Abstract
Many studies have identified associations between adverse health effects and short-term exposure to particulate matter less than 2.5 microns in diameter (PM2.5). These effects, however, are not consistent across geographical regions. This may be due in part to variations in the chemical make-up of PM2.5 resulting from unique combinations of sources, both primary and secondary, in different regions. The Denver Aerosol Sources and Health (DASH) study is a multi-year time series study designed to characterize the daily chemical composition of PM2.5 in Denver, identify the major contributing sources, and investigate associations between sources and a broad array of adverse health outcomes.
Measurement methodology, field blank correction, pointwise uncertainty estimation and detection limit consideration are discussed in the context of bulk speciation for the DASH study. Results are presented for the first 4.5 years of mass, inorganic ion and bulk carbon speciation. The derived measurement uncertainties were propagated using the root sum of squares method and show good agreement with precision estimates derived from bi-weekly duplicate samples collected on collocated samplers. Gravimetric mass has the most uncertainty of any measurement and reconstructed mass generated from the sum of the individual species shows less uncertainty than measured mass on average. The methods discussed provide a good framework for PM2.5 speciation measurements and are generalizable to analysis of other environmental measures.
Keywords: particulate matter, PM2.5, chemical speciation, uncertainty estimation, duplicate precision
1. Introduction
Numerous studies have identified adverse health effects from both short-term and long-term exposures to particulate matter less than 2.5 microns in diameter (PM2.5) including, but not limited to, worsening of asthma (Ko et al., 2007; Rabinovitch et al., 2006) and increased cardiopulmonary mortality (Dominici et al., 2005; Dockery et al., 1993; Pope et al., 1995). Largely because of these health concerns, the Environmental Protection Agency promulgated National Ambient Air Quality Standards (NAAQS) for PM2.5 in 1997 (US-EPA, 1997). Observed health effects with particulate matter, however, are not consistent across geographical regions (Dominici et al., 2005). This may be due in part to variations in sources, both primary and secondary, in different regions. The Denver Aerosol Sources and Health (DASH) study is one of a handful of studies designed to investigate associations between PM2.5 sources and a range of adverse health outcomes including mortality, hospitalizations and asthma control (Vedal et al., 2008). The specific goals of the DASH study are to: 1) measure speciated PM2.5 daily over multiple years in Denver, Colorado, 2) determine the major contributing sources through receptor modeling, and 3) investigate associations between the identified sources and health outcomes.
Since source apportionment is a major aspect of the DASH study, an in-depth exploration of measurement uncertainty was undertaken. Pointwise uncertainty estimates are frequently used in source apportionment models such as Positive Matrix Factorization (PMF) (Paatero, 1997, 2000) and they can have an important effect on the performance of the model (Kim and Hopke, 2007). For long time series studies where sampling protocols and analysis techniques may change with time, pointwise uncertainty estimates are critical for unbiased model results. In this situation, applying a single absolute or relative uncertainty across all measurements may not be appropriate. This paper describes the bulk chemistry measurements and uncertainty estimation tactics used for daily PM2.5 speciation in the DASH study.
The PM2.5 components measured daily over multiple years include nitrate, sulfate, elemental carbon, bulk organic carbon and 84 different organic species which serve as molecular markers for source identification. In addition, analysis for trace metals was performed on a one year subset of the samples. This paper focuses on measurement techniques, uncertainty estimation and results from the bulk PM2.5 speciation (mass, inorganic ions and bulk carbon). The first 4.5 years of daily concentration data for these species are presented and the pointwise propagated uncertainties are compared to precision estimates derived from bi-weekly duplicate measurements using collocated samplers. The propagated uncertainty estimates are broken down by their origin (analytical, field blank correction and volume calculation) revealing areas where methodology improvements would have the most impact. Studies in relatively low concentration cities such as Denver are of particular interest as regulatory agencies consider promulgating ever more stringent air quality standards.
2. Methods
2.1 PM2.5 Sampling Protocol
The core sampling site for the DASH study is located on the rooftop of a two story elementary school in a residential neighborhood 5.3 km east of downtown Denver. This site was chosen for its central location in a highly populated residential region of Denver. The site is far from the influence of any major industrial or point sources. The closest large highway is 5.2 km south-west of the site and the nearest commuter street is 0.6 km away.
Sample collection commenced on July 1, 2002 and is scheduled to continue through 2008. PM2.5 filter samples were collected daily from midnight to midnight with sampling equipment that has been used extensively in air quality measurement campaigns in the past (Bae et al., 2006; Jaeckels et al., 2007; Scheesley et al., 2007). Four identical samplers were located at the site and rotated through the sampling duty. Each sampler was equipped with a sharp cut cyclone (University Research Glassware, Chapel Hill, NC) with a 2.5 μm size cut at a flow rate of 92 L min-1. The air stream leaving the cyclone was split into two parallel sample lines configured to minimize particle losses with 20 L min-1 passing through a 47 mm diameter, 2 μm pore size Teflon (PTFE) filter (Pall Gelman Teflo™) housed in a Teflon filter holder and 72 L min-1 passing through a 90 mm diameter pre-baked quartz fiber filter (Pall Gelman Tissuequartz™) housed in an aluminum filter holder. This flow split ratio provided an equal face velocity for each filter type and was determined by considering the sensitivity of the analytical chemistry techniques to be performed on the two filters. The Teflon filter was used for gravimetric and inorganic analyses (mass, ionic compound speciation and water soluble trace metal speciation) and the quartz fiber filter was used for organic analyses (total elemental and organic carbon and detailed organic molecular marker speciation). The trace metal and detailed organic speciation methodologies will be described in separate papers. The flows were governed by critical orifices and an oilless rotary vane vacuum pump; regular measurements of sample line pressure were taken to ensure that the flow was at the choked condition and thus correctly controlled by the critical orifices. Sample start and stop time was controlled by a mechanical timer (Grasslin, Mahwah, NJ) and the sample duration was recorded with a mechanical elapsed time meter (Redington, Windsor, CT). Sample volume for each filter was directly measured using a calibrated dry gas meter (Elster Amco, Luxembourg City, Luxembourg).
Strict adherence to filter collection, handling and transport methodology and sampler maintenance has been followed throughout the study in a manner consistent with US-EPA quality assurance guidelines for PM2.5 sampling (US-EPA, 1997, 1998). Teflon filters were placed in individually labeled plastic petri dishes and quartz filters were wrapped in clean aluminum foil and pre-baked in an oven at 500 °C before being placed in pre-baked glass petri dishes for transport and pre-baked glass jars for storage. All filter handling was done with clean, solvent rinsed forceps. Filters were collected from the samplers within 72 hours of sampling, transported in coolers with ice packs and stored in a freezer at -25 °C prior to analysis
For quality control and to address contamination concerns, weekly field blank filters were collected, extracted, and analyzed following the same protocols used for the daily sample filters. The field blanks provide a quantitative measurement of contamination coming from filter media, handling techniques and analysis protocols and also provide a check for cross-contamination between samples. In order to assure consistent and robust chemical quantification and to assist in uncertainty estimation, bi-weekly duplicate samples were collected on collocated samplers starting in July, 2004. These duplicate samples were analyzed in parallel with the main samples, thus providing a direct measure of precision for each chemical analysis under investigation. These precision estimates are compared in the Results section with the pointwise propagated uncertainty estimates described later in this section.
2.2 Field Blank Correction
All measurements for the DASH study were field blank corrected. To reduce our sensitivity to occasional outliers in the field blanks, the median rather than the mean value of the field blanks in a given analysis batch was subtracted from all observations in that batch. This batch-by-batch correction provided flexibility for changes in field blank levels over time. Many of the batches showed field blank levels not significantly different from zero. Nevertheless, for consistency across batches and analyses, field blank correction was performed.
