Abstract
An IHC survey using several monoclonal antibodies against different portions of the rat mineralocorticoid receptor (MR) molecule demonstrated significant specific MR immunoreactivity in the ovary, prompting further study of the localization of MR and of determinants of extrinsic MR ligand specificity, 11β-hydroxysteroid dehydrogenase (11β-HSD) types 1 and 2, and hexose-6-phosphate dehydrogenase (H6PDH). MR expression (real-time RT-PCR and Western blot) did not differ significantly in whole rat ovaries at early diestrus, late diestrus, estrus, and a few hours after ovulation. MR immunostaining was most intense in corporal lutea cells, light to moderate in oocytes and granulosa cells, and least intense in theca cells. Light immunoreactivity for 11β-HSD2 occurred in most cells, with some mural granulosa cells of mature follicles staining more strongly. The distribution of immunoreactivity for 11β-HSD1 and H6PDH required to generate NADPH, the cofactor required for reductase activity of 11β-HSD1, was similar, with the most-intense staining in the cytoplasm of corporal lutea and theca cells and light or no staining in the granulosa and oocytes. MR function in the ovary is as yet unclear, but distinct patterns of distribution of 11β-HSD1 and -2 and H6PDH suggest that the ligand for MR activation in different cells of the ovary may be differentially regulated. (J Histochem Cytochem 57:633–641, 2009)
Keywords: corticosterone, cortisol, aldosterone
Ovarian function is regulated by the complex interaction of many hormones, including the adrenal steroids cortisol and corticosterone, the latter being the primary glucocorticoid in rats and other animals lacking the adrenal 17-hydroxylase. An integral part of the regulation of hormonal action is the regulation of the expression and activity of their receptors. Although expression of the glucocorticoid receptor (GR) in the ovary has been studied, less attention has been given to the mineralocorticoid receptor (MR). The intrinsic affinity of the MR is similar for the primary endogenous mineralocorticoid aldosterone and the glucocorticoids cortisol and corticosterone, and 10-fold greater than that of the GR for cortisol and corticosterone (Arriza et al. 1987). Because circulating concentrations of cortisol and corticosterone are normally 100–1000 times those of aldosterone, in the absence of factors that confer extrinsic specificity for aldosterone, for example, in many non-epithelial cells, MRs are primarily occupied by glucocorticoids. The enzyme 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) converts cortisol and corticosterone to inactive metabolites. It is expressed in greatest amounts in aldosterone target cells of transport epithelia of kidney tubules, colon, and salivary gland, allowing aldosterone to access the MR; however, it is also present in the ovary, oviduct, uterus, and placenta of the rat (Roland and Funder 1996). 11β-HSD2 activity requires the cofactor nicotinamide adenine dinucleotide (NAD+). 11β-HSD1 is an enzyme in the endoplasmic reticulum (ER) that has bidirectional activity, but acts as a reductase in most tissues, converting inactive 11-dehydro metabolites into active glucocorticoids and amplifying the impact of circulating levels of glucocorticoids upon the GR and MR (Seckl and Walker 2001). The obligate cofactor for 11β-HSD1 reductase activity is reduced nicotinamide adenine dinucleotide phosphate (NADPH or NADP+). Hexose-6-phosphate dehydrogenase (H6PDH) is required to generate NADPH from NADP within the ER. Without H6PDH activity, 11β-HSD1 acts as a dehydrogenase (Atanasov et al. 2004; Banhegyi et al. 2004; Tomlinson et al. 2004). 11β-HSD enzymes are crucial for the regulation of intracellular concentrations of cortisol and corticosterone, thus glucocorticoid activation of GR, as well as MR.
mRNA for the MR, as well as 11β-HSD1, 11β-HSD2, and GR, have been detected in rat ovaries (Tetsuka et al. 1999). However, the dearth of specific antibodies that consistently recognize the MR protein in both Western blots and IHC has hindered its study until recently (Gomez-Sanchez et al. 2006), and little is known about MR protein expression or function of the MR in the ovary. We produced a panel of mouse monoclonal antibodies against peptides corresponding to several different epitopes of the rat MR, some of which are the same in the human MR, for use in several types of assays, including immunoprecipitation followed by Western blot using antibodies against different epitopes (Gomez-Sanchez et al. 2006). Antibodies producing both specific immunostaining of MR in kidney cortical collecting duct epithelia and a single band in extracts of CHO cells transfected with the rat MR cDNA were used in these studies to demonstrate the distribution of the MR and enzymes known to regulate the ligands that activate the MR in the ovary.
The present studies validate a set of antibodies as tools for future studies of the role of the MR and its primary ligands, corticosterone, cortisol, and aldosterone, in the ovary.
Methods
Animals
Sprague-Dawley rats were housed in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and were used under an approved Veterans Affairs Institutional Animal Care and Use Committee protocol. Rats were deeply anesthetized with isoflurane, the ascending vena cava was cut to exsanguinate the animals, and tissues for the isolation of mRNA and protein were immediately removed, blotted, and frozen in liquid nitrogen. For IHC, the ascending vena cava was cut and the animals were gravity perfused with heparinized saline, followed by STF (Streck's Tissue Fixative, Streck Laboratories; La Vista, NE), a non-crosslinking fixative, before the removal of tissues.
