see panel A of Figure 2). Simple effects tests demonstrated that this difference in attention reached significance within the disease-sensitivity condition, F(1, 249) = 19.30, p<.001, ηp2 = .07, but not within the control condition (p=.20). This functional specificity is consistent with the present theoretical approach and incompatible with alternative explanations built on the simple presumption that people’s attention is held by “novel” cues. Means and standard deviations are presented in Table 1.
Figure 2.
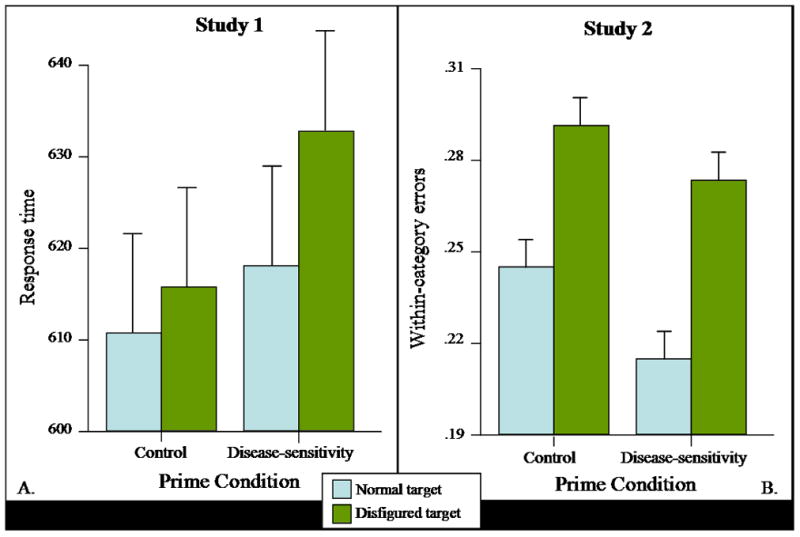
Greater attentional adhesion to disfigured targets (in milliseconds for readability, with standard error bars) (A) does not necessarily translate into better long-term memory storage (B).
Table 1.
Attentional Adhesion (in milliseconds)
| Control Prime | Disease Threat Prime | |||
|---|---|---|---|---|
| Normal targets | Disfigured targets | Normal targets | Disfigured targets | |
| Male targets | 607.10 (117.01) | 614.19 (111.65) | 615.64 (133.67) | 634.58 (145.62) |
| Female targets | 616.08 (116.63) | 615.82 (117.63) | 620.79 (143.83) | 628.66 (140.62) |
|
| ||||
| Total | 611.59a (116.82) | 615.05ab (114.64) | 618.215a (138.75) | 631.62b (143.12) |
Note. Means in the Total row not sharing a subscript are different within rows at p<.05. Standard deviations appear in parentheses. Analyses were conducted on log-transformed scores, but milliseconds are presented for ease of interpretation.
Additionally, a Target Sex × Disfigurement interaction, F(1, 249) = 3.74, p=.05, ηp2 = .02, indicated that the effect of disfigurement across priming conditions was significant for male targets, but not for female targets (see Table 1).1 This sex difference was due to normal female faces holding attention longer than normal male faces, an effect consistent with the relatively higher attention to female faces evident in both infants and adults (e.g., Becker, Neuberg et al., 2008; Quinn, Yahr, Kuhn, Slater, & Pascalis, 2002; Rosenwasser, Adams, & Tansil, 1983). To explore whether the disease-sensitivity manipulation increased attention to both disfigured male and female faces, we investigated the simple effect of disfigurement on attention to faces within each prime condition. In the control condition, attention was captured equally by normal and disfigured female faces (F<1) and marginally more by disfigured male faces than normal male faces, F(1, 249) = 3.09, p=.08, ηp2 = .01. However, in the disease-sensitivity condition, attention was indeed higher for disfigured faces than for normal faces, both with male targets, F(1, 249) = 15.79, p<.001, ηp2 = .06, and (marginally) with female targets, F(1, 249) = 3.48, p=.06, ηp2 = .01. These results suggest a stronger effect of disfigurement on male targets, though when sensitivity to disease is active, both disfigured males and females hold attention.