2.3 Uncertainty Analysis
The numbers reported in this paper are airborne mass concentrations for each species of interest with uncertainties reported as one standard deviation (SD). The root sum of squares (RSS) method was used for propagation of uncertainties through the calculations leading up to the final concentrations. In general, this method provides an estimate of the uncertainty in the calculated output of a function y = f (x⃗) which is dependent on n input variables represented by x⃗ = (x1, x2, x3, …, xn), each with their own predetermined uncertainty estimates δx⃗ = (δx1, δx2, δx3, …, δxn). To a first order approximation, the uncertainty in the calculated output can be derived from a first order Taylor series expansion: the result is the law of propagation of uncertainties shown here (NIST, 1994):
| (1) |
If the input variables xi are independent, then the covariance terms drop out of Equation 1. Some care must be used in applying this technique since the partial derivatives must be defined at the point where the function is being evaluated and higher order terms in the Taylor series expansion must be small for the approximation to hold. Details on the uncertainty propagation specific to each chemical analysis are included in the following sections.
2.4 Sample Volume and Duration Calculation
Each sampler was equipped with two dry gas meters for direct sample volume measurement on the Teflon and quartz filter channels. The gas meters were calibrated in the field using a primary flow meter (DryCal DC-Lite, Bios International Corp., Butler NJ). The uncertainty in this calibration correction was estimated from the standard deviation of 50 separate measurements on each gas meter. To account for the pressure drop present in the sampling lines, an ambient pressure correction of the form 1-ΔP/P was applied to the sample volume using the regularly measured pressure drop (ΔP) observed at the filter surface relative to ambient and the average ambient station pressure (P). The uncertainty in this pressure correction was calculated using the RSS method by incorporating the precision of the pressure gauges and the standard deviation of the ambient pressure readings used to determine the average ambient station pressure. This method provided a direct measure of the actual Teflon and quartz filter sample volumes with corresponding uncertainty for each sample collected.
The use of a seven-day mechanical timer ensured that the samples were restricted to the appropriate 24-hour period. Furthermore, an independent mechanical elapsed time meter wired directly into the vacuum pump provided a direct measurement of the actual sample duration. Samples with durations that fell outside a 24 ± 6 h range were omitted from the dataset (16 of 1710 samples, or less than 1%, were missing or had durations outside this range).
2.5 Gravimetric Analysis
Gravimetric analysis on the Teflon filters was performed using a five-digit LabServe model BP210D microbalance (Sartorius Corporation, Goettingen, Germany) housed in a custom built, low cost temperature and humidity controlled weigh chamber. The weigh chamber was constructed out of aluminum and clear acrylic and humidity was controlled using a design similar to that reported by Allen et al. (2001). Dry compressed air flowed at 2 L min-1 through a HEPA filter and over a saturated solution of magnesium chloride in a humidity control chamber prior to entering the main weigh chamber. The physical dimensions of the humidity control chamber and the residence time of the air in the chamber were designed to achieve 30% relative humidity in accordance with US-EPA guidelines (US-EPA, 1997). The actual relative humidity mean (standard deviation) recorded in the chamber over a 3-year operation period was 32.0 (4.3) %. Temperature was controlled by a heating coil spread uniformly beneath the chamber and regulated by a rheostat. The design temperature was 25 °C and the actual temperature recorded in the chamber over the same 3-year period was 25.3 (1.3) °C. Vibrations were minimized by placing the chamber on a 15 cm thick granite slab and static charge on the filters was reduced by locating two polonium sources in close proximity to the filter being weighed (Weil, 1998).
Filters were allowed to equilibrate in the weigh chamber for 24 hours prior to weighing. The filters were weighed two times each and if those measurements differed by 30 μg or more, they were weighed a third time. This protocol was followed pre and post sampling and the mass accumulated on each filter was determined by the difference in the average pre-weigh mass and the average post-weigh mass. Blank correction was performed next by subtracting the median mass on the field blanks observed in a calendar year. The mass concentration was then obtained by dividing the blank corrected mass by the corresponding Teflon filter sample volume.
Two control filters were weighed between every ten sample filters to ensure consistency over time and to derive an estimate of the gravimetric uncertainty. The control filters never left the weigh chamber and were identical to the sample filters (47 mm Teflon). These filters were retained for as long as their integrity allowed (1.5 to 3 years) and were replaced one at a time to provide continuity. The standard deviation of the mean-removed and linear trend-removed control filter measurements in each calendar year was used to estimate the gravimetric mass uncertainty for all samples collected during that year (mass uncertainties ranged from 13 to 35 μg, depending on the year).
The control filter masses were inspected continuously to look for deviations from the norm. The only period during which the standard deviation changed significantly was from December 15, 2004 to March 10, 2005 when construction was taking place in an adjoining lab. To accommodate for the increased noise in our mass measurements, the uncertainties for all pre- and post-weigh measurements conducted during this period were inflated to 53 μg—the standard deviation of the 157 control filters weighed during the construction period. For all samples, the pre- and post-weigh mass accumulation uncertainties were propagated with the standard deviation of the field blank mass accumulations and the Teflon filter sample volume uncertainty using the RSS method to obtain the final blank corrected mass concentration uncertainty.
2.6 Inorganic Ion Analysis
Upon completion of the gravimetric analysis, each Teflon filter was analyzed for inorganic ions. The ions of primary interest included nitrate and sulfate; other ions including ammonium, chloride, sodium, potassium, magnesium and calcium were also investigated on a subset of the samples. The samples for all years except 2003 (described separately below) were analyzed using ion chromatography (IC). In preparation for IC, the filters were extracted in a vial containing a mixture of 300 μl isopropyl alcohol and 25 ml filtered and deionized (DI) water. The vials were placed on a shaking table for more than 8 hours. A 5 ml portion of each sample extract was analyzed on an ion chromatograph along with nine dilutions of known standards used for quantification and multiple DI water blanks to monitor for contamination. The ion chromatograph (Dionex model DX-120) was equipped with a Dionex AS40 autosampler, Dionex IonPac AS14 4×250 mm column, Dionex IonPac AG14 4×50 mm guard column and 250 mL injection loop. The eluent used consisted of 1.25 mM NaHCO3 and 43.8 mM Na2CO3 in DI water.
The autosampler allowed for consecutive analysis of multiple samples in a sequence. During each sequence, all nine of the known concentration calibration standards were run. The samples were analyzed in batches consisting of between 4 and 14 sequences and incorporating up to one year of samples. Repeat measurements of the calibration standards included in each sequence were used to generate a calibration curve to convert from conductivity peak area output by the PeakNet software to ion mass per filter. The mass concentration for each ion was obtained after dividing by the corresponding Teflon filter sample volume.
Uncertainty estimates for the ions were not calculated by the PeakNet software. Therefore, we derived uncertainty estimates from the fit to the calibration curves using a method similar to that described in NIST/SEMATECH (2006). A good fit to the calibration points was obtained using a quadratic calibration curve of the form:
| (2) |
where P represents the measured conductivity peak area, A represents the known abundance of the ion in the standard, and a, b and c represent scalar fit coefficients. Other functional forms were explored for the IC calibration curves including linear and quadratic with zero intercept, but the full quadratic in Equation 2 provided the best fit to the calibration data using the adjusted-R2 as the model selection criteria. To determine the unknown ion abundance in a sample using the measured peak areas, this equation was inverted to solve for A utilizing the quadratic equation:
| (3) |
For our application, the choice of sign in Equation 3 depends on the curvature of the data and matches the sign of the fit coefficient c. With the equation in this form, the RSS method for error propagation from Equation 1 was used with uncertainty estimates for each of the fit parameters derived from linear regression to estimate the uncertainty in A:
| (4) |
where δA is the propagated uncertainty in A and δP is the uncertainty in P estimated by the residual standard deviation of the regression model. The standard deviation of the model coefficients—δa, δb, and δc—and the covariance between the model coefficients—Cov(a,b), Cov(b,c), and Cov(c,a)—were determined from the regression model. Although the covariance terms contributed an insignificant amount to the overall uncertainty in this analysis (several orders of magnitude less than the other terms in Equation 4), they were kept in the calculation for completeness.
