Introduction
Over the past two decades, clinical and public health recognition of the importance of sleep-disordered breathing (SDB) and other sleep disorders has increased markedly (1–5). Findings from epidemiology studies, many of which were represented at the University of Pennsylvannia-Hershey symposium (November 2007,“Epidemiology of Sleep Disorders: Clinical Implications”) have been critical in identifying the high prevalence of undiagnosed SDB and in linking this disorder with significant morbidity (6–15). Interestingly, identification of the high prevalence of undiagnosed SDB by the population-based studies in the 1990’s contributed to the growing increase in clinical recognition of SDB. The increase in clinical interest, in turn, prompted the need for additional epidemiology studies to quantify the adverse health outcomes of this condition in order to determine the total societal burden of sleep disordered breathing. Thus, the rationale for the Wisconsin Sleep Cohort and other population-based studies as well as the significance of findings, must be explained in the context of the fascinating history of sleep medicine.
Of note, this article is not a general review oof SDB: the assignment for this article was to elaborate on this presentation at the Hershey Symposium. As a report specifically on the Wisconsin Sleep Cohort Study, our findings are referenced primarily, with the exception of other studies that contributed to our design, prior to 1989. Consequently, many ongoing population- based studies have made important contributions that address the overall question of burden of SDB, but are beyond the scope of this paper and could not be included.
Rationale for a population-based cohort study of SDB:1960–1987
1. The emerging need to understand the health burden of SDB
In this paper, SDB refers to the condition of repeated apnea and hypopnea events during sleep, most commonly indicated by the number of apnea and hypopnea events per hour of sleep (apnea-hypopnea index, AHI). * This anomaly of breathing pauses during sleep was documented centuries ago by scholars using colorful case descriptions, usually combined with the common symptom of daytime sleepiness (16,17). However, it was not until European researchers and clinicians in 1966 clearly defined the clinical entity of sleep apnea syndrome as the combination of episodes of obstructive apnea and daytime symptoms, particularly extreme daytime sleepiness (18). At that time, the only effective treatment for SDB was a tracheotomy to provide a patent surgical airway in the cervical trachea. With only an invasive treatment to offer, only the most severe cases of sleep apnea were likely to come to medical attention. Clinical interest in sleep apnea and other sleep disorders remained low in most countries including the US, with noteable exceptions. In the US, Stanford researchers, led by Drs. Dement and Guilleminault, persisted in forming a key research and clinical foundation devoted to sleep disorders, including SDB. With a small group of dedicated researchers, an early professional society was formed and groundwork for a new field was set in place (5). During this critical time, in 1981, Sullivan introduced a revolutionary new treatment for sleep apnea: continuous positive air pressure (CPAP) (19). CPAP, delivered by a small facial mask, effectively kept the upper airway patent and prevented episodes of SDB. Of profound importance, a treatment for SDB that was acceptable to patients had become available: as a feasibly treatable disorder, the significance of SDB greatly increased
Dr. Dement’s efforts to overcome barriers to research and clinical care of sleep disorders were unrelenting, and by 1986 reached the US Congress. The result was a task force and congressional mandate to determine the state of knowledge of sleep disorders and resource needs and to seek new NIH commitment to research (20). An important part of the charge to the task force was to determine the overall public burden of SDB. The Heart, Lung and Blood Institute began to promote research in SDB with workshops to identify research needs, and then, in 1987, requesting grant applications for Specialized Centers for Cardiopulmonary Disorders of Sleep that would combine clinical, experimental and epidemiological research programs. As the epidemiological component of the grant application from the University of Wisconsin, we proposed the Wisconsin Sleep Cohort Study, a longitudinal epidemiology study designed to investigate the natural history of SDB by conducting overnight polysomnography studies on a random sample of the general population.
2. Motivation for population-based studies to determine to determine the total societal burden of SDB
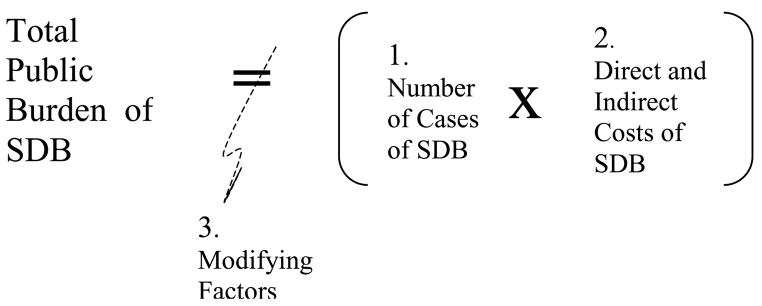
To address the congressional mandate and identify long-term research goals, an accurate description of the public health burden of SDB was needed. As shown in Figure 1, the health burden of a disorder is the product of the prevalence and the proportion of adverse health outcomes that can be attributed to the disorder. Two decades ago, virtually all information about SDB prevalence and outcomes was based on observations of the small number of patients, mostly men, diagnosed with SDB (6,7). Although considered an uncommon disorder, clinic studies linked significant morbidity and mortality with SDB. Clinical researchers found excessive daytime sleepiness, motor vehicle crashes, hypertension, cardiovascular disease and mortality to be more prevalent in patients with SDB (21,22). Thus, two decades ago, estimating the total health burden of SDB was limited by lack of a valid estimate of how many people were affected with this disorder. Furthermore, clinic referral and other biases and limitations in control groups raised concern that the health risks linked with SDB morbidity were overestimated. Although CPAP clearly reduced apnea and hypopnea episodes, outside of the small field of sleep research, the lack of rigorous tials of CPAP efficacy was criticized.