Ancillary analyses
Filler trials (when the shape appeared in the same quadrant as the photo) were used to motivate participants to maintain eye contact with the facial photographs. Although not of interest for attentional disengagement, analysis of responses to filler trials revealed only a main effect of target disfigurement: People were faster to respond to cues that appeared behind disfigured faces than behind normal faces, F(1, 249) = 3.89, p = .05, ηp2 = .02. This supports the conclusion that disfigurement both draws and holds attention.
Discussion
The results of Study 1 show that physical disfigurements hold attention. This is particularly the case when people were primed with disease threat. The attentional adhesion effect also appeared stronger for male targets than for female targets, which may reflect the relatively stronger heuristic association between males and threat (Daly & Wilson, 1999; Maner et al., 2005; Neuberg et al., 2005), or alternately, a relative disregard for normal male faces (Quinn et al., 2002; Rosenwasser et al., 1983). Additionally, the adhesion effect does not appear to be solely a function of cue novelty as it proved significant only when participants were primed with disease-sensitivity.
The elevated attention given to physical disfigurement, as with other types of threat cues, represents an immediate form of threat management. If we expect that attention to disfigurement follows the standard monotonically increasing relationship with memory, we should find that disfigured faces are remembered better than normal faces. This should be especially true when participants are sensitive to disease threats. However, if the processing of disfigured faces is focused on the invariant threat cue and not the individual bearing that cue, we may find evidence for a processing disjunction. That is, memory for disfigured faces may be worse than for normal faces, even in light of elevated attention, and even in light of elevated disease-sensitivity.
Study 2: Socicospatial Memory
Study 2 used a photo matching task to test memory for normal and disfigured faces. This task measures both recognition memory (i.e., the ability to correctly identify previously-seen faces) and memory for the location of faces (Becker, Kenrick, Guerin, & Maner, 2005). We again primed participants with the slideshows used earlier to investigate their role in orienting perceivers toward (heuristic) cues of disease threat.
Method
Participants
One hundred eleven undergraduates (39 female, median age = 20) participated in exchange for course credit.
Materials
The card matching task involved a computerized version of the classic “Concentration” card game (Becker et al., 2005). This game consisted of a 4×16 array with 32 pairs of faces, again varying by sex and disfigurement (only the normal and port wine stain faces from Study 1 were used) with the particular faces bearing disfigurement counterbalanced between participants. At the beginning of the game, all the faces were presented in randomly assigned locations for 10 seconds; this pre-exposure period allowed for the examination of early matches—an index of attention—as well as simplifying the calculation of matching efficiency by ensuring that all of the face locations have an equal chance of being noted. The faces were then concealed behind tiles, and participants attempted to match pairs of faces by clicking the tiles, one at a time, with a computer mouse. If two sequentially selected tiles matched, they remained face up; otherwise, both were hidden again after 2 seconds. The object of the task is to match the faces in as few as trials as possible. See Figure 3 for a sample game.
Figure 3.
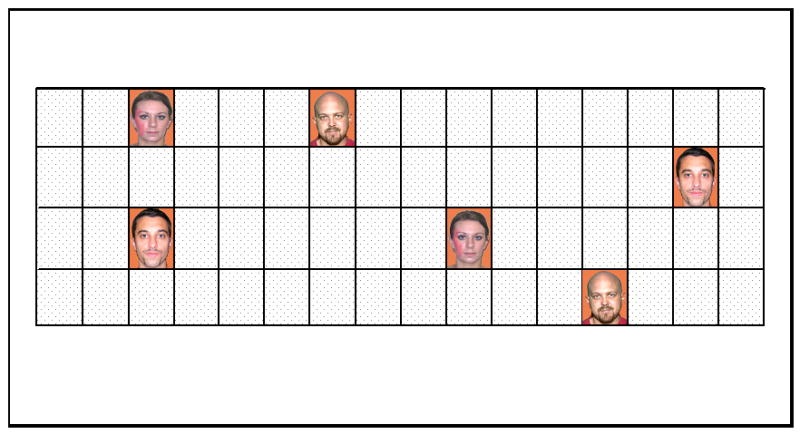
A single run of the concentration game task (Study 2).