An analytical expression for each of the partial derivatives required in Equation 4 was derived and the appropriate value for each parameter was inserted into the resulting equation. This provided a unique estimate of the ion abundance uncertainty, δA, derived from the quality of fit to the quadratic calibration curve using repeat measurements of the known calibration standards. Finally, the ion concentration uncertainties were calculated by incorporating the standard deviation of the field blanks within each batch and the Teflon filter sampler volume uncertainties using the RSS method for error propagation.
The Teflon filters for 2003 were selected for supplemental trace metal analysis and as a result were treated differently than the other filters. In order to maximize the filter media available for the metals analysis, nitrate and sulfate were measured using alternative approaches to IC. These filters were extracted in DI water at the Wisconsin State Laboratory of Hygiene (WSLH) using methods similar to the IC extractions described above. The extracts were then split and analyzed for nitrogen by automated flow injection ion analysis (US-EPA, 1993a, 1993b; WSLH, 1997) and water soluble metals by high resolution inductively coupled plasma mass spectrometry (ICP-MS) (Field and Sherrell, 1998; Herner et al., 2006; Ntziachristos et al., 2007; Vanhaecke and Moens, 1999). The nitrate + nitrite and the ammonia fractions of the nitrogen analysis were used as proxies for nitrate and ammonium, respectively, with uncertainties derived from the instrument's analytical precision (3.5% for nitrate + nitrite, 4.5% for ammonia). The water soluble sulfur from the ICP-MS analysis was used as a proxy for sulfate with uncertainties generated from the standard deviation of three replicate measurements on each sample and replicate analysis of four method blanks run within each batch of samples. The complete results from the supplemental trace metals analysis will be presented in a future paper.
2.7 Carbon Analysis
A 1.5 cm2 punch taken from the quartz filters was used for bulk organic carbon (OC) and elemental carbon (EC) analysis. The punches were sent in batches to WSLH for carbon analysis using the NIOSH 5040 thermal optical transmission (TOT) method (NIOSH, 2003; Schauer et al., 2003) on a Sunset Laboratory ECOC analyzer. The batches ranged in size from 40 to 223 filters with batch size dictated by analytical availability. Table 1 lists the date range and number of filters included in each batch. All weekly field blanks were analyzed in the same batch as their respective daily samples. In addition, three punches of a control filter were sent with each batch for inter-batch characterization and quality control.
Table 1.
ECOC analysis batch information and OC field blank statistics.
| Batch Number | Date Range | Total Number of Filters | Number of Field Blanks | OC Blank Corr. Valuea (μg/m3) | OC Blank Corr. Unc.b (μg/m3) |
|---|---|---|---|---|---|
| 1 | 12/02/2002 - 01/26/2003 | 48 | 6 | 0.49 | 0.21 |
| 2 | 09/23/2002 - 02/16/2003 | 104 | 13 | 0.12 | 0.04 |
| 3 | 07/01/2002 - 09/22/2002 | 94 | 12 | 0.79 | 0.29 |
| 4 | 02/17/2003 - 05/18/2003 | 104 | 13 | 0.07 | 0.02 |
| 5 | 05/19/2003 - 08/17/2003 | 104 | 13 | 0.14 | 0.04 |
| 6 | 08/18/2003 - 11/16/2003 | 104 | 13 | 0.12 | 0.04 |
| 7 | 11/17/2003 - 12/21/2003 | 40 | 5 | 0.14 | 0.07 |
| 8 | 12/22/2003 - 07/04/2004 | 223 | 27 | 0.14 | 0.06 |
| 9 | 07/05/2004 - 12/19/2004 | 203 | 23 | 0.07 | 0.05 |
| 10 | 12/20/2004 - 03/13/2005 | 102 | 12 | 0.10 | 0.03 |
| 11 | 12/13/2004 - 07/03/2005 | 137 | 17 | 0.06 | 0.18 |
| 12 | 07/04/2005 - 09/18/2005 | 93 | 11 | 0.06 | 0.40 |
| 13 | 09/19/2005 - 12/11/2005 | 102 | 12 | 0.21 | 0.17 |
| 14 | 12/12/2005 - 03/05/2006 | 102 | 12 | 0.10 | 0.03 |
| 15 | 03/06/2006 - 05/21/2006 | 94 | 11 | 0.07 | 0.02 |
| 16 | 05/22/2006 - 08/27/2006 | 119 | 14 | 0.16 | 0.04 |
| 17 | 08/28/2006 - 12/03/2006 | 119 | 14 | 0.11 | 0.02 |
OC blank correction value determined from the median of the field blanks within batch and expressed as a concentration using the study average sample volume.
OC blank correction uncertainty determined from the standard deviation of the field blanks within batch and expressed as a concentration using the study average sample volume.
The temperature steps used in the first heating cycle of the TOT analysis were 340, 500, 615 and 900 °C; the second heating cycle ended with a final temperature of 910 °C. The sum of the carbon measured at the four distinct temperature steps in the first heating cycle along with a pyrolized carbon adjustment (OP) made up OC while the carbon measured during the second heating cycle minus OP made up EC. The sum of OC and EC made up total carbon (TC).
The OC, EC and TC amount along with the area of the filter punch and the total deposit area of the quartz filter were used to obtain the mass of carbon on the sample. The final concentration was obtained by dividing by the quartz filter sample volume. OC and EC analytical uncertainties (δOC and δEC, respectively) were provided by WSLH and were based on the following calculations: δOC = 0.20 + 0.05*OC and δEC = 0.05 + 0.05*EC + 0.05*OP. The calculation for δOC was recommended by the instrument manufacturer (Sunset Laboratory) while the calculation for δEC was based on laboratory observations and is discussed in Schauer et al. (2003). Concentration uncertainties were calculated by incorporating the standard deviation of the field blanks within each batch and the quartz filter sampler volume uncertainties using the RSS method for error propagation.
