Abstract
Recently, the focus of interest on the role of the renin angiotensin system in the pathophysiology of hypertension has shifted towards greater emphasis on new developments in local renin angiotensin systems in specific tissues. We have focused our recent investigations on the role of the intrarenal-intratubular RAS in hypertension. All of the components needed for angiotensin II generation are present within the various compartments in the kidney. This brief review is focused on recent evidence that inappropriate activation of renin in distal nephron segments, by acting on angiotensinogen generated in the proximal tubule cells and delivered to the distal nephron may contribute to increased distal intrarenal angiotensin II formation, sodium retention and development and progression of hypertension.
Keywords: Intrarenal RAS, intratubular RAS, distal tubular renin, angiotensinogen, Chronic Ang II-infused rats, 2K1C Goldblatt hypertension
Introduction
The renin angiotensin system (RAS) plays a pivotal role in regulating renal sodium and water excretion and, therefore, in maintaining body sodium and fluid balance. It has generally been held that renin produced by the kidney cleaves liver-derived angiotensinogen (AGT) to form angiotensin (Ang) I in circulating blood which is then converted into Ang II by angiotensin converting enzyme (ACE) located at the luminal side of the endothelium in many tissues. More recently, an ACE homolog, ACE2, has been shown to cleave a single amino acid from Ang I to form Ang 1-9 and from Ang II to form Ang 1-7. (8;9;15)
The kidney possesses all the necessary RAS components to generate Ang II, as evidenced by the fact that its tissues contain much greater Ang II levels than the plasma (75;80) Indeed, intrarenal Ang II contributes to the regulation of transport function and renal hemodynamics. (24;27;50;66;74) The major fraction of Ang II present in the renal tissues is generated from AGT delivered to the kidney as well as from AGT locally produced by proximal tubule cells. (32;34) Although Ang I delivered to the kidney can also be converted into Ang II within the kidney (37;70), the secretion of renin from juxtaglomerular (JG) cells and its delivery to the interstitium and microcirculation can form de novo Ang I in the renal interstitium. (20) Furthermore, the presence of AGT in proximal tubule cells (31) also provides a source for local generation of Ang I in the early part of the nephron. (44;52) In addition, the localization of renin protein and mRNA expression in principal cells of collecting ducts (29;63;69) along with the evidence of AGT in urine (32) and ACE in the distal nephron segments (5;67) indicate that the late part of the nephron is a likely site for intrarenal Ang II formation. Although, the intratubular concentrations of Ang II in the distal nephron remains undetermined, several studies support an important role of Ang II in the regulation of distal nephron sodium reabsorption via activation of AT1 receptors (AT1R) located on the apical aspects of the distal tubules and collecting ducts. (1;37;61) The demonstration of a direct action of Ang II on the luminal amiloride-sensitive epithelial sodium channel (37;61) favors the scenario for a maintained stimulation of sodium reabsorption in the collecting duct and may help to explain the attenuation of the pressure-natriuretic in response to elevations in arterial blood pressure and the development and maintenance of hypertension during Ang II-dependent hypertension. (19;23;45) In this review, we summarize our current understanding of independent regulation of the intrarenal RAS, and discuss the hypothesis that inappropriate activation of renin in distal nephron segments and AGT synthesized and secreted by proximal tubules cells coordinate their actions to increase the formation of angiotensin peptides in the distal nephron segments, and thereby play a role in the development and progression of hypertension.
Augmentation of Intrarenal AGT and Ang II in Hypertension
The proximal tubule is a very active segment that participates in the reabsorption, degradation and secretion of small peptides and bigger molecules such as AGT. Zou et al. demonstrated accumulation of Ang II in the whole kidney that is prevented by treatment with AT1R blockers. (77) The internalization of Ang II in the PT can occur via AT1R into endosomes and intermicrovillar cleft vesicles (76) and mediated by megalin (17) which targets Ang II to degradation protecting the cell against Ang II accumulation. Thus it has been proposed that a balance between these two pathways will ultimately determine intracellular Ang II levels. (17) Chronic Ang II infusions lead to increased intrarenal Ang II content in the rat and mouse Ang II-infused models of hypertension; (79) (18) however local de novo Ang II formation due to enhanced intrarenal AGT production also contributes to the overall Ang II levels in the kidneys. (32;34) In vivo and in vitro studies have shown that Ang II stimulates intrarenal AGT mRNA and protein levels in proximal tubule (PT) cells (26;31;71). Chronic Ang II-infused rats have increases in renal AGT mRNA (31) and protein (30), as well as an enhancement of urinary excretion rate of AGT (32) which occurs in time- and dose-dependent manners.(34) This amplification mechanism may be responsible for the sustained or enhanced generation of AGT leading to continued intrarenal production of Ang II under conditions of elevated circulating Ang II concentrations. Intrarenally formed Ang II may exert an additive effect with the Ang II that is internalized via AT1R both contributing to the overall increased intrarenal Ang II content. AGT urinary excretion rate is closely correlated with systolic blood pressure and kidney Ang II content, but not with plasma Ang II concentration. (32;34) In addition, the increased urinary AGT is not just due to increased proteinuria or the development of hypertension since urinary protein excretion in volume-dependent hypertensive rats was significantly increased more than in Ang II-dependent hypertensive rats. (34) In contrast, urinary AGT excretion was significantly lower in volume-dependent hypertensive rats than in Ang II-dependent hypertensive rats. (34) Furthermore, the finding of intact AGT in urine suggests its presence throughout the nephron and, to the extent that renin and ACE are available along the nephron, there may be continued Ang I generation and Ang II conversion in segments beyond the proximal tubule. (11;12;69)
Kobori et al (33) also examined the relationship between urinary excretion of AGT and kidney AGT levels in Dahl salt sensitive rats (DS). Although this model is characterized by a low activity of circulating RAS, Ang I-converting enzyme inhibitors and AT1R antagonists ameliorate renal dysfunction in DS rats under a high salt diet. (35;57). Kidney AGT levels were significantly increased in DS rats fed a high salt diet compared to DS fed a low salt diet or Dahl salt resistant rats on either high or low salt diets. Urinary excretion of AGT was paradoxically increased in DS rats fed a high salt diet demonstrating an inappropriate response to increases in salt intake. These findings suggest that the intrarenal RAS, when inappropriately activated, contributes to the development of hypertension in DS rats. Thus, the hypertension that results when DS rats are fed a HS diet may be due, in part, to inappropriate and paradoxical increases in intrarenal AGT levels.