Procedure
Participants completed the study in groups of 1–3 using individual computers, and included the same cover story for evaluating slideshow primes (disease-sensitivity or control) as in Study 1. Participants were told that that the study involved memory for people before and after they underwent cosmetic surgery to correct facial disfigurements. The card matching game, and instructions for completing it, were then administered. Following the game, all participants were probed for suspicion, fully debriefed and released.
Results
For each participant, mean error rates for each combination of target sex and disfigurement were calculated, as was the conditional probability that faces were confused with other faces of the same type. These mismatches and within-category errors were calculated with reference to the first face chosen in each turn. That is, if the first face was a diseased male and the second face was not a diseased male, this counted as one mismatch. If that mismatch was a (different) diseased male, this was additionally counted as a within-category error. No participant results necessitated being excluded as outliers.
Primary analyses
A Disease Prime × Target Disfigurement × Target Sex mixed ANOVA revealed a main effect of Disfigurement: Despite the tendency for disfigured faces to hold attention in Study 1, participants made more mismatches with disfigured faces than normal faces, F(1, 109) = 94.93, p<.001, ηp2 = .47. This increased mismatch rate resulted from a relatively high number of within-category errors (see Table 2). That is, participants confused disfigured faces with each other more than with normal faces, F(1, 109) = 32.17, p<.001, ηp2 = .23. Strikingly, this occurred despite the fact that the location of the disfigurement on each face was unique, and thus should have provided an additional memory cue, making faces easier to match.
Table 2.
Sociospatial memory (within-category errors)
| Control Prime | Disease Threat Prime | |||
|---|---|---|---|---|
| Normal targets | Disfigured targets | Normal targets | Disfigured targets | |
| Male targets | .245 (.090) | .298 (.103) | .240 (.098) | .287 (.120) |
| Female targets | .245 (.118) | .287 (.117) | .193 (.091) | .257 (.107) |
|
| ||||
| Total | .245a (.104) | .293b (.110) | .217c (.095) | .272b (.114) |
Note. Means in the Total row not sharing a subscript are different within rows at p<.05. Standard deviations appear in parentheses.
A main effect of the priming manipulation revealed that participants receiving the disease prime generated significantly fewer within-category memory errors relative to control participants (see panel B of Figure 2), F(1, 109) = 7.45, p<.01, ηp2 = .06. The conventional monotonic relationship between attention and memory would suggest that this reduction in errors would occur most strongly for disfigured faces (as these were the faces receiving the most attention in Study 1). However, planned comparisons indicated that the reduction in within-category errors was significant with normal faces, F(1, 109) = 4.94, p<.03, ηp2 = .04, but it was not significant with disfigured faces (p=.11). Thus, it appears that although disease-sensitivity generally prompted more effortful processing of target faces, this occurred primarily for normal and not for disfigured faces. This pattern is inconsistent with conventional predictions, but it is consistent with a processing disjunction—people concerned with disease-related threats focus attention on targets bearing (invariant) cues heuristically associated with disease, but do not spend the cognitive resources to individuate those targets.
In order to determine whether the attentional adhesion found in Study 1 produced any initial encoding advantage for disfigured faces, we also analyzed the first three trials of the game separately. Participants did match significantly more disfigured faces (N = 34) than normal faces (N = 13) over the first three trials (pbinomial < .005). This verifies that attention was attracted to and held by disfigurement cues. Yet despite this early advantage, disfigured faces were ultimately remembered (across all trials) only in terms of their location rather than their individual identity. In fact, although the result of the first three trials mathematically increases the probability that future mistakes would involve between-category errors, our earlier analyses instead revealed that people were more likely to confuse disfigured faces with other disfigured faces.
Finally, we investigated whether a boost in attention to disfigured male faces relative to non-disfigured male faces (as observed in Study 1) might produce later stage memory effects. In fact, a main effect of Target Sex indicated that participants made more within-category memory errors across all male faces relative to female faces (see Table 2), F(1, 109) = 4.57, p<.05, ηp2 = .04. Thus, although disfigurement led to increased attention to male faces in Study 1, especially when participants were primed with disease-sensitivity, this attention did not produce a corresponding increase in memory. This finding is consistent both with the idea of a processing disjunction, and with previous research indicating that female faces are often more memorable than male faces (e.g., Becker, Neuberg et al. 2008; Maner et al., 2003; Rehnman & Herlitz, 2006).