3. Results
3.1 Time Series and Summary Statistics
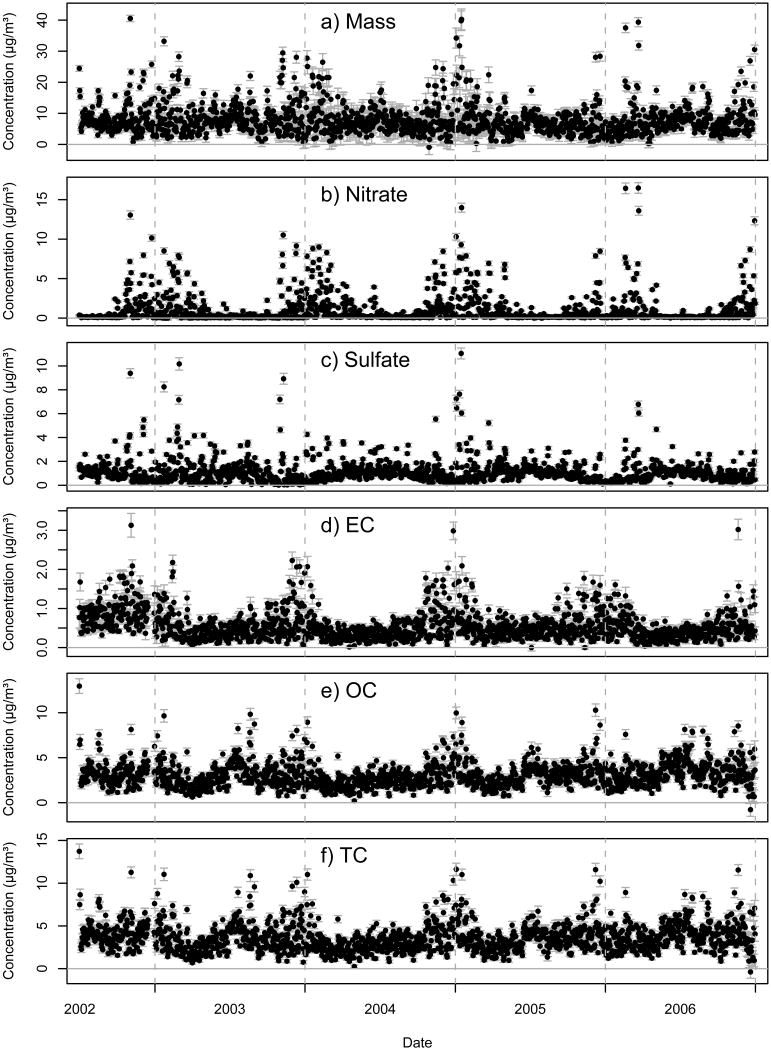
Figure 1 contains concentration time series plots of PM2.5 mass, nitrate, sulfate, EC, OC and TC for the first 4.5 years of the DASH study (July 1, 2002 – December 31, 2006). The error bars depict the pointwise propagated uncertainty estimates (+/- 1 SD) derived using the techniques discussed in this paper. Table 2 contains a list of aggregate statistics for each species covering the 4.5 year period including the mean value of the field blank correction applied to each sample. To help illustrate the relative importance of the different sources of uncertainty, Table 3 contains the average uncertainty resulting from the analytical measurement, field blank correction and volume calculation separately along with the final propagated concentration uncertainty. The uncertainties are expressed as concentrations and since the addition of individual uncertainties is non-linear, the individual contributions do not directly add up to the final concentration uncertainty. Analyses with field blank concentrations distributed near zero resulted in a mean blank correction uncertainty that was larger than the mean value of the correction itself; this is particularly evident for mass where the mean uncertainty in the field blank correction (1.18 μg/m3) is eight times the mean value of the field blank correction (0.14 μg/m3). The signal to noise ratio (S/N, the ratio of the mean concentration to the mean uncertainty) and the percent of values below detection limit (BDL) using a 95% confidence interval are also listed in Table 3.
Figure 1.
Time series plots of PM2.5 mass, nitrate, sulfate, EC, OC and TC. The error bars represent the pointwise propagated measurement uncertainty (+/- 1 SD).
Table 2.
Statistics for each species based on 4.5 years of data (July 1, 2002 to December 31, 2006).
| Chemical Species | Number of Days | Mean (μg/m3) | CV ( )a | Median (μg/m3) | Maximum (μg/m3) | Mean Blank Corr. (μg/m3)b |
|---|---|---|---|---|---|---|
| Mass | 1628 | 7.95 | 0.62 | 6.91 | 40.53 | 0.14 |
| Nitrate | 1628 | 0.97 | 1.86 | 0.21 | 16.48 | -0.01 |
| Sulfate | 1628 | 1.10 | 0.88 | 0.90 | 11.04 | -0.01 |
| EC | 1614 | 0.55 | 0.67 | 0.46 | 3.13 | 0.00 |
| OC | 1614 | 3.07 | 0.45 | 2.88 | 12.96 | -0.21 |
| TC | 1614 | 3.62 | 0.45 | 3.42 | 13.73 | -0.21 |
Coefficient of variation (CV) = standard deviation/mean
Mean value of the field blank corrections expressed as a concentration (negative values indicate blank subtraction).
Table 3.
Propagated uncertainties averaged over 4.5 years (July 1, 2002 to December 31, 2006).
| Chemical Species | Analytical (μg/m3)a | Blank Corr. (μg/m3)b | Volume (μg/m3)c | Final (μg/m3)d | S/N ( )e | BDL (%)f |
|---|---|---|---|---|---|---|
| Mass | 1.28 | 1.18 | 0.07 | 1.76 | 4.5 | 12.3 |
| Nitrate | 0.08 | 0.06 | 0.01 | 0.13 | 7.3 | 38.0 |
| Sulfate | 0.08 | 0.03 | 0.01 | 0.09 | 11.9 | 1.7 |
| EC | 0.08 | 0.00 | 0.01 | 0.09 | 6.4 | 1.4 |
| OC | 0.29 | 0.10 | 0.06 | 0.33 | 9.4 | 0.4 |
| TC | 0.30 | 0.10 | 0.06 | 0.34 | 10.7 | 0.4 |
Uncertainty from the analytical measurement alone expressed as a concentration.
Uncertainty from the blank correction alone expressed as a concentration.
Uncertainty from the volume measurement alone expressed as a concentration.
Final propagated concentration uncertainty.
Signal to noise ratio (mean concentration/mean uncertainty).
Percent of observations below detection limit (BDL) as defined in the text.
3.2 Inorganic Ion Method Comparison
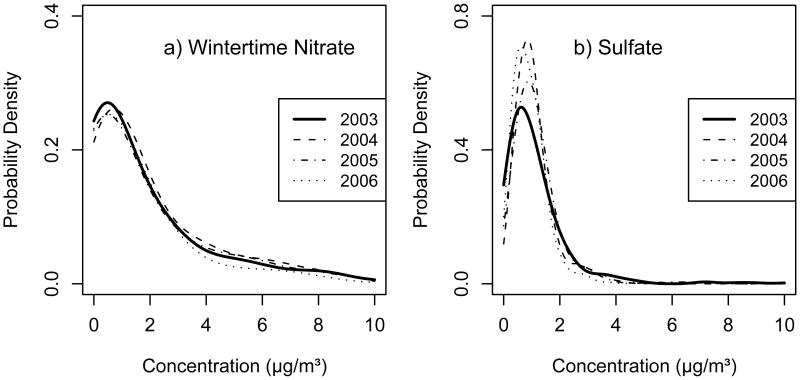
As mentioned in the Methods section, the nitrate data for 2003 was derived from nitrate + nitrite measured using automated flow injection ion analysis rather than from IC as in the other years. There is consistency in the shape of the time series shown in Figure 1b for nitrate across all years including 2003. Figure 2a shows the distribution of the nitrate data by year, limited to the colder months (November – March) when nitrate is regularly above detection limit. The overlapping probability density functions (PDFs) for the four different years demonstrates consistency between 2003 and the rest of the dataset. Although we have no concurrent measurements using these two methodologies in our time series, this evidence suggests that the two nitrate analysis techniques are comparable and that nitrite is negligible compared to nitrate in Denver.
Figure 2.
Probability distribution functions (PDFs) estimated from the data for a) nitrate (wintertime only) and b) sulfate (year-round) for each full year of measurements (2003-2006).
Correspondingly, the sulfate data for 2003 was derived from water soluble sulfur measured using high resolution ICP-MS rather than from IC. The general shape of the time series in Figure 1c shows consistency across years. Furthermore, the annual mean of the 2003 sulfate (1.13 μg/m3) is within the range of the 2004, 2005 and 2006 measurements (1.09, 1.19 and 0.95 μg/m3, respectively). However, Figure 1c reveals that the 2003 data has more frequent low readings during the summer months compared to the other years. Figure 2b shows the distribution of the sulfate data by year. The 2003 PDF shows a slight shift in density to lower concentrations relative to the other years which is consistent with the time series observation noted above. The high concentration region shows better agreement between years. Since the two measurement methods were applied during different years without any overlap, no direct comparison can be made with the current dataset and this could simply be the result of year-to-year variation in sulfate. Further investigation directly comparing these two methods, especially at low concentrations, would shed light on any possible method bias.