Figure 1. The Total Public Burden of SDB is a function of.
- The number of cases of SDB (prevalence), multiplied by
-
The costs of SDB, including:
-
direct costs
- monentary cost of diagnosis and treatment,
- distress, discomfort associated with having SDB to the individual sufferer, including unsatisfactory sleep, low motivation, problems with daytime functioning, quality of life, relationships, etc,
- loss to family and society (loss of potential contributions to community e.g., jobs, volunteer sevices)
- morbidity attributable to SDB (e.g., hypertension, depression)
- loss of years of life attributable to SDB
-
indirect costs
- SDB exacerbation of comorbidity
- worse prognosis for other disorders due to SDB-related behavioral morbidity (e.g., low compliance with therapy for other disorders, lack of exercise)
-
-
Modifying factors
- prevention programs to reduce prevalence and progression of SDB (e.g., weight loss)
- increases in factors that cause or worse SDB (e.g., ongoing obesity epidemic, aging of the popuation)
- treatment of SDB that prevents or reduces adverse outcomes
At the time the WSCS was designed, only a small minority of people with SDB had been diagnosed and treated. Compared to most medical specialties, established in the mid 1800’s, sleep medicine was still in its early years: it was not until 1994 that sleep medicine was recognized by the American Medical Association as a subspecialty. Consequently, general medical training and resources for recognition of SDB and other sleep disorders were rare. Although awareness of SDB has grown, the increase in case-finding has not been uniform, either intra- or internationally.
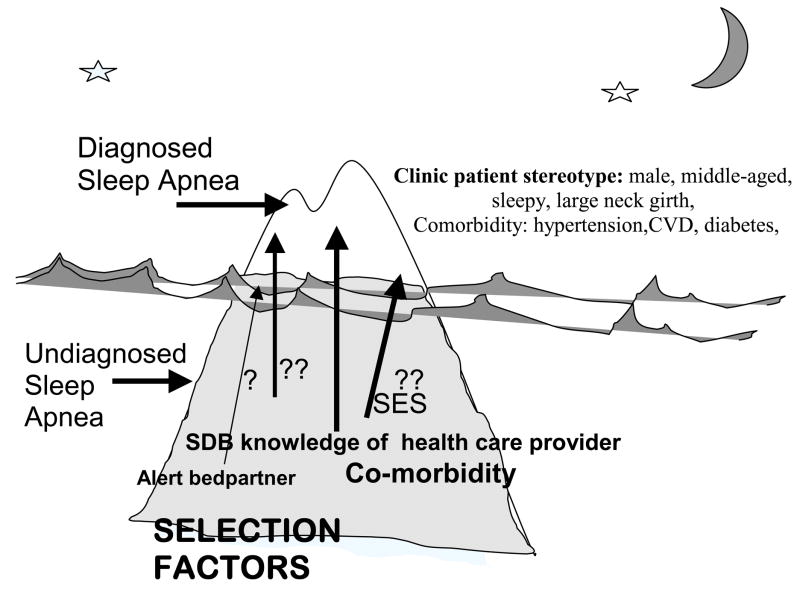
The striking and unpredictable growth in clinical and public recognition of SDB presents a challenge for determining the occurrence of SDB and investigating SDB risk factors and the adverse long-term health consequences. In common with other disorders with low and uneven recognition by the health care system, the patients with SDB who were evaluated and diagnosed have been different in known and unknown ways from the majority of cases of SDB that remain undetected. This phenomenon of selective referral and diagnosis for under-recognized but prevalent disorders is recognized in epidemiology as the “tip of the iceberg” paradigm, whereby the iceberg comprises all cases of SDB and multiple factors determine which cases ultimately become “patients” with diagnosed SDB.