Real-time RT-PCR
To assess the expression of mRNA for ovarian MR, 11β-HSD1, and 11β-HSD2 at different times in the estrus cycle, the cycles of the rats were synchronized with luteinizing hormone-releasing hormone (LHRH) (40 μg/rat; ∼200 μg/kg), a protocol used successfully in our laboratory to prepare recipient dams for embryo transfer, and ovaries were taken from four rats, each at early diestrus, late diestrus, estrus, and late estrus, 8–10 hr after ovulation (Filipiak and Saunders 2006). Ovaries were also taken 8–12 hr after coitus from rats super-ovulated by the administration of LHRH (40 μg/rat; ∼200 μg/kg) on day 4, pregnant mare serum gonadotropin (300 IU/kg) on day 2, and human chorionic gonadotropin (HCG) (300 IU/kg) on day 0 at the time one-celled embryos were harvested from the fallopian tube for another study (Filipiak and Saunders 2006). Total RNA was extracted with Tri-Reagent (MRC; Cincinnati, OH), resuspended in diethyl pyrocarbonate–treated H2O, DNase treated using a TURBO DNA-free kit (Ambion; Austin, TX), and quantified by spectrophotometry. Five μg of RNA was reverse transcribed with 0.5 μg of T12VN primer and Superscript III (Invitrogen; Carlsbad, CA) in a final volume of 20 μl, and the reaction was carried out following the manufacturer's suggested protocol. Primers for MR (sense: 5′-CACGGCTCTTTTGAAGAAGACT-3′; antisense: 5′-ATCTGTTTGGTGTGTGGAGATG-3′; product size: 90 bp) and for 11β-HSD2 (sense: 5′-TCATCACCGGTTGTGACACT-3′; antisense: 5′-CACGCAGTTCTAGAGCACCA-3′; product size: 120 bp) were designed with Primer3 software (Rozen and Skaletsky 2000) and checked for the absence of cross-reactivity by BLAST search. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and 11β-HSD1 primers were previously described (Gomez-Sanchez et al. 2008). Real-time PCR was performed with 1 μl of RT product, 0.1 μM each of primer, 0.2 mM deoxynucleotide triphosphates, SYBR Green I (1:20,000 final concentration; Molecular Probes, Eugene, OR), and 1 μl of Titanium Taq DNA polymerase (Clontech; Palo Alto, CA). Cycling conditions were 1 min at 95C, followed by 50 cycles of 15 sec at 95C, 15 sec at 60C, and 1 min at 72C. The PCR was carried out in an i-Cycler thermal cycler (Bio-Rad Laboratories; Hercules, CA), and real-time data were collected during the extension phase of the PCR cycle. Threshold cycle values were obtained at the log phase of each gene amplification. After PCR amplification, the specificity of the PCR was confirmed by melting temperature determination of the PCR product and electrophoretic analysis in 2% agarose gels. The PCR product was cloned into the pCR2.1 TOPO vector to be used for the standard curve (Invitrogen Life Technologies, Inc.) and were diluted to add 10–108 molecules/PCR. Results are expressed in arbitrary units and standardized by molecules of GAPDH.
Western Blot Analyses
To enrich the Western blot sample for MR, protein was isolated from the postmitochondrial fraction comprising cytosol and microsomes, but not nucleus or mitochondria, of ovaries, kidneys, and hippocampus (positive control) of unsynchronized female rats by homogenizing 1 g of tissue in 5 ml of buffer [25% sucrose with 0.05 M Hepes, 0.05 M molybdate, and protease inhibitors (Roche Diagnostics; Indianapolis, IN), pH 7.36, 4C] and centrifuging at 4C for 10 min at 3000 × g, then for 60 min at 20,000 × g. Western blots in this study were done as described previously (Gomez-Sanchez et al. 2006). Briefly, 7% PAGE gels were transferred to polyvinylidene difluoride (PVDF) membranes using a wet-transfer technique, blocked with 1% Carnation dry skim milk in TBS with 0.05% Tween-20. The membranes were incubated with the primary antibody, monoclonal antibody rMRN 64–82 (clone 2B7) (Gomez-Sanchez et al. 2006), raised against a peptide corresponding to aa 64–82 of the MR molecule, overnight, washed, and developed using a goat anti-mouse peroxidase–labeled antibody (Jackson Immunoresearch; West Grove, PA). The membranes were developed using a chemiluminescence substrate (SuperSignal West Pico; Pierce Biotechnology, Rockford, IL) and recorded using an auto-radiographic film (Fuji). Western blots for 11β-HSD1 and -2 and H6PDH were done as previously described by us (Gomez-Sanchez et al. 2003,2008) using microsomal protein. Microsomes comprising primarily ER and plasma membrane were isolated by differential centrifugation as we have done before (Morita et al. 1996), then solubilized using Laemmli buffer (Laemmli 1970) and run in a 12% PAGE (11β-HSD) or 7.5% PAGE (H6PDH), transferred by semidry blot to a PVDF membrane, dried, and blocked with 1% non-fat milk. The antibodies used were sheep anti-11β-HSD2 and anti-H6PDH polyclonal antibodies produced by us (Gomez-Sanchez et al. 2001,2008) and a rabbit anti-11β-HSD1 polyclonal antibody kindly provided by Zigmund Krozowski (Brereton et al. 2001). The blots were then incubated with peroxidase-labeled donkey anti-rabbit or anti-sheep second antibodies and developed using West Pico chemiluminescence substrate from Pierce Chemical Co. Liver and adipose tissue were the positive controls for 11β-HSD1 and H6PDH; kidney for 11β-HSD2.