General Discussion
How do people allocate cognitive resources to disease-relevant threats? Unlike many other types of threats, disease-causing agents are not directly detectable and perceivers must rely on heuristic indicators, such as physical disfigurement, that are not always diagnostic of contagion. The present studies revealed that disfigurements did indeed capture attention, especially when people were primed to be sensitive to disease threats. However, this increased attunement to disfigured faces did not result in improved memory for those faces over the long-term. Perceivers instead encoded faces with disfigurements as being relatively homogenous, confusing them with one another. Even when people were primed with disease cues, exactly the time when we should expect that memory for disfigured faces improves relative to memory for normal faces, disfigured targets were not remembered better. These seemingly inconsistent effects of attention and memory are indicative of a threat-cued processing disjunction. Thus, when people attend to individuals with invariant threat cues, such as physical disfigurements, they may, to some degree, be “looking without seeing.”
Adaptively tuned cognitive threat processing
Detecting and encoding threats in one’s immediate environment represent the first stages of threat management. A number of studies, including the present one, have shown that visual attention is adaptively tuned in such a way as to facilitate automatic and rapid encoding of dangers (e.g., Fox et al., 2000; Lundqvist & Öhman, 2005). As physical disfigurements are often heuristically treated as cues to disease (e.g., Faulkner et al., 2004; Park et al., 2003), their attention-adhering effects make functional sense. Similarly, attentional adhesion is seen when danger-primed people view outgroup males (Eberhardt et al., 2004) and when males display angry expressions (Becker, Anderson et al., 2008; Fox, Russo, & Dutton, 2001), suggesting a commonality in how people attend to different forms of threat.
However, the later stages of threat management (including memory for dangerous targets) may allow for a greater variety of specialized, but still adaptive, processing strategies. For example, outgroup male faces are typically remembered relatively poorly (Anthony, Copper & Mullen, 1992), but the addition of angry expressions to these faces boosts recognition for these individuals by decreasing the confusions made with other angry faces (Ackerman et al., 2006). Contrast this with the deficits in memory found here for disfigured faces. The relatively high number of within-category confusions for these faces indicates that perceivers did encode the location of faces with physical disfigurement cues, but it appears that further processing did not occur. We suggest that these two findings, though representing seemingly inconsistent patterns of threat processing, jointly indicate functional attunement to an important feature of threat-relevant cues—their degree of invariance. Angry expressions are fleeting, although the interpersonal threat they signal may not be. Therefore, perceivers may need to expend valuable cognitive resources remembering individual features of an angry outgroup member to better remember that person at a later time. Physical disfigurements are not as fleeting. In fact, if such a cue (to contagious disease) were to disappear, it is probable that the underlying threat would have similarly vanished. Thus, if a physical abnormality appears permanent, there may be little immediate need to engage in effortful, individuating processing.
Though it is still preliminary to conclude that invariant threat cues make individuals less memorable relative to variant cues, there is other evidence consistent with this conclusion. Much of this evidence concerns the ubiquitous outgroup homogeneity (or cross-race) effect, typified by relatively lower recognition rates for outgroup members compared to those for ingroup members (Anthony et al., 1992; Ostrom & Sedikides, 1992). Within cross-race contexts that involve an active threat, we find that memory appears to increase when variant cues can be used to encode outgroup members, and decrease when invariant cues can be used. Consider three empirical illustrations. First, participants primed with threat tend to more strongly categorize neutral outgroup male faces using the invariant cue of race, leading to reduced memory for these faces (Miller, Maner, & Becker, 2008). Second, participants who view outgroup males with angry expressions (a variant cue) remember those faces better than they remember neutrally-expressive outgroup faces (Ackerman et al., 2006), suggesting that the presence of a variant cue trumps the typically poor encoding that the invariant cue of race elicits. Third, the addition of another variant cue has similarly been shown to modulate memory for outgroup faces. Ackerman (2007) showed neutrally-expressive Black and White male targets, varying in high or low-status clothing (a variant cue) to threat-primed White participants. The addition of low-status, but not high-status, clothing significantly improved memory for Black males, making them equally as memorable as low-status White males, presumably because stereotypes about low-status men involve a physical threat component but stereotypes about high-status men do not.