As shown in Figure 2, there are many forces that shape referral patterns for SDB, beginning with the individual seeking care. In the past, the symptoms of SDB, including snoring and daytime sleepiness, were not seen by the general public as indicators of a medical problem, but rather as comical characteristics or a nuisance, at best. Thus, individuals told they were loud snorers or always sleeping were unlikely to seek care for SDB symptoms. Until recently, SDB was most likely to diagnosed only incidentally, while a patient was being seen for a different medical complaint. A patient hospitalized for a myocardial infarct, for example, might be observed to stop breathing during sleep, and a consultation with a sleep specialist, if available, might be sought. Consequently, SDB was more likely to be diagnosed in someone who had comorbid conditions, which may or may not have been related to SDB. Further bias is introduced because access to any health care is limited by socioeconomic status, thereby confounding correlates of SDB with those of education and income. The view of the stereotypical patient with SDB was that of an overweight, sleepy, middle-aged, snoring male, resulting in a referral bias against women and older patients. Even with optimal awareness in primary care, the ability to refer a patient is tempered by the perceived severity of SDB symptoms, availability of a sleep clinic, patient willingness and ability to pay. These and other selection biases serve to build in spurious associations of SDB with other characteristics and disorders. Consequently, characteristics of SDB patients are clinic-specific, and using sleep clinic patient samples to address questions regarding risk factors, causes, and consequences of SDB may not be generalizable beyond the specific clinic sample. As a result, epidemiology studies of SDB in population samples, free of clinic selection biases and designed to minimize other biases, were critical to determine the health burden of SDB as well as provide a foundation for developing clinical and public health strategies.
Figure 2.
Selection biases for clinical recognition and diagnosis of sleep apnea. The proportion of all cases of sleep apnea is represented by the iceberg, with clinically diagnosed cases shown in the tip of the iceberg. Clinically recognized sleep apnea represents less than 85% of the total prevalence of sleep apnea cases that would be candidates fo treatment. Factors that favor selection of individuals with unrecognized sleep apnea (below the tip of the iceberg) for clinical referral and diagnosis are shown.
Design of the Wisconsin Sleep Cohort Study
The primary goal of the WSCS was to investigate the natural history of SDB and other sleep disorders, with the long-term goal of better understanding the total societal burden of SDB. Specifically, our aims were to a) describe occurrence, including age and sex-specific prevalence for mild, moderate and severe SDB b) estimate, with longitudinal data, the role of SDB in cardiovascular and behavioral morbidity and mortality and c) identify risk factors for the development and progression of SDB. The fundamental components for the study design were those of a standard epidemiology prospective cohort study, including the identification of a population-based sampling frame, recruitment of a probability sample with sufficient variation in exposure and adequate power for hypothesis testing, and collection of data with sufficient accuracy at baseline and follow-up. A major factor influencing the WSCS study design was the decision to use in-laboratory polysomnography (PSG) for describing SDB. PSG was the clinical diagnostic standard for identifying SDB, thus use of PSG in our population study would provide comparable findings that could be translated to the clinical setting. Furthermore, the extensive data recorded by PSG provides many parameters for measurement accuracy (i.e., breathing by sleep stage), redundancy, and flexibility in operational definitions of breathing events. However, in-laboratory, standard PSG was expensive, labor intensive, and a participant burden. In previous studies, when PSG was the measurement tool of choice, sample sizes were generally smaller; large studies tended to use objective monitoring with fewer signals or subjective indicators of SDB, such as self-reported snoring.
1. Influence of early population-based studies
To help plan several aspects of the WSCS study design, we relied on the few pioneering studies of SDB in the population published prior to 1988 (8,11–15). These studies provided the first impressions of the occurrence of undiagnosed SDB and revealed general and unique methodological problems in quantifying SDB prevalence and investigating associations of SDB with adverse health consequences
Bliwise and colleagues reported on the first population cohort of 198 middle-aged and older people screened for SDB by in-laboratory polysomnography and followed over time (12). In addition to finding a high prevalence, the authors also investigated night-to-night variability in SDB, thereby first bringing attention to the need for study designs to accommodate this measurement error (13). Bliwise et al also noted the high prevalence with age, the progression in the respiratory distress index (breathing events per hour of sleep) over a 10 year period, and the risk of cardiovascular death (14). In Europe, in 1983, Lavie reported findings from a 2-stage approach to screen for SDB symptoms in Israeli industrial workers. The group was surveyed for SDB symptoms, with the expectation that almost all cases of SDB would be concentrated in the group reporting symptoms (11). Polysomnography was then performed on the symptomatic sample to obtain a “minimum” prevalence. A prevalence of 1% resulted.
Using in-home monitoring without EEG, Ancoli- Israel and colleagues screened sleep and breathing in a sample of 358 elderly community dwelling volunteers with a mean age of 72 years (15). Reported in 1987, the prevalence of obstructive sleep apnea was estimated at 31% in men and 19% in women. Central sleep apnea was found in 6% of the sample.