3.3 Collocated Duplicate Samples
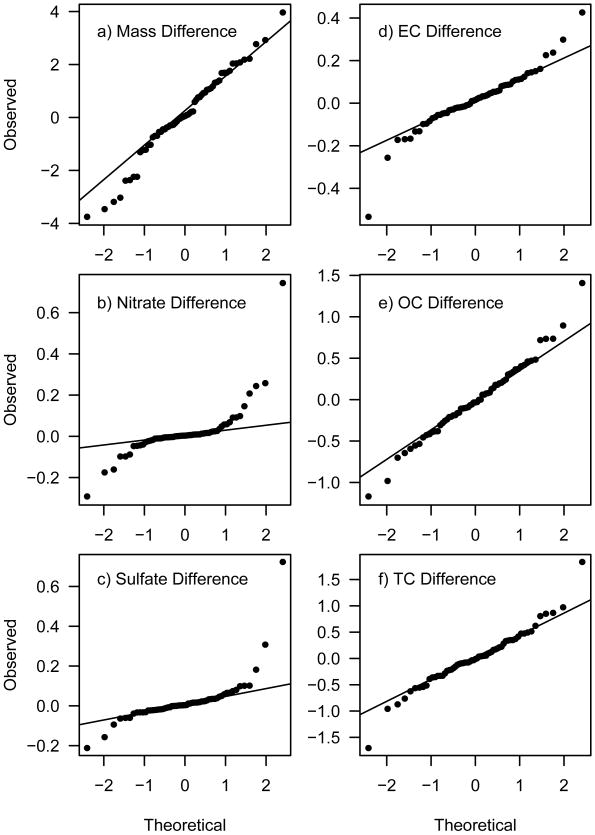
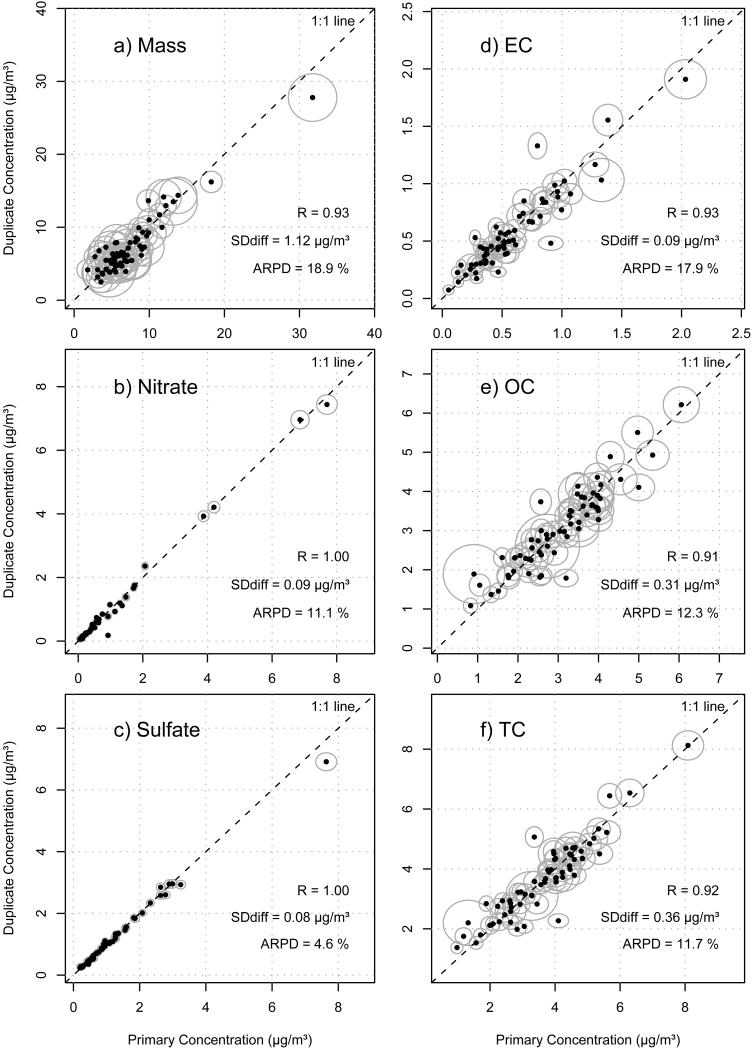
The collection of bi-weekly duplicate samples on collocated samplers started in July, 2004 and provided a total of 63 sample pairs in the 4.5 year dataset. Figure 3 contains normal probability plots of the paired differences between the collocated concentrations for mass, nitrate, sulfate, EC, OC and TC. These plots provide a visual test for normality in the distribution of the differences (NIST/SEMATECH, 2006). Figure 4 contains scatter plots for the same species with confidence ellipses illustrating the propagated uncertainty for both the primary and duplicate measurements. Also included in Figure 4 is the Pearson's correlation coefficient (R), the pooled standard deviation of the paired differences (SDdiff) and the average relative percent difference (ARPD). The latter two are defined as follows:
Figure 3.
Normal probability plots of the differences between the bi-weekly collocated measurements for each species.
Figure 4.
Scatter plots comparing bi-weekly collocated measurements for each species with Pearson's correlation coefficient (R), pooled standard deviation of the difference (SDdiff, defined in Equation 5) and average relative percent difference (ARPD, defined in Equation 6). Confidence ellipses represent one standard deviation.
| (5) |
| (6) |
where n is the total number of collocated sample pairs, is the ith species concentration measured by the primary sampler and is the corresponding ith species concentration measured on the collocated duplicate sampler. SDdiff and ARPD can be used to estimate measurement uncertainty derived from duplicate data (Flanagan et al., 2006; Skoog and West, 1969).
The larger confidence ellipses for PM2.5 mass in Figure 4a correspond to the measurements performed during lab construction as discussed earlier. The two largest confidence ellipses for EC in Figure 4d are a result of both high EC and a large pyrolized carbon correction (OP) for these samples. The two largest confidence ellipses for OC in Figure 4e (and, consequently, TC in Figure 4f) observed at low and mid-level concentrations were part of an analysis batch containing several high value field blanks. As a result, error propagation incorporating the blank correction caused these points to receive high uncertainties.
To quantitatively compare the propagated uncertainties to the precision of the collocated samplers, we used SDdiff and ARPD (from Equations 5 and 6). To facilitate a comparison in absolute terms, the ARPD was multiplied by the average concentration of all 126 collocated samples, thereby providing an uncertainty estimate in μg/m3. Table 4 shows a comparison of the mean propagated uncertainty with the two duplicate derived precision estimates.
Table 4.
Comparison of propagated uncertainties to precision estimates derived from bi-weekly collocated duplicate samples.
| Chemical Species | Number of Paired Observations (μg/m3) | Mean of All Paired Observations (μg/m3) | Mean Propagated Uncertaintya (μg/m3) | SDdiff Duplicate Uncertaintyb (μg/m3) | ARPD Duplicate Uncertaintyc (μg/m3) |
|---|---|---|---|---|---|
| Mass | 63 | 7.35 | 1.84 | 1.12 | 1.39 |
| Nitrate | 63 | 0.77 | 0.08 | 0.09 | 0.08 |
| Sulfate | 63 | 1.16 | 0.10 | 0.08 | 0.05 |
| EC | 63 | 0.58 | 0.08 | 0.09 | 0.10 |
| OC | 63 | 3.05 | 0.33 | 0.31 | 0.38 |
| TC | 63 | 3.64 | 0.35 | 0.36 | 0.42 |
Derived from propagation of errors.