When intrarenal AGT formation is increased, some of the AGT is secreted into the tubular fluid and spills over into the distal tubule and eventually into the urine. (32;34) The presence of substrate allows for continued Ang II generation at distal nephron sites dependent on availability of renin and ACE. This issue has raised our interest in a more detailed investigation of renin present at distal nephron segments and on its role in Ang II-dependent hypertension.
Enhancement of Collecting Duct Renin in Ang II-dependent Hypertension
Renin in distal nephron segments may provide a possible pathway for Ang I generation from proximally delivered AGT. Although it is well established that plasma renin is primarily derived from the cells of the juxtaglomerular (JG) apparatus; renin transcript and protein have also been detected in renal tubular segments. (13;25;63;69;73) Renin is expressed in principal cells of connecting tubules and cortical and medullary collecting ducts of kidneys of normal rats and Ang II-dependent hypertensive rats (Figure 1). Several studies have shown that renin expressed by the tubular segments is differentially regulated renin synthesized in JG cells (22;29;46;63;69). CD renin protein and message are upregulated in chronic Ang II infused rats (63) and these increases are prevented by AT1R blockers (ARB) indicating an AT1R-mediated mechanism. (64) More recently, Kang et al (29) confirmed these findings using M-1 cells, a cortical collecting duct cell line of mouse origin, treated with Ang II and an AT1R blocker, olmesartan. These authors suggested that ARB treatment may exerts a dual beneficial effect, by permitting stimulation of renin production at its JG cells localization while inhibiting Ang II-mediated renin stimulation at the CD cells. (29)
Figure 1. Renin Immunoreactivity in Cortical and Medullary Collecting Ducts of Rat Kidneys.
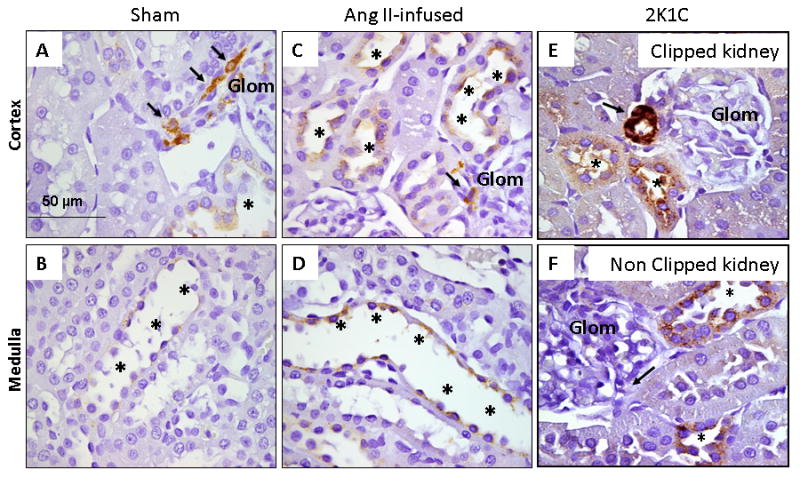
Panels A-F show the cortex (Panels A, C, and E) and medulla (Panels B and D) of kidney sections (3 μm) with specific renin immunostaining in sham rats (panels A-B), chronic Ang II-infused rats (panels C-D) and 2K1C Goldblatt hypertensive rats (panels E-F). Arrows show renin immunoreactivity in juxtaglomerular cells localization (DAB chromogen) in a sham (panel A), chronic Ang II-infused rat (panel C) and in the clipped kidney (panel E) and non-clipped kidney (panel F) from Goldblatt rats. Higher renin immunoreactivity (asterisks; DAB chromogen) are shown in the collecting ducts of the renal cortexes of chronic Ang II-infused rats (panel C) and both, clipped (panel E) and non-clipped (panel F) kidneys relative to sham kidney section (panel A). Glom: Glomerulus.