Immunohistochemistry
Tissues were emersion fixed for another 18–24 hr after in situ perfusion before embedding in paraffin. Sections of kidney for MR and 11β-HSD2 and liver for 11β-HSD1 and H6PDH were included on each slide as a positive control; a negative control (no primary antibody) was included for each preparation. Primary monoclonal antibodies against peptides comprising the aa sequences 64 to 82, (rMR64-82-2D6) and 79 to 97 (rMR79-97-3F10) of the MR are presented. Several other MR antibodies were also used successfully with similar results. The section was blocked with 1% skim milk and donkey anti-mouse IgG was used as second antibody. The antibodies against 11β-HSD2, 11β-HSD1, and H6PDH were those used for the Western blot analyses described above, followed by donkey anti-rabbit or anti-sheep biotin-labeled antibodies and detected using the streptavidin-peroxidase system (Zymed Laboratories; San Francisco, CA) and DAB (Sigma; St. Louis, MO) and counterstained with Gil hematoxylin.
Results
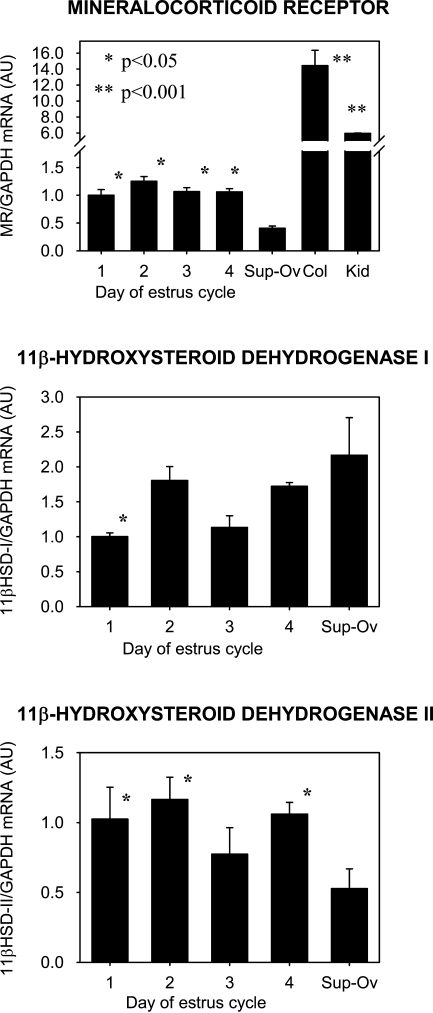
Real-time RT-PCR results are expressed as the ratio of the mRNA of interest to that of GAPDH. In Figure 1, relative amounts of mRNA for MR are shown in the top graph, 11β-HSD1 in the middle, and 11β-HSD2 in the bottom graph. Considerable amounts of MR mRNA are expressed in the ovary, albeit in concentrations that are less than those in the colon or kidney, p<0.001 (Figure 1, top graph). There was no significant difference in MR message detected at four different times of the estrus cycle in synchronized rats. The concentration of MR mRNA was significantly reduced in super-ovulated ovaries collected a few hours after ovulation and fertilization, when one-celled embryos are still in the fallopian tube, compared with that in estrus-synchronized but not super-ovulated ovaries, p<0.05. 11β-HSD1 mRNA was significantly increased in the super-ovulated ovaries, compared with day 1 of estrus, whereas 11β-HSD2 mRNA in the super-ovulated ovaries was decreased, compared with days 1, 2, and 4, p<0.05. However, variability in message levels was great.
Figure 1.
Densitometric summary, expressed as the ratio of mRNA for mineralocorticoid receptor (MR) (top), 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) (middle), and 11β-HSD2 (bottom) to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in rat ovaries (on days 1–4 of the estrus cycle), super-ovulated ovaries, colon, and kidney. Estrus cycles of the rats were synchronized with luteinizing hormone-releasing hormone (LHRH), and ovaries were taken at day 1 (early diestrus), day 2 (late diestrus), day 3 (estrus), and day 4 (late estrus). Sup-Ov: super-ovulated ovaries taken from rats 8–12 hr after coitus after super-ovulation induced by the administration of LHRH (40 μg/rat; ∼200 μg/kg on day 4), pregnant mare serum gonadotropin (300 IU/kg on day 2), and human chorionic gonadotropin (300 IU/kg on day 0). Col: colon; Kid: kidney. Error bars represent standard error of the mean. *Denotes significance compared with the values of mRNA from super-ovulated ovaries.