In 1987, Gislason and Taube meticulously described the statistical considerations and methodological concerns that shaped the design of an investigation of SDB prevalence in Swedish men (23). Limited by resources for in-laboratory monitoring, the researchers determined the enriched sampling scheme needed that would result in adequate variation in a subsample of 60 men symptomatic for SDB. Taking these calculations and participation rate into account, a postal survey was sent to 4,064 men. Men who reported snoring sometimes or more often and daytime sleepiness formed the high risk group; a total of 166 were identified from the survey responses and recruited for the overnight study. Taking a conservative approach that all SDB cases were captured in the high risk category, results from the 61 participants were extrapolated to the sampling frame of 30–69 year old men, concluding that the minimal prevalence of SDB was 1.3% (8). Thus, at the time the WSCS was designed, the sparse information available suggested that SDB prevalence was markedly different in the US and European populations, and differed by age. It was not clear if the wide variability in prevalence was due to participant characteristics including age and gender, sampling error, or differences in methods to quantify SDB. Based on the previous studies, it was clear that to address our aims we needed a probability sampling scheme to yield a final cohort sample enriched for SDB risk of approximately 800 middle-aged men and women. We choose an age range of 30–60, with the expectation that we would be able to monitor SDB prevalence from middle to older age with overnight studies at baseline and at follow-up intervals of 4 years. Drawing on the methods of Gislason, we planned a two stage sampling scheme to increase variability in SDB and thereby increase study power (24).
2. Sample construction
Identification of a sampling frame, or enumeration of individuals with a known chance of being sampled, was our first requirement. For this, we chose the payroll files of Wisconsin State employees in the year 1988. The sampling frame had several advantages. It comprised a complete range of job titles from unskilled to professional, included sociodemographic data on the entire sampling frame for targeted recruitment and for eventual comparison of responders and nonresponders. Like other employed groups, the sample would be traced more easily, an important advantage for longitudinal studies. Furthermore, cohorts based on defined employee groups often have a positive identity that increases commitment to the study. All employees had equal access to health care, an advantage in reducing potential bias in health outcomes. The payroll file data included contact information, social security number, details on job, pay rate, sex, birthdate, race and other factors.
All employees, ages 30–60 in 1988, living or working in a defined area of south central Wisconsin were eligible for sample selection. Using a 2-stage scheme, a mailed survey was sent to a random sample of the eligible sampling frame and a subsample was recruited from the respondents for the longitudinal cohort study. The survey included questions on sociodemographics, life style, health habits, and sleep characteristics. A variable for SDB high risk was based on answers to questions on snoring frequency and loudness, and breathing pauses. A survey respondent was considered to be high risk if they reported snoring sometimes or more frequently, or very loud snoring or had witnessed breathing pauses, the remainder were considered low risk. We did not introduce sleepiness into the risk definition as this would hinder assessment of the indepemdent role of SDB in daytime impairment. All high risk respondents and an age and sex frequency matched random sample of low risk respondents were recruited with approximately 1.5:1 weighting of high:low risk. This technique is commonly used for increasing study power. The weighted sampling scheme is accounted for with specialized software such as SUDAAN.
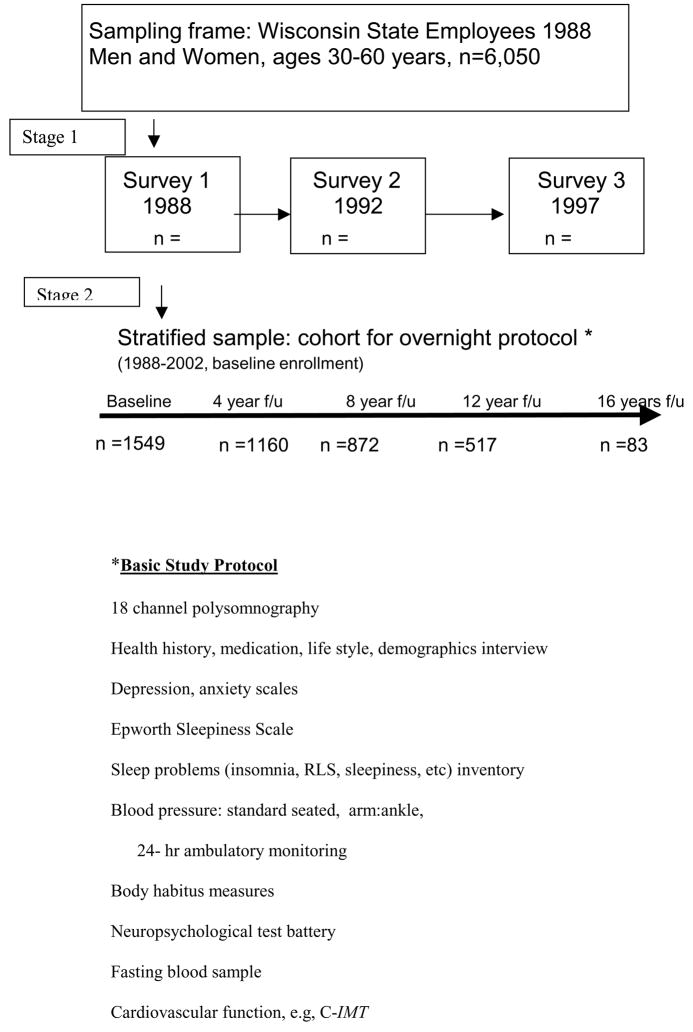
After constructing the cohort sample, the potential participants were recruited for the cohort overnight study protocol by repeated mailed invitations and by telephone, at a rate to perform 8 in-laboratory studies per week. To meet our target enrollment, we anticipated baseline studies would be performed continuously for about 3–4 years, after which time we would begin 4-year follow-up studies. The sample design and baseline protocol is shown in Figure 3. Over the next several years, other protocols were added and ancillary studies were conducted. We continued enrollment beyond the original target of 900, for a total of 1550 men and women. This sample continues to cycle through follow-up studies, in synchrony with the rolling recruitment over the first three years. As a result, the earliest participants have had the opportunity for 5 follow-up studies, while later partcipants are being recruited currently for their third study..