Immunohistochemistry studies specifically localized renin in the principal cells of CD but not in intercalated cells. The presence of renin immunoexpression as well as its transcript has been identified exclusively in principal cells of cortical and medullary CD and connecting tubules. (29;64;69) Using a sophisticated confocal imagine technique with multiphoton excitation fluorescence microscopy, Peti-Peterdi and associates reported quinacrine-labeled renin granular content in JG cells and CD cells. (59;60) Using this approach these authors reported the visualization of renin exocytosis was visualized in real time and at the individual renin granule level in response to a number of stimuli including Ang II. (29) Moreover, Rohrwasser et al. suggested in vitro renin secretion by isolated connecting tubules (69) and demonstrated renin and prorenin in urine from mice treated with high salt diet and amiloride. (68) Because of its small molecular size, renin can be partially filtered through the glomerular membrane and most of the filtered load is reabsorbed by the proximal tubule cells under normal physiological conditions. (41) Although plasma renin concentration seems to parallel renin urinary excretion, (42) it has been suggested that this depends on the balance between the filtered load and the Tm for renin reabsorption in the proximal tubule. In addition, Lalouel and associates have proposed that during manipulation of dietary sodium the renin excretion is inversely related to more moderated changes in plasma renin content, (39) therefore, they suggested that the presence of significant amounts of prorenin and renin in final urine indicates that renin and/or prorenin can be secreted through apical side of the cells into the lumen of distal nephron segments. (68)
The presence of renin mRNA in the renal medullary tissues of Ang II-dependent hypertensive rats also supports local synthesis. (64;65) We found in renal medullary tissues of chronic Ang II-infused rats (64) and in 2K1C Goldblatt hypertensive rats (65) increased renin transcript levels compared to control rats. However, it cannot be ruled out that some renin protein uptake by the CD cells may also contribute to the increased renin in CD cells. One possibility that could be implicated in mediating renin or prorenin uptake by CD cells is the recently cloned prorenin receptor -(P)RR- which has been demonstrated in mesangial cells, cortical renal arteries, and distal tubules of the kidney. (6;10;54). Muller et al (48) demonstrated the presence of (P)RR in kidneys of 2K1C Goldblatt hypertensive rats but there were no changes in (P)RR expression in either of the two kidneys compared to control. Therefore it is more likely that the upregulation of CD renin in both kidneys of 2K1C rats is due to an increased formation as suggested by the increased renin mRNA. (65) Nevertheless, the presence of (P)RR would be expected to increase Ang II tissue generation by binding either renin or prorenin and increasing the catalytic efficiency. (10;38;54) Along with the increased renin synthesis and secretion from the principal cells of the collecting ducts, (29;65) it is possible that (P)RR located in the distal nephron segments (62) may play a pivotal role to increase the efficiency of intratubular angiotensin peptide generation by anchoring renin or prorenin synthesized and released by principal cells. Nevertheless, accordingly with Nguyen et al., it is still an issue whether the affinity of the (P)RR is sufficient for binding renin in picomolar concentration ranges, given the fact that only ≈1% of soluble renin is expected to bind to the receptor. (4;54) Nguyen et al (53) described the immunoexpression of this receptor on the basolateral side of distal tubular cells as well as macula densa cells which may be particularly significant in regulating interstitial Ang II levels. Increases in renal interstitial fluid Ang II levels have been reported for two models of hypertension. Siragy and Carey (72) found that renal interstitial Ang II is increased in the wrapped kidney of rats with two-kidney, one-wrap Grollman hypertension. Nishiyama et al (55;56) reported that renal interstitial fluid Ang II concentrations are increased in rats infused with Ang II for 2 weeks. Because the renal interstitial values are greater than can be explained on the basis of equilibration with the plasma concentrations, it seems likely that local regulation of Ang II formation in the renal interstitial compartment and enhanced production of interstitial Ang II might be secondary to specialized Ang II-forming pathways or accumulation mechanisms. (55) The presence of (P)RR at the basolateral side of distal tubular segments cells may contribute to the pool of Ang II in this renal compartment.