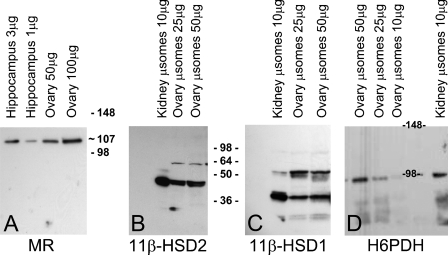
Representative Western blot analyses for MR, 11β-HSD1, 11β-HSD2, and H6PDH are presented in Figure 2. MR immunoreactivity of 1 μg and 3 μg of cytosolic protein from hippocampus and 50 μg and 100 μg from ovary for MR is seen in Figure 2A. Although quantities are only estimated by Western blot analysis, expression of the MR in the ovary is significant and approximately one tenth that in the hippocampus, the tissue with the highest expression of MR, and of the same order of magnitude as that in the kidney (data not shown). The blots shown in Figures 2B–2D were stained with antibodies against 11β-HSD1, 11β-HSD2, and H6PDH in microsomal protein of ovaries and kidneys. Strong bands were detected at ∼36 kDa and 45 kDa, as expected, for 11β-HSD1 and -2, respectively. These are both polyclonal antibodies, and it is not clear what the additional stained bands represent. Expression of both 11β-HSD enzymes is only slightly less in the ovary than in the kidney, that of H6PDH, at ∼98 kDa, is approximately one fifth that of the kidney.
Figure 2.
Western blot analysis for MR in hippocampus and ovary (A), and for 11β-HSD2 (B), 11-βHSD1 (C), and H6PDH (D) in kidney and ovary. Whole-tissue protein was used for gel A, microsomal protein was used for gels B–D.
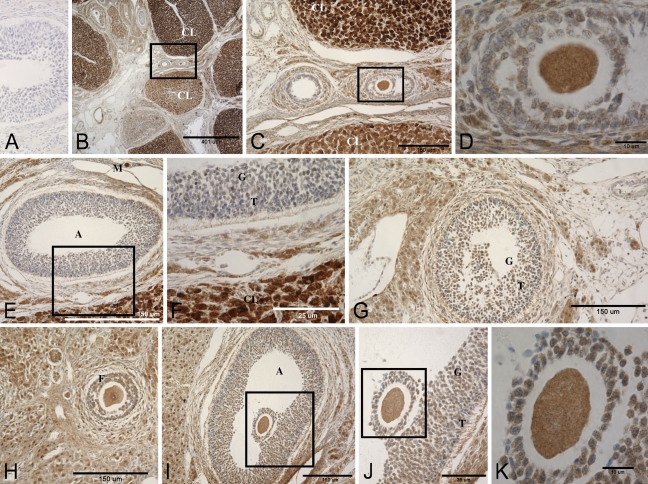
Immunohistochemistry for the MR and the three enzymes is shown in Figures 3 and 4. Figure 3 is a composite of representative photomicrographs of rat ovary sections stained with two different antibodies against the MR molecule, showing an almost identical distribution of staining. Negative controls were uniformly negative. Corpora lutea (CL) in different stages of dissolution from several cycles are seen in Figures 3B and 3C (Navarro 2005). MR immunostaining in the steroidogenic cells of the youngest most-active CL was more intense than that of follicular cells, granulosa and theca. Large cells with abundant cytoplasm in the stroma, probably hilar or Berger cells, also expressed MR abundantly. Due to processing, oocytes in more-mature follicles tended to be slightly shrunken and retracted from the cumulus and granulosa cells. The cytoplasm and nuclei of oocytes at various stages of development had fine granular MR immunostaining that did not change appreciably as the ova matured. Less-intense, primarily cytoplasmic MR immunoreactivity was seen in granulosa and cumulus cells of developing follicles, with lighter to no staining in theca cells. As in other tissues, MR immunoreactivity was found in macrophages and vascular smooth muscle of veins and arteries (Gomez-Sanchez et al. 2006).
Figure 3.
Photomicrographs of immunohistochemical detection of the MR in a rat ovary using two different monoclonal antibodies. (A) Control, no primary antibody. (B–F) Antibody against aa 79–97 of the MR sequence. (G–K) Antibody against aa 64–82. (C,D) Higher magnifications of the boxed areas in B and C, respectively. (F) Higher magnification of the boxed area in E. (J,K) Successive magnifications of I and J. A, follicular antrum; CL, corpora lutea; T, theca cells; G, granulosa cells; M, macrophage.
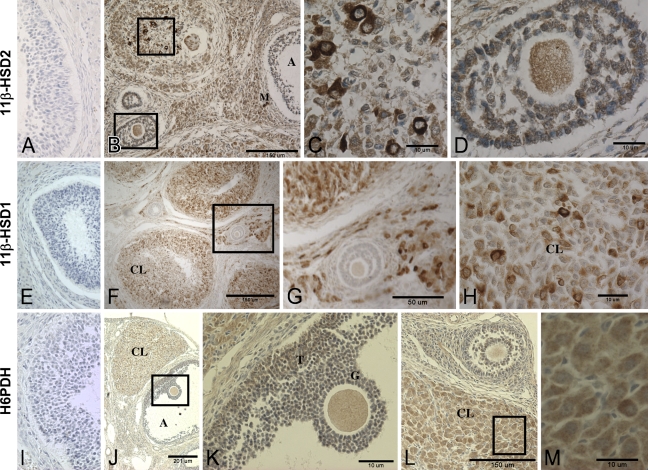
Figure 4.