Figure 3.
Wisconsin Sleep Cohort Study Design and Protocol
A two-stage sampling design was used to obtain the WSCS sample for baseline and 4-year follow-up overnight protocols. The basic study protocol is listed.
The defined sampling frame for the first stage sample allowed us to examine potential participation bias on sociodemographic factors and other data that could be linked, including mortality records. The survey respondents comprised the second stage sampling frame, from which we recruited the cohort participants. The more detailed data from the survey on the entire sampling frame was vital in comparing nonparticipants, participants, and those who dropped out of the cohort. Participation rate was 82% for the survey stage, nonrespondants did not differ from respondents on the sociodemographic variables. Participation for the baseline overnight protocol rose from 50% to 54% by completion. Based on survey data, participants showed a typical healthy volunteer bias, with less self-reported hypertension and slightly higher education. Comparisons of participants and nonparticipants have been analysis-specific, e.g., stratified by gender, by SDB findings, and many other factors (24–26). In a study of SDB and mortality, we were able in explore possible retention bias by comparing mortality of participants who withdrew from the longitudinal study (26). Mortality was higher for survey participants who did not participate in the cohort, compared with the rate of the participants. The elevated mortality rate was found for both risk groups of the nonparticipants (high and low SDB risk, based on survey data). Thus, a healthy volunteer bias, commonly seen in epidemiology cohort studies, has been consistent at all stages of the study. The “better health” bias did not differ by the important study factors of SDB, so it is unlikely that our findings overestimated health risks of SDB. However, it is likely that having a slightly healthier cohort resulted in a loss of study power and the ability to detect small differences in outcomes by SDB status. In addition, it is possible that prevalence of SDB was underestimated at the baseline.
Findings from the Wisconsin Sleep Cohort Study
In keeping with the original long term goal of determining the total societal burden of SDB, the most relevant findings from the WSCS are described below, organized into the three components of the total burden: the number of affected people, the cost of SDB and the effects of modifying factors.
1. The number of people affected with SDB: prevalence by age and sex (24)
Our estimates of prevalence required careful extrapolation to account for the two stage sampling procedure to increase SDB variance (i.e., oversampling habitual, loud snorers and those with reported breathing pauses). As reported in 1993, with SDB severity indicated by the apnea-hypopnea index (AHI), we found a wide severity spectrum, with AHI ranging from 0 to 92. Using commonly used cutpoints (AHI at 5 and 15) to indicate mild, moderate and severe SDB, we estimated the age and sex specific prevalence as weighted averages from the high and low risk strata. The age and sex specific prevalence estimates could then be applied to any other population with different age and sex distributions. The overall prevalence for AHI 5–15 and AHI>15 based on the cohort distribution of age was also calculated, showing a markedly high prevalence for SDB for both men and women. Prevalence (95% confidence interval) of having SDB with an AHI >5 was 9% (5.6,12)for women and 24% (19,28) for men, and for AHI>15 was 4% (1.5,6.6) for women and 9% (6.4,11) for men. We also calculated prevalence of SDB with daytime sleepiness, as a surrogate for clinically diagnosed sleep apnea syndrome. For this, we used a strict definition of sleepiness: using three standard questions about sleepiness (falling asleep against wishes, not feeling rested regardless of hours of sleep and sleepiness that affects daily functioning), participants were categorized as having excessive daytime sleepiness (EDS) if they reported positively to all three questions. The prevalence of “SAS”, based on AHI>5 and “ EDS” was 2% for women and 4% for men. It is important to note that the prevalence for “SAS” would have been higher if we had used a less stringent definition of EDS. Similarly, in our study, a 4% desaturation is required for a hypopnea event; prevalence would obviously be higher if our definition included events with a 3% desaturation.. As other researchers have formally reported, prevalence of any disorder is highly dependent on definitions and cutpoints.
Describing the high prevalence and wide severity spectrum was an important step toward addressing the societal burden of SDB. Since our report, several other studies, using comparable methods, have reported similar SDB prevalence (9,10,27). The high prevalence of screen-detected SDB, compared with the small number of patients diagnosed with SDB, indicated that a large proportion of people with SDB who would meet clinical criteria for treatment were not being diagnosed. Furthermore, the findings revealed a gender bias: although SDB was more prevalent than expected in general, this was particularly striking for women. In sleep clinic populations, the ratio of men to women with SDB was approximately 9:1, but in the general population, at equal severity, the ratio was 2–3 to 1. This difference indicated that there had been a strong bias against women being diagnosed with SDB (25).