Regulation of Renin in the Collecting Duct during Ang II-dependent Hypertension
The mechanisms responsible for the upregulation of renin in the CD during Ang II-dependent hypertension remain unclear. We have examined the gene expression of renin in the CD cells of different models of Ang II-dependent hypertension. (63-65) In chronic Ang II-infused rats, renin expressed by principal cells of cortical connecting tubules and cortical and medullary CD is augmented. (63) The increases in CD renin transcript as well as enzymatic activity in the medullary tissues of these rats indicates local synthesis and an adequate source of the enzyme available for cleaving AGT delivered into the tubular fluid from the proximal tubule segment. (64;65) Importantly, the coexistence of suppressions of JG renin and PRA in the chronic Ang II-infused rat model argues against a recapture effect being the major determinant of augmented renin in medullary regions. Thus in contrast to the inhibitory effect that Ang II exerts on JG renin, chronic Ang II infusions stimulate renin in the CD cells. (29;63)
Treatment with AT1R blockers also reduces arterial pressure; thus, data from the chronic Ang II-infused rats treated with ARB do not distinguish between the stimulatory effects of Ang II versus those due to the associated increases in arterial blood pressure. To determine the potential effects of arterial pressure, the two-kidney, one-clip (2K1C) Goldblatt hypertensive rat model was used to dissect the effects of high Ang II levels, which are present in both kidneys of 2K1C rats, from the effects of exposure to elevated arterial pressure, which is restricted to the non-clipped kidney (NCK). During 2K1C Goldblatt hypertension, a cascade of events initiated by the increased renin secretion from the clipped kidney (CK) and followed by early increases in circulating Ang II, leads to an increased blood pressure and inhibition of JG renin synthesis and release in the NCK. The elevated circulating Ang II levels return back towards normal after 2-3 weeks of renal unilateral clipping. (19) Recent studies demonstrated that there is augmented gene expression of renin in the collecting ducts of inner medullary tissues from both kidneys in 2K1C Goldblatt hypertensive rats indicating that the enhancement of local synthesis and stimulation of renin in the distal nephron segments occurs independently of blood pressure. The observation that the NCK is highly Ang II dependent even when circulating Ang II levels returned towards normal suggests that there is dissociation between the circulating Ang II levels and the renal Ang II dependency. Importantly, the fact that the Ang II content of the NCK is elevated even at a time when JG renin content and its transcript levels are markedly decreased (19;43;47) suggests a unique mechanism responsible for the enhanced intrarenal Ang II in NCK.
It is unlikely that circulating Ang II sustains the upregulation of distal nephron renin since plasma Ang II concentrations in 2K1C hypertensive rats return towards control levels. (19) However, because increases in plasma Ang II concentration occur during the early phases following renal unilateral clipping, (75) this could play a role in initiating the increases in intrarenal Ang II by augmentation of intrarenal AGT (30) and AT1R-mediated uptake. (78) Accordingly, the initial event caused by unilateral renal artery clipping may initiate the cascade responsible for intrarenal Ang II augmentation in the NCK. (7;19) Furthermore, it is also unlikely that circulating renin or prorenin is the stimulus for the upregulation of renin in the CD cells since chronic Ang II-infused rats exhibit stimulation of renin in distal nephron segments in a setting of marked suppression of PRA.(63) It is conceivable that the local amplification mechanism of intrarenal Ang II on distal nephron renin may allow a moderate increase in Ang II to further augment the intratubular and interstitial Ang II levels in order to achieve rapid homeostatic regulation of sodium balance as needed in a setting of volume depletion. Although this effect appears to be a positive feedback under pathologic conditions, the physiologic consequences of this mechanism would be to prevent or minimize volume and sodium depletion by enhancing sodium reabsorption to re-establish sodium balance and ultimately, to allow renin to return to normal levels. Thus, we have suggested (65) that in physiological conditions, this represents a feed forward mechanism that anticipates and prevents volume depletion. However, during overactivation of the RAS such as following unilateral renal artery clipping, or chronic exogenous infusions of Ang II; this stimulus would be sustained leading to further increases in local Ang II levels through the coordinated actions of AGT in the proximal tubule cells and renin in the distal nephron segments. We recognize that other possible downstream mediators such as distal nephron sodium reabsorption, changes in distal sodium delivery, and aldosterone might also be involved in the regulation of CD renin during Ang II-dependent hypertension.
Potential Role of Enhanced Collecting Duct Renin in Ang II-dependent Hypertension
There is evidence for a compartmentalized and regional distribution of Ang II within the kidney. (49;55) Renal interstitial fluid concentrations of Ang I and Ang II are much higher than in plasma. (2;55) Early studies showed that medullary Ang II levels are higher than the cortical levels in normal rats. (51) This concentrations increase even further in Ang II-infused hypertensive rats (Figure 2). It has been suggested that increases in Ang II content in association with high density of AT1 receptors may account for major actions of Ang II in regulating hemodynamics and tubular function in the renal medulla (21;58) Increased Ang II content in the renal medulla suggest a particular Ang II-forming pathway, or accumulation mechanism in medullary tissues that are subject to local regulation. We have hypothesized that the enhancement of renin gene and protein expression as well as its activity in the collecting duct cells is a driving force to increase intrarenal Ang II content in the medullary regions. (65) In human and rat kidneys it has been shown that ACE2, a homolog of ACE that metabolizes Ang II to generate Ang 1-7, is predominantly distributed in the deep renal cortex and outer medulla. (3;40) Angiotensin 1-7 opposes the actions of Ang II. (14) Recent evidence demonstrating that Ang II upregulates ACE and downregulates ACE2 in vitro and in vivo (16;28;36) highlights the importance of upregulated renin in the CD cells in the increased formation of Ang I and ultimately Ang II in the distal tubular segments. A reduction in ACE2 has been reported in hypertensive animal models such as the Sabra hypertensive rat and the spontaneously hypertensive rat. (9) Thereby, during Ang II-dependent hypertension, it is likely that the downregulation of ACE2 will decrease Ang II degradation and further increase intrarenal content of Ang II in the renal medulla. The promotion of an imbalance in the angiotensin peptide intrarenal content of Ang II and Ang 1-7 will contribute further to the progression of high blood pressure during hypertension. More studies using specific renin inhibitors during upregulation of CD renin conditions or specific targeting of the renin gene at the principal cells of the CD will help to elucidate the functional role of renin in the distal nephron segments during Ang II-dependent hypertension.