Photomicrographs of immunohistochemical detection of 11β-HSD1, 11β-HSD2, and H6PDH in a rat ovary. (A,E,I) Negative controls for 11β-HSD1, 11β-HSD2, and H6PDH, respectively. (A–D) 11β-HSD2. (C,D) Higher magnifications of areas boxed in B. The follicle in upper left of B appears to be atretic and includes several active macrophages. (E–H) 11β-HSD1. (G) Higher magnification of boxed area in F. (H) Higher magnification of a corpora lutea showing intense 11β-HSD1 immunoreactivity in a few cells. (I–M) H6PDH. (K,M) Higher magnifications of boxed areas in J and L, respectively, showing the oocyte with preovulatory cumulus cells (K) and corpora lutea (M). A, follicular antrum; CL, corpora lutea; T, theca cells; G, granulosa cells; M, macrophage.
As shown in Figure 4, all three microsome-associated enzymes were detected in the cytoplasm, not nuclei. 11β-HSD2 immunoreactivity had a distribution similar to that of the MR in the ovary, with a few corpora lutea cells staining much more intensely than others and moderate staining of the follicular cells and ova. 11β-HSD1 staining was primarily of the corpora lutea and stromal cells, with much lighter staining in the ova and very little in the follicular cells. H6PDH immunoreactivity was similar in distribution to that of 11β-HSD1, also with the most-prominent staining in the corpora lutea and ova, some in the theca cells, and little in the granulosa cells.
Discussion
The MR, like other members of the steroid hormone transcription factor superfamily, has both genomic and more rapid non-genomic effects through common signaling pathways (Haseroth et al. 1999; Rossol-Haseroth et al. 2004; Fuller and Young 2005; Funder 2005; Grossmann et al. 2005; Karst et al. 2005). Its conformation, and thus the epitopes exposed to the detection antibody, changes depending upon the type of cell and the structural, chaperone, and cofactor proteins to which it is bound in different locations within the cell (Viengchareun et al. 2007). This explains the slightly different intracellular patterns of staining in the same cell type by the MR antibody against aa 64–82, which detects protein in nuclei and cytoplasm fairly equally, and the MR 79–97 antibody, which preferentially stains cytoplasmic MR (Gomez-Sanchez et al. 2006).
Aldosterone target cells would be expected to express MR and 11β-HSD2 and/or 11β-HSD1 with no H6PDH, resulting in net dehydrogenase activity and the conversion of active to inactive glucocorticoid (Atanasov et al. 2004; Banhegyi et al. 2004; Tomlinson et al. 2004). Glucocorticoids play a major role in ovarian function (Alliston et al. 2000). However, whether they act through the GR, the MR, or both GR and MR, as they do in most non-epithelial tissues (De Kloet et al. 1998; Brennan and Fuller 2000; Seckl and Walker 2001; Michael et al. 2003), is not clear.
Cyclical changes in adrenal steroid receptors, 11β-HSD1, and/or 11β-HSD2 message in total RNA isolated from a variety of species have been reported, but the results are not consistent, probably due to differences in species and conditions of study (Tetsuka et al. 1999,2003; Thurston et al. 2003a,b; Fru et al. 2006; Jonas et al. 2006). Ovaries of animals such as the rat, in which many ova mature at once and the estrus cycle is relatively short, comprise many corpora from several cycles in various stages of degeneration. Tetsuka et al. (1999) found that gonadotropin treatment of immature non-cycling rats increased ovarian mRNA for 11β-HSD1, decreased that for 11β-HSD2 and MR, and left GR mRNA levels unchanged . We did not find consistent cyclical changes in message for MR, 11β-HSD1, or 11β-HSD2, probably because our animals were postpubertal rats whose ovaries comprised corpora from several previous cycles in various stages of involution; nor was there a measurable difference in protein by Western blot for the 11β-HSD enzymes, although this is not a very quantitative method.
Our IHC studies demonstrated the presence of both 11β-HSD enzymes, as well as H6PDH, in many of the same types of ovarian cells as the MR, and thus did not clearly differentiate between aldosterone target cells and cells in which MRs are primarily activated by glucocorticoids. Immunoreactivity for 11β-HSD2 was consistently greater than for 11β-HSD1 in rat granulosa-theca cells, with little to no immunostaining for H6PDH in granulosa cells. MR staining was seen in both, but generally greater in granulosa cells, compared with theca cells. This suggests that MR in granulosa cells may be activated by aldosterone. In contrast, bovine and human granulosa cells were found to express 11β-HSD1, but not 11β-HSD2 (Tetsuka et al. 2003; Chandras et al. 2007). Other studies showed that human granulosa cells expressed 11β-HSD2 message during the follicular phase, and upon stimulation, ceased to express 11β-HSD2 and began to express 11β-HSD1 (Thurston et al. 2003a). As in our rats, both HSD enzymes were reported in the bovine corpora lutea, with 11β-HSD2 expression increasing in comparison to that of 11β-HSD1 as the corpora lutea matured (Tetsuka et al. 2003). In our study, MR immunoreactivity and 11β-HSD2 immunoreactivity were particularly intense in several large cells in mature follicles and larger, more-active corpora lutea, suggesting that MR in these cells might be activated by aldosterone and that GR activation would be reduced. MR and both 11β-HSD enzymes were expressed in the ovarian stroma comprising various cell types, including hilar cells that produce androgens and macrophages that participate in the dissolution of old corpora lutea and produce cytokines important for the regulation of steroidogenesis in the ovary (Bornstein et al. 2004). Changes in 11β-HSD1 and -2 message and even protein expression do not necessarily translate into altered enzyme activity (Gomez-Sanchez et al. 2001,2003). Both message and protein for 11β-HSD1 were reduced to very low levels by estradiol in the rat kidney, whereas those for 11β-HSD2 were greatly increased. Notwithstanding, in vivo activity, as measured by the ratio of urinary corticosterone to 11-dehydrocorticosterone, was not different between kidneys of control and estrogen-treated rats, apparently owing to the formation of inactive dimers (Gomez-Sanchez et al. 2001,2003), suggesting that 11β-HSD2 activity may be regulated in vivo by dimer formation. Whether dimerization occurs only in those cells in which the estrogen-induced increase in 11β-HSD2 is not adaptive is not known.