It is important to recognize that SDB prevalence varies with population differences in the prevalence of SDB risk factors, including overweight/obesity (a strong causal factor) and age. Consequently, SDB prevalence in a cohort will change over time, as well as vary across population with differences in age and sex distribution, and in the proportion of overweight people. Of particular concern, adults and children in the US and other countries are experiencing an increase in overweight and obesity (28,29). With our longitudinal data over the past 20 years, we have observed a increase in BMI in the WSCS corresponding to national trends in the obesity epidemic. And, matching this epidemic, SDB prevalence has increased markedly (27).
2. The cost of SDB: health care costs, well-being, morbidity, and mortality
With cross-sectional data, we have explored the associations of SDB with hypertension, quality of life, motor vehicle accidents, and stroke (7). Our analyses, controlling for potential confounding factors, have shown that SDB is associated with significant negative health outcomes, but the cross-sectional data limit a determination of what proportions of the outcomes are attributable to SDB. As our longitudinal data increase, we have been able to explore differences in incidence of some health outcomes by SDB status among those free of the specific outcome at baseline. The adverse outcomes predicted by SDB from longitudinal data, summarized in Table 1, provide better estimates for understanding the health and well-being burden that may be attributed to SDB (i.e., likely to have a casual role).
Table 1.
Wisconsin Sleep Cohort Study: Longitudinal associations of baseline sleep-disordered breathing with development of adverse health outcomes
| Outcome (citation) | Follow up time(mean) | Adjustment variables | Odds Ratio (95% Confidence Interval) for Outcome and SDB severity level* | |
|---|---|---|---|---|
| Moderate vs none | Severe vs none | |||
| Incident Hypertension (30) >140/90 mm Hg or use of antihypertensives. | 4 yrs | Age, sex, BMI, waist, hip girth, health hx, BP, smoking, alcohol | 2.0 (1.2,3.2) | 2.9 (1.5,5.6) |
| Incident ”non-dipping” (31) loss of ≥ 10% drop in sys BP from wake to sleep | 4 yrs | Age, sex, BMI, BP, smoking, alcohol, sleep duration, antihypertensive meds | 3.1 (1.3,7.7) | 4.4 (1.2,16) |
| Incident Depression (32) Zung score>50 | 4 yrs | Age, sex, BMI, alcohol, education | 2.0 (1.4, 2.9) | 2.6 (1.7, 3.9) |
| Incident stroke (33) | 4 yrs | age, sex | n.s. | 4.5(1.3,,15) |
| All-cause mortality (26) | 14 yrs | Age, sex, BMI, | n.s | 3.0 (1.4,6.3) |
| All cause mortality, (26) CPAP users excluded | 14 yrs | Age, sex, BMI, | n.s | 3.8 (1.6,9.0) |
| Cardiovascular mortality, (26) CPAP users excluded | 14 yrs | Age, sex, BMI, | n.s | 5.2 (1.4, 19) |
no SDB was defined as AHI<5, moderate SDB was defined as AHI 5–15, and severe SDB was defined as AHI>30 for mortality outcomes, AHI>20 for stroke, and AHI>15 for all other outcomes. Odds ratios were estimated with AHI <5 as the reference category,
Regardless of how blood pressure was measured, we have found significant associations between SDB and hypertension or elevated BP. With a prospective design, we excluded all WSCS participants with existing hypertension (defined as measured BP of >140/90 or using antihypertensive medication), and followed the group free of hypertension at baseline, for 4–8 years to determine the incidence of new hypertension (30). After controlling for age, sex, BMI, initial BP and other confounding factors, we found a dose-response increased risk of developing hypertension with SDB. The 4-year incidence of hypertension was 2.9 greater for participants with AHI>15 vs <5 at baseline.
Using 24 hour ambulatory BP monitoring, we found participants with SDB had higher BP levels before, during and after sleep compared to those without SDB (31). Longitudinally, we repeated ambulatory blood pressure monitoring at 4-year intervals to determine the incidence of developing an abnormal nighttime BP pattern, described by the lack of 10% or greater dip in BP with sleep. This condition, referred to as “non-dipping”, has been linked to poor prognosis for cardiovascular disease and death. We found SDB severity at baseline predicted an increase in the incidence of non-dipping: participants with AHI>15 vs <5 at baseline had a 4-fold greater odds of developing non-dipping nocturnal BP.
Other longitudinal analyses of the WSCS data have linked SDB with the development of depression, measured by the Zung self-rating depression scale (32) and incident stroke (33). Most recently, we have assessed the 18-year mortality rate by SDB status at baseline. The rate of all-cause mortality was 3-fold higher for participants with severe SDB, with AHI>30, compared to those without SDB (26).
Longitudinal analyses with the WSCS data support the hypothesis that SDB has a role in increasing significant cardiovascular morbidity, depression, and mortality. After accounting for confounding factors, persons with SDB, particularly severe, untreated SDB, had 3–5 times greater incidence of the leading causes of poor health and well being, and mortality. Corroboration from other population studies is needed, but our findings suggest that the burden of SDB is large, due to a high prevalence of untreated SDB and potentially high attributable risk for significant adverse health and well-being outcomes.