Processing variance and invariance more generally
We have thus far limited our consideration of cue variance to the context of threat management. However, functionally selective cognitive processing is evident across a range of situations and problems for which cue variance may be important (e.g., Ackerman & Kenrick, 2008; Cosmides & Tooby, 1994; Kenrick et al., 2003; McArthur & Baron, 1983).
Consider the role physical attractiveness (a relatively invariant cue) plays in romantic partner choice. Both men and women prefer physically attractive sexual partners (Li & Kenrick, 2006), but female attractiveness is a central indicator of longer-term romantic suitability whereas male attractiveness is less so (Buss, 1989; Gangestad & Simpson, 2000; Li, Bailey, Kenrick, & Linsenmeier, 2002). Interestingly, physically attractive faces draw visual attention regardless of their gender, yet attractive female faces are well remembered whereas attractive male faces are not (Becker et al., 2005; Maner et al., 2003). This is evidence of another processing disjunction, and one that suggests that perceivers can individuate targets according to invariant cues (e.g., female attractiveness) but typically do not expend the cognitive resources to do so when it is relatively unimportant. Thus, people do not remember attractive men under normal circumstances because doing so would produce little bang for the cognitive buck. Suggestively, evidence indicates that people may be better at remembering men of high status (a central indicator of romantic suitability for males; Li et al., 2002) when status is expressed by the variant cue of clothing (Maner, DeWall, & Gailliot, 2008) than when it is expressed by an invariant cue such as physical attractiveness (Maner et al., 2003). These examples suggest that memory for faces is probably not impaired by a limited capacity to process relevant information, but rather a (nonconscious) disinclination to do so (see also Rodin, 1987).
Implications for stereotypic processing
The current studies suggest a number of implications for intergroup processing and stigmatization. The heuristic association of physical disfigurement with disease may often lead to the social and physical isolation of people bearing such features. Our data indicate that this stigmatization begins at an early stage of cognition. As with individuals defined by other stigmatized characteristics (e.g., race), perceivers tend to confuse such individuals with one another—an outgroup homogeneity effect. However, unlike many of the more innocuous cues that define outgroup membership, the threat associated with disfigurement grabs attention, possibly facilitating further avoidance and segregation.
An active motivation to avoid disease may increase the potential for stigmatization, even in those people without disfigurements. In Study 2, priming disease-sensitivity made non-disfigured faces more memorable. This effect may indicate that active concerns about health promote allocation of cognitive resources toward individuals who bear no physical abnormalities, but may nonetheless be perceived as potential carriers of disease. This possibility is consistent with the finding that people motivated to avoid disease endorse more negative attitudes about, and behaviors toward, targets stereotypically associated with contagion threat, including foreign immigrants (Faulkner et al., 2004) and homosexual men (Crandall, Glor, & Britt, 1997).
Conclusions
Basic cognitive mechanisms show specialized and adaptive attunements to threatening stimuli. These attunements may lead to disjunctions in the expected linear relationship between attention and memory depending on the particular qualities of the particular threats. One such quality is the variant nature of the threat cue. Here, we found that people process facial disfigurement (a relatively invariant threat cue) in a functional manner, and yet very differently from the functional manner in which they process angry facial expressions (a relatively variant threat cue). These patterns have important implications outside of the experimental environment. Consider that each year in the U.S., 6,800 children are born with orofacial clefts (CDC, 2006), just one of many physical disfigurements. Understanding basic cognitive reactions to such individuals is an important step in addressing the stigmatization they will one day face.
Acknowledgments
Supported by National Institute of Mental Health Grant MH64734 to Douglas Kenrick and Steven Neuberg.
Footnotes
Because of the Target Sex effect, we also ran an analysis including Participant Sex in the model. A main effect of Participant Sex did emerge, F(1, 247) = 7.77, p < .01, η p2 = .03, with women holding ttention to all faces longer than men, but no other effects involving Participant Sex were significant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joshua M. Ackerman, Yale University
D. Vaughn Becker, Arizona State University, Polytechnic Campus.
Chad R. Mortensen, Arizona State University
Takao Sasaki, Arizona State University.