It has been suggested that the net dehydrogenase activity found in mature human and bovine ovarian follicles (Michael et al. 1993; Tetsuka et al. 2003) may be due to the competition of other steroidogenic P450 enzymes in the granulosa cells for NADPH (Michael et al. 2003). However, a decrease in cortisol in follicles of these species is not a universal finding (Acosta et al. 2005; Fru et al. 2006). In our present studies, IHC did not detect H6PDH in rat granulosa cells, which would further decrease the availability of NADPH. The resulting decrease in local cortisol concentrations was thought to be important in the late follicular phase (Thurston et al. 2003a,2007). An endogenous inhibitor of 11β-HSD reductase activity produced by granulosa-lutein cells has been proposed (Thurston et al. 2003b), and inappropriate production of this inhibitor has been postulated to be the cause of infertility in some women (Michael et al. 1996). Expression in the ovary of 11β-hydroxylase, the unique and last enzyme in the synthesis of glucocorticoids, providing for the de novo synthesis of cortisol or corticosterone in the ovary, was reported over 30 years ago (Maschler et al. 1975) and more recently (Yazawa et al. 2008), although not in macaque ovaries (Fru et al. 2006). Clearly, the normal agonist for the MR in the different cells of ovary has not yet been defined. However, addition of the MR antagonist spironolactone to cultured granulosa cells from macaque follicles prevented HCG-induced progesterone synthesis (Fru et al. 2006).
Progesterone is synthesized in granulosa, theca, and corpora lutea cells. It is a competitive antagonist of the MR that circulates in quantities that are significantly greater than those of aldosterone, especially during the luteal phase of the estrus cycle and pregnancy (Quinkler et al. 2002). Although its affinity for the recombinant MR in vitro is similar to that of aldosterone, corticosterone, and cortisol (Rupprecht et al. 1993; Myles and Funder 1996), in vivo, it is approximately two orders of magnitude less than that of aldosterone and corticosterone (Arriza et al. 1987; Hultman et al. 2005). MR activity in the kidney is not disrupted, in particular, during pregnancy, when levels exceed those of aldosterone by an order of magnitude, owing perhaps to the existence of progesterone receptors in the kidney, but primarily to the rapid metabolism of progesterone in this aldosterone target organ (Quinkler et al. 2001; Bumke-Vogt et al. 2002). Whether progesterone reaches concentrations competitive with those of adrenal corticoids for the MR in the ovary, allowing it to regulate MR-mediated activities, including its own production, remains to be tested.
In summary, our study, using real time RT-PCR, Western blot, and IHC, demonstrates that the MR is expressed in several cell types of the rat ovary, along with enzymes that regulate intracellular glucocorticoid concentrations and confer ligand specificity to the MR. This suggests that occupation of the MR and GR in the ovary by adrenal steroids is stringently regulated. However, unlike classic aldosterone target epithelial cells of the kidney tubules or colon, where 11β-HSD2, but not 11β-HSD1, is expressed, the identity of the “preferred” ligand for the MR in specific ovarian cells remains to be determined and is an important topic for future research.
Acknowledgments
This work was supported by medical research funds from the Department of Veterans Affairs and National Institutes of Health Grants HL-27255 and HL-75321. M.T.G-S. was a recipient of a pre-doctoral fellowship from the American Heart Association, Southeast Affiliate.