Derived from the pooled standard deviation of the collocated sample differences (SDdiff, defined in Equation 5).
Derived from the average relative percent difference (ARPD, defined in Equation 6) of the collocated samples and converted to μg/m3 by multiplying by the mean of all paired observations.
4. Discussion
4.1 PM2.5 Bulk Chemistry in Denver
The source apportionment and health modeling goals of the DASH study both rely heavily on accurate measurement of speciated PM2.5 as well as careful determination of pointwise uncertainty estimates. This paper has provided a first look at the bulk chemical make-up and seasonality of the PM2.5 measured daily for 4.5 years at the DASH receptor site in Denver. It has also outlined the measurement methods and uncertainty estimation techniques used in the mass, inorganic ion and bulk carbon measurements.
Episodic wintertime spikes are observed for all species in the time series plotted in Figure 1. These are driven by the meteorology of the region where frequent but short lived wintertime atmospheric temperature inversions occur (Neff, 1997). These events result in stagnant air with little mixing that can last for several days and result in significant increases in pollutant concentrations. In addition to the wintertime spikes, several of the pollutants including sulfate and OC show summertime increases as a result of increased photochemistry and/or emissions.
The annual average OC concentration measured during 2003 was 3.1 μg/m3 compared to 8.6 μg/m3 for PM2.5 mass. Mass closure was used to derive an average scaling factor from OC (carbon mass only) to organic mass (OM) including other elements (e.g., hydrogen, oxygen, nitrogen). A factor of 1.53 resulted in optimum mass closure which falls within the range of values reported in the literature (Pang et al., 2006; Turpin and Lim, 2001; White and Roberts, 1977). After multiplying OC by this scaling factor, organic carbon made up just over half the PM2.5 mass during 2003. Adding in the average EC concentration during 2003 (0.5 μg/m3) brings the estimated total carbon contribution up to 61% of the PM2.5 mass which is in line with prior estimates for the region (US-EPA, 2003).
4.2 Blank Correction, Uncertainty Estimation and Detection Limits
As discussed in the Methods section, one field blank was collected each week during the study and the median value of all field blanks contained in each analysis batch was used for blank correction. In Table 2, mass was the only measurement that required blank addition on average over the study period. This is the result of normally distributed mass on the field blanks with a slight mass loss on average across all batches. The field blank corrections were small and well within our experimental uncertainty for mass. Nitrate, sulfate and EC required negligible blank correction. OC and TC required the largest correction with an average blank subtraction of 0.21 μg/m3 each. This amounts to roughly seven percent of the mean OC concentration on the sample filters.
The use of field blanks to account for OC sampling artifacts can result in an underestimate of the correction if incomplete and variable saturation of available adsorption sites occurs in the quartz filter used for the field blank. Other methods exist for in situ estimation of the OC artifact, most commonly involving the addition of a quartz backup filter. Both positive (Turpin et al., 2000) and negative (Pang et al., 2001) artifacts have been identified using this approach. Besides obvious cost and analysis time challenges, unresolved issues exist when using backup filters as well including alteration in the absorption equilibrium of the downstream filter (Kirchstetter et al., 2001). Therefore, we applied field blank correction to address OC sampling artifacts which also provided a degree of consistency with the other analysis tracks.
The field blanks played a variable role in the propagated uncertainties as can be seen in Table 3 where the concentration uncertainties are broken down by their origin. For mass and nitrate, the field blank correction contributed nearly as much uncertainty on average to the final concentration as the analytical measurement itself. This is a result of noisy mass measurements and frequently small nitrate measurements during the summer months. For sulfate, OC and TC, the field blank correction played a much smaller relative role in the propagated uncertainties and for EC the field blank correction was essentially zero and therefore had negligible effect on the propagated uncertainty. For all species, the sample volume uncertainty played a very small role in the overall uncertainty propagation.
Since mass had by far the largest uncertainty of the bulk measurements, we explored whether reconstructed mass generated from the individual species measurements would contain less uncertainty. This analysis was limited to 2003 since that is the only year containing metals data. The reconstructed mass was generated by summing the contributions from nitrate, sulfate, ammonium, EC, OM and metals. The 1.53 annual average scaling factor from OC to OM discussed earlier was used in this calculation with an estimated uncertainty of 0.20 based on the results of Turpin and Lim (2001). Adjustments to the metals data were made to account for the mass of oxygen contained in common metal oxides (Lonati et al., 2005) with uncertainties in these correction factors assumed to be negligible since their mass makes up a small fraction of the PM2.5. For 2003, the average reconstructed mass uncertainty was 0.8 μg/m3 (10%) compared to 1.5 μg/m3 (18%) for the measured mass. As a result, reconstructed mass generated from the sum of individual species had approximately half the uncertainty compared to measured mass using our relatively simple gravimetric methodology. Since organic carbon makes up the majority of the PM2.5 in Denver, additional uncertainty in the OC scaling factor could significantly increase the reconstructed mass uncertainty. However, a very large scaling factor uncertainty (on the order of 0.5) would be required to bring the reconstructed mass uncertainty up to the level of the measured mass uncertainty. Therefore, we conclude that reconstructed mass exhibits less uncertainty than measured mass for this study.
In many instances, environmental measurements are below the detection limits imposed by the instrumentation or methodology. This is frequently the case in air quality studies where trace level compounds are being measured and contamination is a concern. For source apportionment modeling purposes, it is common practice to replace below detection limit values by half the DL and impose an uncertainty equal to some multiple of the DL (Polissar et al., 1998; Reff et al., 2007). However, instrument sensitivity can vary substantially over time which adds to the complexity of choosing a single value of the DL for each chemical analysis procedure. This is especially important in time series studies such as the DASH study where the chemical analyses are being performed over a long span of time. With accurate pointwise uncertainty estimates, we have the option of using a confidence interval approach for determining if a value is above detection. This approach involves flagging all values as BDL whose confidence intervals incorporate zero with some pre-determined level of confidence. Table 3 shows 12.3% of the mass measurements are BDL (using a 95% confidence interval assuming normality) resulting from frequent low mass days and a low S/N ratio. Nitrate has 38.0% of the measurements BDL which is not surprising since nitrate is not present in the particle phase at any significant level in the warmer summer months. The remaining species have far fewer values flagged as BDL using the confidence interval approach resulting from their higher S/N ratios and year-round abundance in Denver.
4.3 Duplicate Precision Analysis
Validation of the propagated uncertainties was the primary reason for initiating the collocated sampling campaign. The normal probability plots shown in Figure 3 demonstrate that the differences between the collocated samplers are normally distributed with nitrate and sulfate having slightly exaggerated tails (i.e., a few outliers in the differences). The scatter of the data shown in Figure 4 indicate that the gravimetric and ECOC analyses are less precise (0.91 ≤ R ≤ 0.93) than the IC analysis (R = 1.00). A recent analysis using collocated monitors within the EPA's Speciation Trends Network (STN) showed similar findings with mass, EC and OC less precise than nitrate and sulfate (US-EPA, 2006). In our case, lower mass precision can be attributed to our use of a relatively simple gravimetric setup for inherently difficult mass measurements. For ECOC, there are many factors that go into the measurements which increase uncertainty including the application of the pyrolized carbon adjustment. Furthermore, the ECOC analysis is performed on a small 1.5 cm2 punch which only incorporates 3% of the total quartz filter deposit area. If the mass deposited on the primary and duplicate filters aren't perfectly uniform, this could result in additional noise in the comparison. Hyslop and White (2008) analyzed collocated measurements from the Interagency Monitoring of Protected Visual Environments (IMPROVE) network and found a similar result: whole filter analyses for mass, nitrate and sulfate showed better precision (4-10%) than partial filter analyses for EC, OC and TC (17-22%).