Steven L. Neuberg, Arizona State University
Douglas T. Kenrick, Arizona State University
References
- Ackerman JM. What you see isn’t what you get: Differential processing across the cognitive stream. Arizona State University; Tempe, Arizona: 2007. Unpublished doctoral dissertation. [Google Scholar]
- Ackerman JM, Kenrick DT. The costs of benefits: Help-refusals highlight key trade-offs of social life. Personality and Social Psychology Review. 2008;12:118–140. doi: 10.1177/1088868308315700. [DOI] [PubMed] [Google Scholar]
- Ackerman JM, Shapiro JR, Neuberg SL, Kenrick DT, Becker DV, Griskevicius, et al. They all look the same to me (unless they’re angry): From out-group homogeneity to out-group heterogeneity. Psychological Science. 2006;17:836–840. doi: 10.1111/j.1467-9280.2006.01790.x. [DOI] [PubMed] [Google Scholar]
- Anthony T, Copper C, Mullen B. Cross-racial facial identification: A social cognitive integration. Personality and Social Psychology Bulletin. 1992;18:296–301. [Google Scholar]
- Atkinson RC, Shiffrin RM. Human memory: A proposed system and its control processes. In: Spence KW, Spence JT, editors. The psychology of learning and motivation. Vol. 8. London: Academic Press; 1968. [Google Scholar]
- Barrett HC, Kurzban R. Modularity in cognition: Framing the debate. Psychological Review. 2006;113:628–637. doi: 10.1037/0033-295X.113.3.628. [DOI] [PubMed] [Google Scholar]
- Becker DV, Anderson US, Mortensen CR, Robertson TE, Tybur J, Delton AW, Shapiro JR, Ackerman JM. Angry Faces Demand Attention: Evidence of Attentional Adhesion Across Four Paradigms. 2008 Manuscript submitted for publication. [Google Scholar]
- Becker DV, Kenrick DT, Guerin S, Maner JK. Concentrating on beauty: Sexual selection and sociospatial memory. Personality and Social Psychology Bulletin. 2005;12:1643–1652. doi: 10.1177/0146167205279583. [DOI] [PubMed] [Google Scholar]
- Becker DV, Neuberg SL, Maner JK, Shapiro JR, Ackerman JM, Schaller M, Kenrick DT. Interpersonal Threats Generate Disease Prime-Specific Encoding Benefits. 2008 Manuscript submitted for publication. [Google Scholar]
- Buss DM. Sex differences in human mate preferences: Evolutionary hypotheses tested in 37 cultures. Behavioral and Brain Sciences. 1989;12:1–49. [Google Scholar]
- Centers for Disease Control and Prevention. Improved National Prevalence Estimates for 18 Selected Major Birth Defects—United States, 1999–2001. MMWR. 2006;54:1301–1305. [PubMed] [Google Scholar]
- Crandall CS, Glor J, Britt TW. Aids-related stigmatization: Instrumental and symbolic attitudes. Journal of Applied Social Psychology. 1997;27:95–123. [Google Scholar]
- Cosmides L, Tooby J. Origins of domain specificity: The evolution of functional organization. In: Hirschfeld LA, Gelman SA, editors. Mapping the Mind: Domain Specificity in Cognition and Culture. Cambridge, MA: MIT Press; 1994. pp. 85–116. [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- Daly M, Wilson M. An evolutionary psychological perspective on homicide. In: Smith MD, Zahn MA, editors. Homicide: A sourcebook of social research. Thousand Oaks, CA: Sage Publications; 1999. pp. 58–71. [Google Scholar]
- Eberhardt JL, Goff PA, Purdie VJ, Davies PG. Seeing Black: Race, Crime, and Visual Processing. Journal of Personality and Social Psychology. 2004;87:876–893. doi: 10.1037/0022-3514.87.6.876. [DOI] [PubMed] [Google Scholar]
- Faulkner J, Schaller M, Park JH, Duncan LA. Evolved Disease- Avoidance Mechanisms and Contemporary Xenophobic Attitudes. Group Processes & Intergroup Relations. 2004;7:333–353. [Google Scholar]
- Ferguson MJ, Bargh JA. Liking is for doing: The effects of goal pursuit on automatic evaluation. Journal of Personality and Social Psychology. 2004;87:557–572. doi: 10.1037/0022-3514.87.5.557. [DOI] [PubMed] [Google Scholar]