References
- Acosta TJ, Tetsuka M, Matsui M, Shimizu T, Berisha B, Schams D, Miyamoto A (2005) In vivo evidence that local cortisol production increases in the preovulatory follicle of the cow. J Reprod Dev 51:483–489 [DOI] [PubMed] [Google Scholar]
- Alliston TN, Gonzalez-Robayna IJ, Buse P, Firestone GL, Richards JS (2000) Expression and localization of serum/glucocorticoid-induced kinase in the rat ovary: relation to follicular growth and differentiation. Endocrinology 141:385–395 [DOI] [PubMed] [Google Scholar]
- Arriza JW, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM (1987) Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science 237:268–275 [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Nashev LG, Schweizer RA, Frick C, Odermatt A (2004) Hexose-6-phosphate dehydrogenase determines the reaction direction of 11beta-hydroxysteroid dehydrogenase type 1 as an oxoreductase. FEBS Lett 571:129–133 [DOI] [PubMed] [Google Scholar]
- Banhegyi G, Benedetti A, Fulceri R, Senesi S (2004) Cooperativity between 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase in the lumen of the endoplasmic reticulum. J Biol Chem 279:27017–27021 [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Rutkowski H, Vrezas I (2004) Cytokines and steroidogenesis. Mol Cell Endocrinol 215:135–141 [DOI] [PubMed] [Google Scholar]
- Brennan FE, Fuller PJ (2000) Rapid upregulation of serum and glucocorticoid-regulated kinase (sgk) gene expression by corticosteroids in vivo. Mol Cell Endocrinol 166:129–136 [DOI] [PubMed] [Google Scholar]
- Brereton PS, van Driel RR, Suhaimi Fb, Koyama K, Dilley R, Krozowski Z (2001) Light and electron microscopy localization of the 11beta-hydroxysteroid dehydrogenase type I enzyme in the rat. Endocrinology 142:1644–1651 [DOI] [PubMed] [Google Scholar]
- Bumke-Vogt C, Bahr V, Diederich S, Herrmann SM, Anagnostopoulos I, Oelkers W, Quinkler M (2002) Expression of the progesterone receptor and progesterone-metabolising enzymes in the female and male human kidney. J Endocrinol 175:349–364 [DOI] [PubMed] [Google Scholar]
- Chandras C, Harris TE, Bernal AL, Abayasekara DR, Michael AE (2007) PTGER1 and PTGER2 receptors mediate regulation of progesterone synthesis and type 1 11beta-hydroxysteroid dehydrogenase activity by prostaglandin E2 in human granulosa lutein cells. J Endocrinol 194:595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19:269–301 [DOI] [PubMed] [Google Scholar]
- Filipiak WE, Saunders TL (2006) Advances in transgenic rat production. Transgenic Res 15:673–686 [DOI] [PubMed] [Google Scholar]
- Fru KN, VandeVoort CA, Chaffin CL (2006) Mineralocorticoid synthesis during the periovulatory interval in macaques. Biol Reprod 75:568–574 [DOI] [PubMed] [Google Scholar]
- Fuller PJ, Young MJ (2005) Mechanisms of mineralocorticoid action. Hypertension 46:1227–1235 [DOI] [PubMed] [Google Scholar]
- Funder JW (2005) The nongenomic actions of aldosterone. Endocr Rev 26:313–321 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP (2006) Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147:1343–1348 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ganjam V, Chen YJ, Liu Y, Clark SA, Gomez-Sanchez CE (2001) The 11beta hydroxysteroid dehydrogenase 2 exists as an inactive dimer. Steroids 66:845–848 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Ganjam V, Chen YJ, Liu Y, Zhou MY, Toroslu C, Romero DG, et al. (2003) Regulation of 11 beta-hydroxysteroid dehydrogenase enzymes in the rat kidney by estradiol. Am J Physiol Endocrinol Metab 285:E272–E279 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez EP, Romero DG, de Rodriguez AF, Warden MP, Krozowski Z, Gomez-Sanchez CE (2008) Hexose-6-phosphate dehydrogenase and 11beta-hydroxysteroid dehydrogenase-1 tissue distribution in the rat. Endocrinology 149:525–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann C, Benesic A, Krug AW, Freudinger R, Mildenberger S, Gassner B, Gekle M (2005) Human mineralocorticoid receptor expression renders cells responsive for nongenotropic aldosterone actions. Mol Endocrinol 19:1697–1710 [DOI] [PubMed] [Google Scholar]
- Haseroth K, Gerdes D, Berger S, Feuring M, Gunther A, Herbst C, Christ M, et al. (1999) Rapid nongenomic effects of aldosterone in mineralocorticoid-receptor-knockout mice. Biochem Biophys Res Commun 266:257–261 [DOI] [PubMed] [Google Scholar]
- Hultman ML, Krasnoperova NV, Li S, Du S, Xia C, Dietz JD, Lala DS, et al. (2005) The ligand-dependent interaction of mineralocorticoid receptor with coactivator and corepressor peptides suggests multiple activation mechanisms. Mol Endocrinol 19:1460–1473 [DOI] [PubMed] [Google Scholar]
- Jonas KC, Chandras C, Abayasekara DR, Michael AE (2006) Role for prostaglandins in the regulation of type 1 11beta-hydroxysteroid dehydrogenase in human granulosa-lutein cells. Endocrinology 147:5865–5872 [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M (2005) Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102:19204–19207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Maschler I, Salzberger M, Finkelstein M (1975) 11 Beta-hydroxylase with affinity to C-21-deoxysteroids from ovaries of patients with polycystic ovary syndrome. J Clin Endocrinol Metab 41:999–1002 [DOI] [PubMed] [Google Scholar]