The comparison between propagated uncertainty estimates and those derived from the collocated duplicate samples (Table 4) demonstrates that we are capturing most sources of random error in the propagation steps and that these methods for uncertainty estimation are comparable in aggregate. Mass is the only measurement where the mean propagated uncertainty (1.84 μg/m3) is appreciably larger than both duplicate-derived uncertainty estimates (SDdiff = 1.12 μg/m3 and ARPD uncertainty = 1.39 μg/m3). Since the duplicate filter pairs are weighed in parallel (i.e., in the same weigh session), it is possible that differences between sessions are being captured by the error propagation that are not captured by the duplicate precision calculations. Sensitivity to outliers in the field blanks (a significant source of the propagated mass uncertainty as shown in Table 3) could also be contributing to this discrepancy. The remaining species, however, show good agreement between propagated uncertainties and the duplicate-derived precision estimates. While collecting duplicate samples may require extra effort and cost, it encompasses all forms of random error from sampling to analysis without requiring an intimate knowledge of these sources. It also eliminates the reliance on reported uncertainties coming from outside laboratories which may not always be accurate or available. This method, however, may be less flexible compared to propagation of error when it comes to adjusting uncertainty estimates on a point-by-point basis to account for protocol changes or instrument variability. This flexibility is quite important in long time series studies such as the DASH study when not all the filters are being analyzed in a single batch.
Flanagan et al. (2006) used the ARPD while comparing collocated STN samples from six cities (Bakersfield, Riverside, Boston, New Brunswick, Cleveland and Houston). Our gravimetric ARPD is twice what they report (18.9 % compared to 9.3 %). This is due to a combination of low mass loadings in Denver relative to most of the cities investigated by Flanagan and coauthors and the limitations imposed by our five digit microbalance and relatively simple gravimetric weigh chamber. In contrast, our sulfate measurements have a lower ARPD than what they observed for the STN data (4.6 % compared to 8.2 %). This could be partially attributed to the extra handling required for the filters being collected at multiple sites in the STN network compared to our single site collection and local IC analysis. Finally, the ARPD we observed for OC is similar to the STN data (12.3 % compared to 14.2 %) which is reassuring given that both rely on the NIOSH TOT method for carbon analysis. Flanagan and coworkers did not report on nitrate, EC or TC.
4.4 Concluding Remarks
The 4.5 year average PM2.5 concentration measured at our receptor site in Denver is 7.95 μg/m3 which is well below the current annual NAAQS of 15 μg/m3 (US-EPA, 2007). Despite being in compliance with the PM2.5 standards, Denver is a valuable location for a health effect study such as the DASH study. The bulk data shows substantial variability in PM2.5 mass and chemical make-up: in addition to the obvious seasonal variation, several short-term patterns are present in the data and will be the subject of a future paper. Furthermore, the chemical composition of the PM2.5 is relatively simple. Sufficient ammonium is usually present to fully neutralize nitrate and sulfate as NH4NO3 and (NH4)2SO4, respectively, and mass closure is demonstrated when the metals data are incorporated into the data set. This relatively simple chemical composition will improve the interpretability of source apportionment and health findings. Finally, carbon makes up more than half of the PM2.5 in Denver and is prevalent in all regions across the country (US-EPA, 2003). This is why carbon speciation was chosen as the focus of the DASH study and why the findings from this study have the potential to play an important role in identifying significant PM2.5 sources and associated health effects.
Acknowledgments
This research is supported by NIEHS research grant number RO1 ES010197. Additional support for student assistance was provided by NSF Research Experience for Undergraduates award number EEC 0552895. We would like to thank Brendon Rudack for his help constructing the weigh chamber; Dan Williams, James Schroeder, Bobby Irmiger, Brian Cone, Rachel Bryant and Toni Newville for their contributions to the mass measurements; Paul Schuster, Fatimah Matalkah, Mary Beth Oshnack and Stacy Louie for their contributions to the IC analyses; Dr. Martin Shafer and Jeff DeMinter for their contributions to the nitrogen, ICP-MS and ECOC analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen R, Box M, Liu LJS, Larson TV. A cost-effective weighing chamber for particulate matter filters. Journal of the Air & Waste Management Association. 2001;51:1650–1653. doi: 10.1080/10473289.2001.10464392. [DOI] [PubMed] [Google Scholar]
- Bae MS, Schauer JJ, Turner JR. Estimation of the monthly average ratios of organic mass to organic carbon for fine particulate matter at an urban site. Aerosol Science and Technology. 2006;40:1123–1139. [Google Scholar]
- Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: Mortality among residents of 90 cities. Journal of Toxicology and Environmental Health-Part a-Current Issues. 2005;68:1071–1092. doi: 10.1080/15287390590935932. [DOI] [PubMed] [Google Scholar]
- Field MP, Sherrell RM. Magnetic sector ICPMS with desolvating micronebulization: Interference-free subpicogram determination of rare earth elements in natural samples. Analytical Chemistry. 1998;70:4480–4486. doi: 10.1021/ac980455v. [DOI] [PubMed] [Google Scholar]
- Flanagan JB, Jayanty RKM, Rickman EE, Peterson MR. PM2.5 speciation trends network: Evaluation of whole-system uncertainties using data from sites with collocated samplers. Journal of the Air & Waste Management Association. 2006;56:492–499. doi: 10.1080/10473289.2006.10464516. [DOI] [PubMed] [Google Scholar]
- Herner JD, Green PG, Kleeman MJ. Measuring the trace elemental composition of size-resolved airborne particles. Environmental Science & Technology. 2006;40:1925–1933. doi: 10.1021/es052315q. [DOI] [PubMed] [Google Scholar]
- Hyslop NP, White WH. An evaluation of interagency monitoring of protected visual environments (IMPROVE) collocated precision and uncertainty estimates. Atmospheric Environment. 2008;42:2691–2705. [Google Scholar]
- Jaeckels JM, Bae MS, Schauer JJ. Positive matrix factorization (PMF) analysis of molecular marker measurements to quantify the sources of organic aerosols. Environmental Science & Technology. 2007;41:5763–5769. doi: 10.1021/es062536b. [DOI] [PubMed] [Google Scholar]
- Kim E, Hopke PK. Comparison between sample-species specific uncertainties and estimated uncertainties for the source apportionment of the speciation trends network data. Atmospheric Environment. 2007;41:567–575. [Google Scholar]
- Kirchstetter TW, Corrigan CE, Novakov T. Laboratory and field investigation of the adsorption of gaseous organic compounds onto quartz filters. Atmospheric Environment. 2001;35:1663–1671. [Google Scholar]
- Ko FWS, Tam W, Wong TW, Lai CKW, Wong GWK, Leung TF, Ng SSS, Hui DSC. Effects of air pollution on asthma hospitalization rates in different age groups in Hong Kong. Clinical and Experimental Allergy. 2007;37:1312–1319. doi: 10.1111/j.1365-2222.2007.02791.x. [DOI] [PubMed] [Google Scholar]