3. Modifiers
Factors that alter the prevalence or the adverse consequences of SDB are important in determining the total societal burden. Identification of causal factors that have a direct role in initiating the development of SDB or that worsen progression may justify intervention programs only if the factors can be reduced. Body weight is an established risk factor for SDB (6,7). Longitudinal analyses indicate weight is a modifiable risk factor (34, 35). Relative to stable weight over a 4-year period, a 10% loss in weight was associated with a decrease in SDB severity of a 23% reduction in AHI; a 10% gain in weight was associated with a 6-fold (2.2, 17.0) greater risk of developing moderate or worse SDB, and a 32% increase in AHI progression (34). As a modifiable risk factor, weight loss should hold the greatest promise as a means to reduce SDB prevalence. However, as a result of the ongoing strong trends in weight gain in both adults and children, the opposite result is likely: SDB prevalence is bound to increase. Using the relative risks from the WSCS analysis, data on US obesity trends and BMI distributions by age and sex in the US over 1992–2008, we estimated that prevalence of SDB would nearly double, and that the attributable proportion of SDB prevalence at a severity level of AHI>15 would rise from 56% to 69% by 2008 (27).
Effective treatment of SDB is an extremely important modifier of the total social burden. It has been clear to clinicians within the field of sleep medicine that CPAP effectively prevented apnea and hypopnea episodes, and represented a significant way to reduce the adverse sequelae of SDB (5). Consequently, if all SDB could be diagnosed and treated, the total burden would be equal to the direct cost of care for SDB—a small fraction of the potential burden of untreated SDB. However, in 1990, Wright and others pointed out the lack of randomized trials of CPAP, and suggested that outside the field of sleep medicine, effective therapy for SDB was yet to be proven (36). This led to swift action in the sleep field to promote proposals for CPAP trials. Two ongoing randomized, placebo controlled clinical trials of CPAP (APPLES, centered at Stanford University (37) and CATNAP, centered at University of Pennsylvania (38)) will be critical to quantify the burden of SDB that can be reduced by treatment.
Conclusion
In summary, findings from the WSCS and other population studies, indicate:
The first component of total social or public burden of SDB (Figure 1) poses a significant concern: the number of persons with untreated SDB is large, with at least 12–18 million affected adults. Of additional concern, the prevalence will rise markedly on the coat-tails of the obesity epidemic. Similarly, as the US population ages, prevalence of SDB will increase, due to accumulation of cases and the likelihood that incidence is higher in older age.
Limited longitudinal findings from the WSCS, supporting a causal role of SDB in increased morbidity and mortality, indicate that the second part of the total burden of SDB is significant. SDB is likely to contribute to increased cases of hypertension, cardiovascular disease, stroke, depression, and mortality. Adjusted relative risks and hazard ratios indicate moderate to large effect size (e.g., Table 1, point estimates of risk of significant health outcomes with severe SDB range from 2.5–5)
The burden of SDB reflected by the large number of persons with this disorder multiplied by the cost of adverse consequences that can be attributed to SDB is likely to be staggering. The burden could be decreased by preventing SDB through risk factor reduction, with weight loss as the most likely candidate. However, national and international trends predict the opposite: it is unlikely that reduction in SDB prevalence and severity will occur in the near future. Modification of the total burden by diagnosis and treatment with CPAP holds the greatest hope for reduction of the SDB burden. Results from the forthcoming clinical trials on the proportion of SDB adverse effects that can be reduced with CPAP treatment will greatly increase our understanding of the burden of treated and untreated SDB. These data, in conjunction with 1) robust estimates of the number of affected people, according to age, sex, and other subgroups, and 2)the proportion of morbidity and mortality than can be attributed to SDB will provide a solid basis for developing appropriate health policy and its rapid translation to health care, to eventually reduce the total public burden of SDB.
Acknowledgments
This work was supported by grants R01HL62252, RR03186, and R01AG14124 from the National Institutes of Health.