Figure 2. Angiotensin II levels in rat plasma and kidney cortex and medulla tissues.
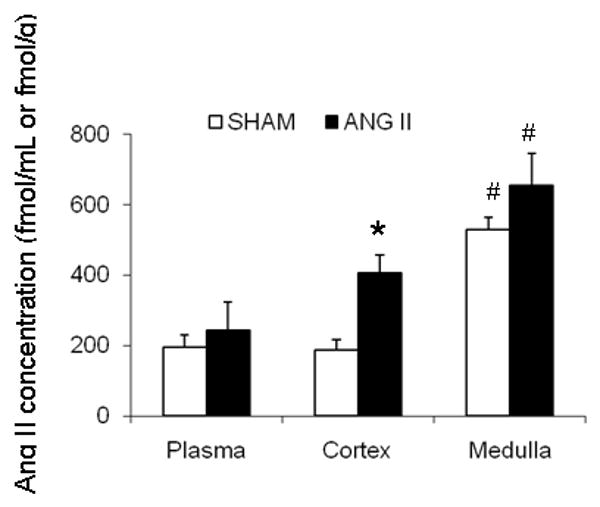
The levels of Ang II were measured by radioimmunoanalysis in plasma and kidney cortex and medulla samples from sham rats (n=8) and chronic Ang II-infused rats (n=8). Values are expressed in fmol/mL (plasma) or fmol/g (tissue). Values are means ± S.E. * p< 0.05 versus sham. # p< 0.05 kidney cortex versus kidney medulla.
Concluding Comments
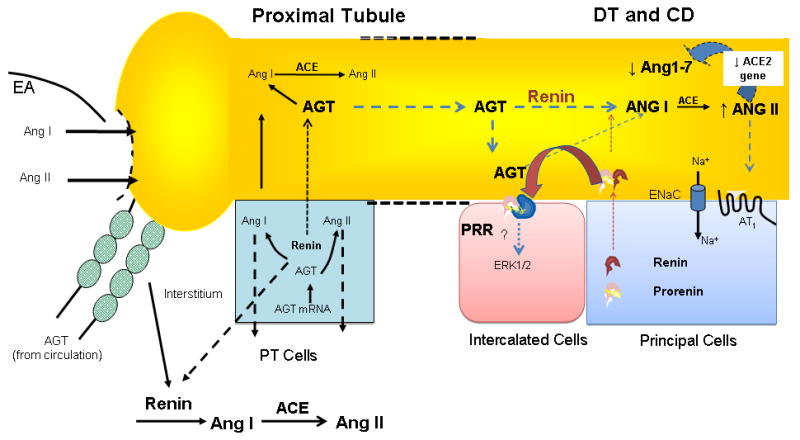
Emerging data indicate that collecting duct renin can be regulated independently from blood pressure. In the Figure 3) These data help to explain how, even during states of JG renin suppression, the non-clipped kidney can still maintain de novo intrarenal Ang II formation. From a functional perspective, enhanced AGT in urine of Ang II dependent hypertensive rats reflects spillover of AGT from proximal nephron segments and substrate availability throughout the nephron. Thus, augmented renin in the collecting ducts along with the local presence of the (P)RR and increased levels of Ang I suggest that distal nephron renin provides a pathway to increase the generation of this peptide from proximally delivery AGT. The presence of (P)RR at the surface of the collecting duct cells by increasing the catalytic activity for Ang I generation and anchoring renin may reduce washout of prorenin or renin into the urine. The availability of ACE in distal nephron segments along with reduction in ACE2 supports subsequent enhanced formation of Ang II. The enhancement of renin in the collecting ducts along with the presence of (P)RR lead to increased intrarenal Ang II formation and consequently decrease its degradation by favoring the downregulation of ACE2. Accordingly, in Ang II-dependent hypertension, renin and ACE in distal nephron segments may provide a critical final mechanism for Ang II formation which can act on transport systems to stimulate sodium reabsorption and consequently play a major role in the development and maintenance of high blood pressure.
Figure 3. Hypothetical Model of Intrarenal RAS.
Acknowledgments
We thank to Dr. Tadashi Inagami, PhD (Vanderbilt University) for providing renin antibody used for these investigations. The authors acknowledge excellence technical assistance from Victoria L Martin and Dale M. Seth. Digital images of histological specimens were obtained at the Imaging Core Facility of the Hypertension and Renal Center, Department of Physiology at Tulane University.