- Lonati G, Giugliano M, Butelli P, Romele L, Tardivo R. Major chemical components of PM2.5 in Milan (Italy) Atmospheric Environment. 2005;39:1925–1934. [Google Scholar]
- Neff WD. The Denver Brown Cloud studies from the perspective of model assessment needs and the role of meteorology. Journal of the Air & Waste Management Association. 1997;47:269–285. doi: 10.1080/10473289.1997.10464447. [DOI] [PubMed] [Google Scholar]
- NIOSH. NIOSH Manual of Analytical Methods (NMAM) Fourth. National Institute of Occupational Safety and Health; Cincinnati, OH: 2003. Method 5040, issue 3: Diesel particulate matter (as elemental carbon) pp. 1–5. [Google Scholar]
- NIST. Technical note 1297, 1994 edition: Guidelines for evaluating and expressing the uncertainty of NIST measurement results. National Institute of Standards and Technology; 1994. http://physics.nist.gov/Pubs/guidelines/appa.html. [Google Scholar]
- NIST/SEMATECH. E-handbook of statistical methods. National Institute of Standards and Technology/SEMATECH; 2006. http://www.itl.nist.gov/div898/handbook/mpc/section3/mpc3671.htm. [Google Scholar]
- Ntziachristos L, Ning Z, Geller MD, Sheesley RJ, Schauer JJ, Sioutas C. Fine, ultrafine and nanoparticle trace element compositions near a major freeway with a high heavy-duty diesel fraction. Atmospheric Environment. 2007;41:5684–5696. [Google Scholar]
- Ostro B, Broadwin R, Green S, Feng WY, Lipsett M. Fine particulate air pollution and mortality in nine California counties: Results from CALFINE. Environmental Health Perspectives. 2006;114:29–33. doi: 10.1289/ehp.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paatero P. Least squares formulation of robust non-negative factor analysis. Chemometrics and Intelligent Laboratory Systems. 1997;37:23–35. [Google Scholar]
- Paatero P. Users guide for positive matrix factorization programs PMF2 and PMF3. University of Helsinki; Finland: 2000. [Google Scholar]
- Pang YB, Ren Y, Obeidi F, Hastings R, Eatough DJ, Wilson WE. Semi-volatile species in PM2.5: Comparison of integrated and continuous samplers for PM2.5 research or monitoring. Journal of the Air & Waste Management Association. 2001;51:25–36. doi: 10.1080/10473289.2001.10464252. [DOI] [PubMed] [Google Scholar]
- Pang Y, Turpin BJ, Gundel LA. On the importance of organic oxygen for understanding organic aerosol particles. Aerosol Science and Technology. 2006;40:128–133. [Google Scholar]
- Polissar AV, Hopke PK, Paatero P. Atmospheric aerosol over Alaska - 2. Elemental composition and sources. Journal of Geophysical Research-Atmospheres. 1998;103:19045–19057. [Google Scholar]
- Pope CA, 3rd, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW., Jr Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- Rabinovitch N, Strand M, Gelfand EW. Particulate levels are associated with early asthma worsening in children with persistent disease. American Journal of Respiratory and Critical Care Medicine. 2006;173:1098–1105. doi: 10.1164/rccm.200509-1393OC. [DOI] [PubMed] [Google Scholar]
- Reff A, Eberly SI, Bhave PV. Receptor modeling of ambient particulate matter data using positive matrix factorization: Review of existing methods. Journal of the Air & Waste Management Association. 2007;57:146–154. doi: 10.1080/10473289.2007.10465319. [DOI] [PubMed] [Google Scholar]
- Schauer JJ, Mader BT, Deminter JT, Heidemann G, Bae MS, Seinfeld JH, Flagan RC, Cary RA, Smith D, Huebert BJ, Bertram T, Howell S, Kline JT, Quinn P, Bates T, Turpin B, Lim HJ, Yu JZ, Yang H, Keywood MD. ACE-Asia intercomparison of a thermal-optical method for the determination of particle-phase organic and elemental carbon. Environmental Science & Technology. 2003;37:993–1001. doi: 10.1021/es020622f. [DOI] [PubMed] [Google Scholar]
- Sheesley RJ, Schauer JJ, Meiritz M, DeMinter JT, Bae MS, Turner JR. Daily variation in particle-phase source tracers in an urban atmosphere. Aerosol Science and Technology. 2007;41:981–993. [Google Scholar]
- Skoog DA, West DM. Fundamentals of analytical chemistry. Holt; New York, NY: 1969. [Google Scholar]
- Turpin BJ, Saxena P, Andrews E. Measuring and simulating particulate organics in the atmosphere: problems and prospects. Atmospheric Environment. 2000;34:2983–3013. [Google Scholar]
- Turpin BJ, Lim HJ. Species contributions to PM2.5 mass concentrations: Revisiting common assumptions for estimating organic mass. Aerosol Science and Technology. 2001;35:602–610. [Google Scholar]
- US-EPA. Method 353.2, revision 2.0: Determination of nitrate-nitrite nitrogen by automated colorimetry. Environmental Monitoring Systems Laboratory, Office of Research and Development, U.S. Environmental Protection Agency; Cincinnati, OH: 1993a. pp. 1–13. [Google Scholar]
- US-EPA. Method 350.1, revision 2.0: Determination of ammonia nitrogen by semi-automated colorimetry. Environmental Monitoring Systems Laboratory, Office of Research and Development, U.S. Environmental Protection Agency; Cincinnati, OH: 1993b. pp. 1–14. [Google Scholar]
- US-EPA. 40 CFR part 50, 1997 edition: National primary and secondary ambient air quality standards. Code of Federal Regulations, Environmental Protection Agency; Washington, DC: 1997. pp. 5–84. [Google Scholar]
- US-EPA. Quality Assurance Guidance Document 2.12: Monitoring PM2.5 in ambient air using designated reference or class I equivalent methods. Human Exposure and Atmospheric Sciences Division, National Exposure Research Laboratory, U.S. Environmental Protection Agency; Research Triangle Park, NC: 1998. [Google Scholar]
- US-EPA. Publication 454/R-03-005: National air quality and emissions trends report: 2003 special studies edition. Office of Air Quality and Standards, Air Quality Strategies and Standards Division, U.S. Environmental Protection Agency; Research Triangle Park, NC: 2003. pp. 1–190. [Google Scholar]
- US-EPA. Annual data summary report for the chemical speciation of PM2.5 filter samples project: January 1 through December 31, 2005. Office of Air Quality Planning and Standards, U.S. Environmental Protection Agency; Research Triangle Park, NC: 2006. [Google Scholar]
- US-EPA. 40 CFR part 50, 2007 edition: National primary and secondary ambient air quality standards. Code of federal Regulations, Environmental Protection Agency; Washington, DC: 2007. pp. 5–127. [Google Scholar]
- Vanhaecke F, Moens L. Recent trends in trace element determination and speciation using inductively coupled plasma mass spectrometry. Fresenius Journal of Analytical Chemistry. 1999;364:440–451. doi: 10.1007/s002160100801. [DOI] [PubMed] [Google Scholar]
- Vedal S, Hannigan MP, Dutton SJ, Miller SL, Milford JB, Rabinovitch N, Kim SY, Sheppard L. The Denver Aerosol Sources and Health (DASH) study: overview and early findings. Atmospheric Environment. 2008 doi: 10.1016/j.atmosenv.2008.12.017. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J. Technical note: Static control for balances. Cahn Instruments, Inc.; Cerritos, CA: 1998. http://www.epa.gov/ttn/amtic/files/ambient/pm25/qa/static.pdf. [Google Scholar]
- White WH, Roberts PT. On the nature and origins of visibility-reducing aerosols in the Los Angeles air basin. Atmospheric Environment. 1977;11:803–812. [Google Scholar]
- WSLH. ESS method 220.3: Ammonia nitrogen and nitrate + nitrite nitrogen, automated flow injection analysis method. Wisconsin State Laboratory of Hygiene, Environmental Sciences Section, Inorganic Chemistry Unit; Madison, WI: 1997. http://www.epa.gov/grtlakes/lmmb/methods/methd220.pdf. [Google Scholar]