- Fox E, Lester V, Russo R, Bowles RJ, Pichler A, Dutton K. Facial expressions of emotion: Are angry faces detected more efficiently? Cognition & Emotion. 2000;14:61–92. doi: 10.1080/026999300378996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Dutton K. Evidence for delayed disengagement from emotional faces. Cognition & Emotion. 2001;16:355–379. doi: 10.1080/02699930143000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Gangestad SW, Buss DM. Pathogen prevalence and human mate preferences. Ethology & Sociobiology. 1993;14:89–96. [Google Scholar]
- Gangestad SW, Simpson JA. The evolution of human mating: Trade-offs and strategic pluralism. Behavioral and Brain Sciences. 2000;23:573–587. doi: 10.1017/s0140525x0000337x. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. The ecological approach to visual perception. Boston: Houghton Mifflin; 1979. [Google Scholar]
- Green MJ, Phillips ML. Social threat perception and the evolution of paranoia. Neuroscience & Biobehavioral Reviews. 2004;28:333–342. doi: 10.1016/j.neubiorev.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Greenhouse S. Lifetime Affliction Leads to a U.S. Bias Suit. The New York Times. 2003 March 30;:A08. [Google Scholar]
- Heinemann W, Pellander F, Vogelbusch A, Wojtek B. Meeting a deviant person: Subjective norms and affective reactions. European Journal of Social Psychology. 1981;11:1–25. [Google Scholar]
- Houston V, Bull R. Do people avoid sitting next to someone who is facially disfigured? European Journal of Social Psychology. 1994;24:279–284. [Google Scholar]
- Kenrick DT, Delton AW, Robertson TE, Becker DV, Neuberg SL. How the mind warps. In: Forgas JP, Haselton MG, Von Hippel W, editors. The Evolution of the social mind: Evolutionary psychology and social cognition. New York: Psychology Press; 2007. pp. 49–68. [Google Scholar]
- Kenrick DT, Li NP, Butner J. Dynamical evolutionary psychology: Individual decision rules and emergent social norms. Psychological Review. 2003;110:3–28. doi: 10.1037/0033-295x.110.1.3. [DOI] [PubMed] [Google Scholar]
- Kurzban R, Leary MR. Evolutionary origins of stigmatization: The functions of social exclusion. Psychological Bulletin. 2001;127:187–208. doi: 10.1037/0033-2909.127.2.187. [DOI] [PubMed] [Google Scholar]
- Li NP, Bailey JM, Kenrick DT, Linsenmeier JA. The necessities and luxuries of mate preferences: Testing the trade-offs. Journal of Personality & Social Psychology. 2002;82:947–955. [PubMed] [Google Scholar]
- Li NP, Kenrick DT. Sex similarities and differences in preferences for short-term mates: What, whether, and why. Journal of Personality & Social Psychology. 2006;90:468–489. doi: 10.1037/0022-3514.90.3.468. [DOI] [PubMed] [Google Scholar]
- Low BS. Marriage systems and pathogen stress in human societies. American Zoologist. 1990;30:325–339. [Google Scholar]
- Lundqvist D, Öhman A. Emotion regulates attention: The relation between facial configurations, facial emotion, and visual attention. Visual Cognition. 2005;12:51–84. [Google Scholar]
- McArthur LZ, Baron RM. Toward an ecological theory of social perception. Psychological Review. 1983;90:215–238. [Google Scholar]
- Maner JK, DeWall CN, Gailliot MT. Selective attention to signs of success: Social dominance and early stage interpersonal perception. Personality and Social Psychology Bulletin. 2008;34:488–501. doi: 10.1177/0146167207311910. [DOI] [PubMed] [Google Scholar]
- Maner JK, Gailliot MT, Rouby DA, Miller SL. Can’t take my eyes off you: Attentional adhesion to mates and rivals. Journal of Personality and Social Psychology. 2007;93:389–401. doi: 10.1037/0022-3514.93.3.389. [DOI] [PubMed] [Google Scholar]