Sources of Funding: National, Lung, and Blood Institute (HL-26371), P20RR-017659 from the Institutional Developmental Award Program of the National Center for Research Resources, American Heart Association (0325269B).
Footnotes
The authors report no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barreto-Chaves MLM, Mello-Aires M. Effect of luminal angiotensin II and ANP on early and late cortical distal tubule HCO3- reabsorption. Am J Physiol-Renal Physiol. 1996;271:F977–F984. doi: 10.1152/ajprenal.1996.271.5.F977. [DOI] [PubMed] [Google Scholar]
- 2.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am J Physiol-Renal Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. [DOI] [PubMed] [Google Scholar]
- 3.Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R, et al. Enhanced renal immunocytochemical expression of ANG-(1-7) and ACE2 during pregnancy. Hypertension. 2003 Oct;42(4):749–53. doi: 10.1161/01.HYP.0000085220.53285.11. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DJ. Critical Review of Prorenin and (Pro)renin Receptor Research. Hypertension. 2008 May 1;51(5):1259–64. doi: 10.1161/HYPERTENSIONAHA.108.110924. [DOI] [PubMed] [Google Scholar]
- 5.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol-Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 6.Catanzaro DF. Physiological relevenace of renin/prorenin binding and uptake. Hypertens Res. 2005;28:97–105. doi: 10.1291/hypres.28.97. [DOI] [PubMed] [Google Scholar]
- 7.Cervenka L, Wang CT, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension. 1999;33:102–7. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- 8.Chappell MC, Tallant EA, Diz DI, Ferrario CM. The Renin-Angiotensin System and Cardiovascular Homeostasis. In: Husain A, Graham RM, editors. Drugs, Enzymes and Receptors of the Renin-Angiotensin System: Celebrating a Century of Discovery. Harwood Academic Publishers; 2000. pp. 3–22. [Google Scholar]
- 9.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002 Jun 20;417(6891):822–8. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 10.Danser AHJ, van Kats JP, Admiraal PJJ, Derkx FHM, Lamers JMJ, Verdouw PD, et al. Cardiac renin and angiotensins. Uptake from plasma versus in situ synthesis. Hypertension. 1994;24:37–48. doi: 10.1161/01.hyp.24.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;1:3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 12.Ding Y, Davisson RL, Hardy DO, Zhu LJ, Merrill DC, Catterall JF, et al. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem. 1997;272:28142–8. doi: 10.1074/jbc.272.44.28142. [DOI] [PubMed] [Google Scholar]
- 13.Dzau VJ. Significance of the vascular renin-angiotensin pathway. Hypertension. 1986 Jul;8(7):553–9. doi: 10.1161/01.hyp.8.7.553. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario CM. Angiotension-(1-7) and antihypertensive mechanisms. J Nephrol. 1998 Nov;11(6):278–83. [PubMed] [Google Scholar]
- 15.Ferrario CM. Contribution of Angiotensin-(1-7) to cardiovascular physiology and pathology. Curr Hypertens Rep. 2003 Apr;5(2):129–34. doi: 10.1007/s11906-003-0069-y. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol. 2006 Feb;290(2):C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol. 2005 Feb 1;288(2):F420–F427. doi: 10.1152/ajprenal.00243.2004. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, et al. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008 Sep 1;295(3):F772–F779. doi: 10.1152/ajprenal.00019.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan S, Fox J, Mitchell KD, Navar LG. Angiotensin and angiotensin converting enzyme tissue levels in two-kidney, one clip hypertensive rats. Hypertension. 1992;20:763–7. doi: 10.1161/01.hyp.20.6.763. [DOI] [PubMed] [Google Scholar]
- 20.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, Physiology, and Molecular Biology of Renin Secretion. Physiol Rev. 1990;70:1067–116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 21.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT(1) receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002 Jan;282(1):F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27:1337–40. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- 23.Huang WC, Navar LG. Effects of unclipping and converting enzyme inhibition on bilateral renal function in 2 kidney 1 clip Goldblatt hypertensive rats. Kidney Int. 1983;23:816–22. doi: 10.1038/ki.1983.100. [DOI] [PubMed] [Google Scholar]
- 24.Imig JD, Navar GL, Zou LX, O'Reilly KC, Allen PL, Kaysen JH, et al. Renal endosomes contain angiotensin peptides, converting enzyme, and AT1A receptors. Am J Physiol-Renal Physiol. 1999;277:F303–F311. doi: 10.1152/ajprenal.1999.277.2.F303. [DOI] [PubMed] [Google Scholar]
- 25.Inagami T, Okamura T, Clemens D, Celio MR, Naruse K, Naruse M. Local generation of angiotensin in the kidney and in tissue culture. Clin Exp Hypertens A. 1983;5(78):1137–49. doi: 10.3109/10641968309048847. [DOI] [PubMed] [Google Scholar]