- Michael AE, Gregory L, Thaventhiran L, Antoniw JW, Cooke BA (1996) Follicular variation in ovarian 11 beta-hydroxysteroid dehydrogenase (11 beta HSD) activities: evidence for the paracrine inhibition of 11 beta HSD in human granulosa-lutein cells. J Endocrinol 148:419–425 [DOI] [PubMed] [Google Scholar]
- Michael AE, Pester LA, Curtis P, Shaw RW, Edwards CR, Cooke BA (1993) Direct inhibition of ovarian steroidogenesis by cortisol and the modulatory role of 11 beta-hydroxysteroid dehydrogenase. Clin Endocrinol (Oxf) 38:641–644 [DOI] [PubMed] [Google Scholar]
- Michael AE, Thurston LM, Rae MT (2003) Glucocorticoid metabolism and reproduction: a tale of two enzymes. Reproduction 126:425–441 [DOI] [PubMed] [Google Scholar]
- Morita H, Zhou MY, Foecking MF, Gomez-Sanchez EP, Gomez-Sanchez CE (1996) 11 Beta-hydroxysteroid dehydrogenase type 2 cDNA stably transfected into Chinese hamster ovary cells: specific inhibition by 11 alpha-hydroxyprogesterone. Endocrinology 137:2308–2314 [DOI] [PubMed] [Google Scholar]
- Myles K, Funder JW (1996) Progesterone binding to mineralocorticoid receptors: in vitro and in vivo studies. Am J Physiol 270:E601–E607 [DOI] [PubMed] [Google Scholar]
- Navarro A, Torrejón R, Bández MJ, López-Cepero JM, Boveris A (2005) Mitochondrial function and mitochondria-induced apoptosis in an overstimulated rat ovarian cycle. Am J Physiol Endocrinol Metab 289:E1101–1109 [DOI] [PubMed] [Google Scholar]
- Quinkler M, Johanssen S, Bumke-Vogt C, Oelkers W, Bahr V, Diederich S (2001) Enzyme-mediated protection of the mineralocorticoid receptor against progesterone in the human kidney. Mol Cell Endocrinol 171:21–24 [DOI] [PubMed] [Google Scholar]
- Quinkler M, Meyer B, Bumke-Vogt C, Grossmann C, Gruber U, Oelkers W, Diederich S, et al. (2002) Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. Eur J Endocrinol 146:789–799 [DOI] [PubMed] [Google Scholar]
- Roland BL, Funder JW (1996) Localization of 11 beta-hydroxysteroid dehydrogenase type 2 in rat tissues: in situ studies. Endocrinology 137:1123–1128 [DOI] [PubMed] [Google Scholar]
- Rossol-Haseroth K, Zhou Q, Braun S, Boldyreff B, Falkenstein E, Wehling M, Losel RM (2004) Mineralocorticoid receptor antagonists do not block rapid ERK activation by aldosterone. Biochem Biophys Res Commun 318:281–288 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365–386 [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, van Steensel B, Spengler D, Soder M, Berning B, Holsboer F, et al. (1993) Pharmacological and functional characterization of human mineralocorticoid and glucocorticoid receptor ligands. Eur J Pharmacol 247:145–154 [DOI] [PubMed] [Google Scholar]
- Seckl JR, Walker BR (2001) Minireview: 11 beta-hydroxysteroid dehydrogenase type 1: a tissue-specific amplifier of glucocorticoid action. Endocrinology 142:1371–1376 [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Milne M, Simpson GE, Hillier SG (1999) Expression of 11 beta-hydroxysteroid dehydrogenase, glucocorticoid receptor, and mineralocorticoid receptor genes in rat ovary. Biol Reprod 60:330–335 [DOI] [PubMed] [Google Scholar]
- Tetsuka M, Yamamoto S, Hayashida N, Hayashi KG, Hayashi M, Acosta TJ, Miyamoto A (2003) Expression of 11 beta-hydroxysteroid dehydrogenases in bovine follicle and corpus luteum. J Endocrinol 177:445–452 [DOI] [PubMed] [Google Scholar]
- Thurston LM, Abayasekara DR, Michael AE (2007) 11 Beta-hydroxysteroid dehydrogenase expression and activities in bovine granulosa cells and corpora lutea implicate corticosteroids in bovine ovarian physiology. J Endocrinol 193:299–310 [DOI] [PubMed] [Google Scholar]
- Thurston LM, Chin E, Jonas KC, Bujalska IJ, Stewart PM, Abayasekara DR, Michael AE (2003a) Expression of 11 beta-hydroxysteroid dehydrogenase (11betaHSD) proteins in luteinizing human granulosa-lutein cells. J Endocrinol 178:127–135 [DOI] [PubMed] [Google Scholar]
- Thurston LM, Jonas KC, Abayasekara DR, Michael AE (2003b) Ovarian modulators of 11 beta-hydroxysteroid dehydrogenase (11beta HSD) activity in follicular fluid from bovine and porcine large antral follicles and spontaneous ovarian cysts. Biol Reprod 68:2157–2163 [DOI] [PubMed] [Google Scholar]
- Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, et al. (2004) 11 Beta-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev 25:831–866 [DOI] [PubMed] [Google Scholar]
- Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombes M (2007) The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal 5:e012. Published online November 30, 2007 (DOI: 10.1621/nrs.05012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa T, Uesaka M, Inaoka Y, Mizutani T, Sekiguchi T, Kajitani T, Kitano T, et al. (2008) Cyp11b1 is induced in the murine gonad by luteinizing hormone/human chorionic gonadotropin and involved in the production of 11-ketotestosterone, a major fish androgen: conservation and evolution of the androgen metabolic pathway. Endocrinology 149:1786–1792 [DOI] [PubMed] [Google Scholar]