- Maner JK, Kenrick DT, Becker DV, Delton AW, Hofer B, Wilbur CJ, et al. Sexually selective cognition: Beauty captures the mind of the beholder. Journal of Personality and Social Psychology. 2003;85:1107–1120. doi: 10.1037/0022-3514.85.6.1107. [DOI] [PubMed] [Google Scholar]
- Maner JK, Kenrick DT, Becker DV, Robertson TE, Hofer B, Neuberg SL, Delton AW, Butner J, Schaller M. Functional projection: How fundamental social motives can bias interpersonal perception. Journal of Personality and Social Psychology. 2005;88:63–78. doi: 10.1037/0022-3514.88.1.63. [DOI] [PubMed] [Google Scholar]
- Miller SL, Maner JK, Becker DV. Self-Protective Biases in Group Categorization: What Shapes the Psychological Boundary between “Us” and “Them”? . 2008 doi: 10.1037/a0018086. Manuscript in preparation. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Natural selection and the regulation of defenses: A signal detection analysis of the smoke detector principle. Evolution and Human Behavior. 2005;26:88–105. [Google Scholar]
- Neuberg S, Kenrick DT, Maner JK, Schaller M. From evolved motives to everyday mentation: Evolution, goals, and cognition. In: Forgas J, Williams KD, Laham SM, editors. Social motivation: Conscious and unconscious processes. New York: Cambridge University Press; 2005. pp. 133–152. [Google Scholar]
- Ostrom TM, Sedikides C. Out-group homogeneity effects in natural and minimal groups. Psychological Bulletin. 1992;112:536–552. [Google Scholar]
- Park JH, Faulkner J, Schaller M. Evolved disease-avoidance processes and contemporary anti-social behavior: Prejudicial attitudes and avoidance of people with physical disabilities. Journal of Nonverbal Behavior. 2003;27:65–87. [Google Scholar]
- Park JH, Schaller M, Crandall CS. Pathogen-avoidance mechanisms and the stigmatization of obese people. Evolution and Human Behavior. 2007;28:410–414. [Google Scholar]
- Quinn PC, Yahr J, Kuhn A, Slater AM, Pascalis O. Representation of the gender of human faces by infants: A preference for female. Perception. 2002;31:1109–1121. doi: 10.1068/p3331. [DOI] [PubMed] [Google Scholar]
- Rehnman J, Herlitz A. Higher face recognition ability in girls: Magnified by own-sex and own-ethnicity bias. Memory. 2006;14:289–296. doi: 10.1080/09658210500233581. [DOI] [PubMed] [Google Scholar]
- Richeson JA, Trawalter S. The threat of appearing prejudiced and race-based attentional bias. Psychological Science. 2008;19:98–102. doi: 10.1111/j.1467-9280.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- Rodin MJ. Who is memorable to whom: A study of cognitive disregard. Social Cognition. 1987;5:144–165. [Google Scholar]
- Rosenwasser SM, Adams V, Tansil K. Visual attention as a function of sex and apparel of stimulus object: Who looks at whom? Social Behavior and Personality. 1983;11:11–15. [Google Scholar]
- Schaller M, Park JH, Faulkner J. Prehistoric dangers and contemporary prejudices. European Review of Social Psychology. 2003;14:105–137. [Google Scholar]
- Zebrowitz LA, Collins M. Accurate social perception at zero acquaintance: The affordances of a Gibsonian approach. Personality and Social Psychology Review. 1997;1:204–223. doi: 10.1207/s15327957pspr0103_2. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Fellous JM, Mignault A, Andreoletti C. Trait impressions as overgeneralized responses to adaptively significant facial qualities: Evidence from connectionist modeling. Personality and Social Psychology Review. 2003;7:194–215. doi: 10.1207/S15327957PSPR0703_01. [DOI] [PubMed] [Google Scholar]
- Zebrowitz LA, Rhodes G. Sensitivity to “Bad Genes” and the Anomalous Face Overgeneralization Effect: Cue Validity, Cue Utilization, and Accuracy in Judging Intelligence and Health. Journal of Nonverbal Behavior. 2004;28:167–185. [Google Scholar]