- 26.Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, et al. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol-Renal Physiol. 1999;276:F218–F227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 27.Ingert C, Grima M, Coquard C, Barthelmebs M, Imbs JL. Contribution of angiotensin II internalization to intrarenal angiotensin II levels in rats. Am J Physiol Renal Physiol. 2002 Nov;283(5):F1003–F1010. doi: 10.1152/ajprenal.00322.2001. [DOI] [PubMed] [Google Scholar]
- 28.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004 May;43(5):970–6. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 29.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The Collecting Duct Is the Major Source of Prorenin in Diabetes. Hypertension. 2008 Jun 1;51(6):1597–604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–35. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001 Mar;12:431–9. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002 Feb;61(2):579–85. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003 Mar;41(3):592–7. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension. 2003 Jan;41(1):42–9. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kodama K, Adachi H, Sonoda J. Beneficial effects of long-term enalapril treatment and low-salt intake on survival rate of dahl salt-sensitive rats with established hypertension. J Pharmacol Exp Ther. 1997 Nov;283(2):625–9. [PubMed] [Google Scholar]
- 36.Koka V, Huang XR, Chung ACK, Wang W, Truong LD, Lan HY. Angiotensin II Up-Regulates Angiotensin I-Converting Enzyme (ACE), but Down-Regulates ACE2 via the AT1-ERK/p38 MAP Kinase Pathway. American Journal of Pathology. 2008 May 1;172(5):1174–83. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, et al. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003 Aug;42(2):195–9. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 38.Krebs C, Hamming I, Sadaghiani S, Steinmetz OM, Meyer-Schwesinger C, Fehr S, et al. Antihypertensive therapy upregulates renin and (pro)renin receptor in the clipped kidney of Goldblatt hypertensive rats. Kidney Int. 2007 Jun 27; doi: 10.1038/sj.ki.5002408. [DOI] [PubMed] [Google Scholar]
- 39.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, et al. Effects of dietary sodium and genetic background on angiotensinogen and Renin in mouse. Hypertension. 2002 May;39(5):1007–14. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 40.Lely AT, Hamming I, van Goor H, Navis GJ. Renal ACE2 expression in human kidney disease. J Pathol. 2004 Dec;204(5):587–93. doi: 10.1002/path.1670. [DOI] [PubMed] [Google Scholar]
- 41.Leyssac PP. Micropuncture study of renin release at the single nephron level. Evidence for some release directly into the circulating blood. Renal Physiol. 1978;1:61–73. [Google Scholar]
- 42.Lumbers ER, Skinner SL. The occurrence and assay of renin in human urine. Aust J Exp Biol Med Sci. 1969 Apr;47(2):251–62. doi: 10.1038/icb.1969.26. [DOI] [PubMed] [Google Scholar]
- 43.Mendelsohn FAO. Failure of suppression of intrarenal angiotensin II in the contralateral kidney of one clip, two kidney hypertensive rats. Clin Exp Pharmacol Physiol. 1980;7:219–23. doi: 10.1111/j.1440-1681.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell KD, Jacinto SM, Mullins JJ. Proximal tubular fluid, kidney, and plasma levels of angiotensin II in hypertensive ren-2 transgenic rats. Am J Physiol-Renal Physiol. 1997;273:F246–F253. doi: 10.1152/ajprenal.1997.273.2.F246. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell KD, Navar LG. Intrarenal actions of angiotensin II in the pathogenesis of experimental hypertension. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. 2. New York: Raven Press, Ltd.; 1995. pp. 1437–50. [Google Scholar]
- 46.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, et al. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–9. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morishita R, Higaki J, Okunishi H, Tanaka T, Ishii K, Nagano M, et al. Changes in gene expression of the renin-angiotensin system in two-kidney, one clip hypertensive rats. J Hypertens. 1991;9:187–92. doi: 10.1097/00004872-199102000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Muller DN, Klanke B, Feldt S, Cordasic N, Hartner A, Schmieder RE, et al. (Pro)Renin Receptor Peptide Inhibitor “Handle-Region” Peptide Does Not Affect Hypertensive Nephrosclerosis in Goldblatt Rats. Hypertension. 2008 Mar 1;51(3):676–81. doi: 10.1161/HYPERTENSIONAHA.107.101493. [DOI] [PubMed] [Google Scholar]
- 49.Navar LG, Harrison-Bernard LM, Imig JD. Compartmentalization of intrarenal angiotensin II. In: Ulfendahl HR, Aurell M, editors. Renin-Angiotensin. London: Portland Press; 1998. pp. 193–208. [Google Scholar]
- 50.Navar LG, Harrison-Bernard LM, Wang CT, Cervenka L, Mitchell KD. Concentrations and actions of intraluminal angiotensin II. J Am Soc Nephrol. 1999;10:S189–S195. [PubMed] [Google Scholar]
- 51.Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Sem Nephrol. 1997;17:412–22. [PubMed] [Google Scholar]
- 52.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol. 1994;5:1153–8. doi: 10.1681/ASN.V541153. [DOI] [PubMed] [Google Scholar]