Footnotes
Because the majority of apnea and hypopnea events detected in population studies are due to upper airway collapse and increased airway resistance, with few events due to lack of repiratory muscle activation (central apnea), SDB is used in this report to reflect mainly obstructive sleep apnea.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colten H, Altevogt B, editors. Committee on Sleep Medicine and Research, Board on Health Sciences Policy:Sleep disorders and sleep deprivation: an unmet public health problem. Washington DC: Institute of Medicine/National Academies Press; 2006. pp. 20–32. [PubMed] [Google Scholar]
- 2.Somers VK, White DP, Amin R, et al. Sleep Apnea and Cardiovascular Disease. An American Heart Association/American College of Cardiology Foundation Scientific Statement. Circulation. 2008 Jul 28 [Google Scholar]
- 3.Namen AM, Dunagan DP, Fleischer A, Tillett J, Barnett M, McCall WV, Haponik EF. Increased physician-reported sleep apnea: the National Ambulatory Medical CareSurvey. Chest. 2002;121(6):1741–7. doi: 10.1378/chest.121.6.1741. [DOI] [PubMed] [Google Scholar]
- 4.Ball EM, Simon RD, Jr, Tall AA, Banks MB, Nino-Murcia G, Dement WC. Diagnosis and treatment of sleep apnea within the community. The Walla Walla Project. Arch Intern Med. 1997;157(4):419–24. [PubMed] [Google Scholar]
- 5.Shepard JW, Jr, Buysse DJ, Chesson AL, Jr, et al. History of the development of sleep medicine in the United States. J Clin Sleep Med. 2005;1(1):61–82. [PMC free article] [PubMed] [Google Scholar]
- 6.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apne:a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 8.Gislason T, Almqvist M, Eriksson G, et al. Prevalence of sleep apnea syndrome among Swedish men--an epidemiological study. J Clin Epidemiol. 1988;41(6):571–6. doi: 10.1016/0895-4356(88)90061-3. [DOI] [PubMed] [Google Scholar]
- 9.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 10.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–13. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 11.Lavie P. Incidence of sleep apnea in a presumably healthy working population: a significant relationship with excessive daytime sleepiness. Sleep. 1983;6(4):312–8. [PubMed] [Google Scholar]
- 12.Bliwise DL, Bliwise NG, Partinen M, et al. Sleep apnea and mortality in an aged cohort. Am J Public Health. 1988;78(5):544–7. doi: 10.2105/ajph.78.5.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bliwise D, Carskadon M, Carey E, Dement W. Longitudinal development of of sleep-related respiratory disturbance in adult humans. J Gerontol. 1984;39(3):290–3. doi: 10.1093/geronj/39.3.290. [DOI] [PubMed] [Google Scholar]
- 14.Ancoli-Israel S, Kripke DF, Mason W. Characteristics of obstructive and central sleep apnea in the elderly: an interim report. Biol Psychiatry. 1987;22(6):741–50. doi: 10.1016/0006-3223(87)90206-x. [DOI] [PubMed] [Google Scholar]
- 15.Bliwise DL, Carey E, Dement WC. Nightly variation in sleep-related respiratory disturbance in older adults. Exp Aging Res. 1983;9(2):77–81. doi: 10.1080/03610738308258429. [DOI] [PubMed] [Google Scholar]
- 16.Kryger MH. Sleep apnea. From the needles of Dionysius to continuous positive airway pressure. Arch Intern Med. 1983 Dec;143(12):2301–3. doi: 10.1001/archinte.143.12.2301. [DOI] [PubMed] [Google Scholar]
- 17.Lavie P. Who was the first to use the term Pickwickian in connection with sleepy patients? History of sleep apnoea syndrome. Sleep Med Rev. 2008;12(1):5–17. doi: 10.1016/j.smrv.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res. 1966;1(2):167–86. doi: 10.1016/0006-8993(66)90117-x. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 20.National Commission on Sleep Disorders Research. Wake up America: a national sleep alert 1993.
- 21.Kales A, Cadieux RJ, Bixler, et al. Severe obstructive sleep apnea--I: Onset, clinical course, and characteristics. J Chronic Dis. 1985;38(5):419–25. doi: 10.1016/0021-9681(85)90137-7. [DOI] [PubMed] [Google Scholar]
- 22.Kales A, Caldwell AB, Cadieux RJ, Vela, et al. Severe obstructive sleep apnea--II: Associated psychopathology and psychosocial consequences. J Chronic Dis. 1985;38(5):427–34. doi: 10.1016/0021-9681(85)90138-9. [DOI] [PubMed] [Google Scholar]
- 23.Gislason T, Taube A. Prevalence of sleep apnea syndrome--estimation by two stage sampling. Ups J Med Sci. 1987;92(2):193–203. doi: 10.3109/03009738709178689. [DOI] [PubMed] [Google Scholar]
- 24.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 25.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med. 1995;156:2445–51. [PubMed] [Google Scholar]
- 26.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005 Oct;99(4):1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 28.Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35(1):93–9. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- 29.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291(23):2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 30.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 31.Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal Association of Sleep-Disordered Breathing and Nondipping of Nocturnal Blood Pressure in the Wisconsin Sleep Cohort Study. SLEEP. 2008;31(6):795–800. doi: 10.1093/sleep/31.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166(16):1709–15. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 34.Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 35.Newman AB, Foster G, Givelber R, et al. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165(20):2408–13. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 36.Wright J, Johns R, Watt I, Melville A, Sheldon T. Health effects of obstructive sleep apnoea and the effectiveness of continuouspositive airways pressure: a systematic review of the research evidence. BMJ. 1997 Mar 22;314(7084):851–60. doi: 10.1136/bmj.314.7084.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kushida CA, Nichols DA, Quan SF, et al. The Apnea Positive Pressure Long-term Efficacy Study (APPLES): rationale, design, methods, and procedures. J Clin Sleep Med. 2006;2(3):288–300. [PubMed] [Google Scholar]
- 38.Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]