- 53.Nguyen G. Increased cyclooxygenase-2, hyperfiltration, glomerulosclerosis, and diabetic nephropathy: put the blame on the (pro)renin receptor? Kidney Int. 2006;70(4):618–20. doi: 10.1038/sj.ki.5001723. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002 Jun;109(11):1417–27. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid angiotensin I and angiotensin II concentrations during local angiotensin-converting enzyme inhibition. J Am Soc Nephrol. 2002 Sep;13(9):2207–12. doi: 10.1097/01.asn.0000026610.48842.cb. [DOI] [PubMed] [Google Scholar]
- 56.Nishiyama A, Seth DM, Navar LG. Renal interstitial fluid concentrations of angiotensins I and II in anesthetized rats. Hypertension. 2002;39:129–34. doi: 10.1161/hy0102.100536. [DOI] [PubMed] [Google Scholar]
- 57.Otsuka F, Yamauchi T, Kataoka H, Mimura Y, Ogura T, Makino H. Effects of chronic inhibition of ACE and AT1 receptors on glomerular injury in Dahl salt-sensitive rats. Am J Physiol Regulatory Integrative Comp Physiol. 1998;274:R1797–R1806. doi: 10.1152/ajpregu.1998.274.6.R1797. [DOI] [PubMed] [Google Scholar]
- 58.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol. 2006 Jun;290(6):F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 59.Peti-Peterdi J. Multiphoton imaging of renal tissues in vitro. Am J Physiol Renal Physiol. 2005 Jun;288(6):F1079–F1083. doi: 10.1152/ajprenal.00385.2004. [DOI] [PubMed] [Google Scholar]
- 60.Peti-Peterdi J, Fintha A, Fuson AL, Tousson A, Chow RH. Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol. 2004 Aug;287(2):F329–F335. doi: 10.1152/ajprenal.00420.2003. [DOI] [PubMed] [Google Scholar]
- 61.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002 May;13(5):1131–5. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 62.Prieto-Carrasquero MC, Botros FT, Martin VL, Gonzalez-Villalobos RA, Sato R, Kobori R, et al. Prorenin Receptor in Distal Nephron Segments of 2K1C Goldblatt Hypertensive Rats. Hypertension. 2008 Oct 1;52(4):e34–131. [Google Scholar]
- 63.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004 Aug;44(2):223–9. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005 May 3;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE, et al. Collecting Duct Renin Is Upregulated in Both Kidneys of 2-Kidney, 1-Clip Goldblatt Hypertensive Rats. Hypertension. 2008 Jun 1;51(6):1590–6. doi: 10.1161/HYPERTENSIONAHA.108.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Re RN. The intracrine hypothesis and intracellular peptide hormone action. Bioessays. 2003 Apr;25(4):401–9. doi: 10.1002/bies.10248. [DOI] [PubMed] [Google Scholar]
- 67.Redublo Quinto BM, Camargo de Andrade MC, Ronchi FA, Santos EL, ves Correa SA, Shimuta SI, et al. Expression of angiotensin I-converting enzymes and bradykinin B2 receptors in mouse inner medullary-collecting duct cells. International Immunopharmacology. 2008 Feb;8(2):254–60. doi: 10.1016/j.intimp.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 68.Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T, et al. Renin and kallikrein in connecting tubule of mouse. Kidney Int. 2003 Dec;64(6):2155–62. doi: 10.1046/j.1523-1755.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 69.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999 Dec;34(6):1265–74. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 70.Rosivall L, Navar LG. Effects on renal hemodynamics of intra-arterial infusions of angiotensins I and II. Am J Physiol. 1983;245:F181–F187. doi: 10.1152/ajprenal.1983.245.2.F181. [DOI] [PubMed] [Google Scholar]
- 71.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. American Journal of Physiology-Endocrinology and Metabolism. 1992;263:E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 72.Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–42. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- 73.Taugner R, Hackenthal E, Inagami T, Nobiling R, Poulsen K. Vascular and tubular renin in the kidneys of mice. Histochemistry. 1982;75(4):473–84. doi: 10.1007/BF00640599. [DOI] [PubMed] [Google Scholar]
- 74.van Kats JP, Schalekamp MA, Verdouw PD, Duncker DJ, Danser AH. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int. 2001 Dec;60(6):2311–7. doi: 10.1046/j.1523-1755.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 75.Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physiol-Renal Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 76.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002 Jan;39(1):116–21. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 77.Zou L, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension. 1996;27(part 2):658–62. doi: 10.1161/01.hyp.27.3.658. [DOI] [PubMed] [Google Scholar]
- 78.Zou L, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal ANG II augmentation in ANG II-infused rats. Hypertension. 1996;28:669–77. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 79.Zou LX, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension. 1996;28:669–77. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 80.Zou LX, Hymel A, Imig JD, Navar LG. Renal accumulation of circulating angiotensin II in angiotensin II-infused rats. Hypertension. 1996;27:658–62. doi: 10.1161/01.hyp.27.3.658. [DOI] [PubMed] [Google Scholar]


