Abstract
The larynx is the most common site of malignancy in the upper aerodigestive tract. In Brazil, malignant laryngeal lesions represent 2% of all cancers, with ∼3000 annual deaths. The association between human papillomavirus (HPV) and laryngeal cancer is still controversial. The aim of the present retrospective study was to determine the expression of galectin-3 immunoperoxidase in laryngeal carcinoma by examining paraffin-embedded larynx biopsies from 65 patients, 10 in situ laryngeal carcinomas, 27 laryngeal carcinomas without metastases, and 28 with metastases. Twenty-eight cervical lymph nodes from patients with metastatic lesions were also evaluated. Nested PCR was performed to detect and type HPV DNA. Galectin-3 expression was assessed by immunohistochemistry using a computer-assisted system. Among 65 patients, 55 (84.6%) were positive to beta-globin (internal control); 10 (15.4%) patients were beta-globin negative and were excluded from the HPV evaluation. Thus, 7 (12.7%) out of 55 patients were HPV positive and 48 (87.3%) out of 55 patients were HPV negative. High expression of galectin-3 was observed in invasive laryngeal tumors, suggesting that galectin-3 could be associated with the invasiveness and aggressiveness of laryngeal carcinomas. (J Histochem Cytochem 57:665–673, 2009)
Keywords: galectin-3, human papillomavirus, laryngeal carcinoma, immunohistochemistry, PCR
Head and neck squamous cell carcinomas are the sixth most common form of cancer worldwide, with a total of 640,000 new cases a year. The larynx is part of the head and neck, with a total of 159,000 new cases of carcinoma per year, more commonly affecting males (Almadori et al. 2005; Hunter et al. 2005; Koskinen et al. 2007). In Brazil, laryngeal tumors represent ∼2.0% of all cancers, corresponding to ∼8000 new cases annually (Wünsch-Filho 2004).
Alcohol and tobacco are the two best-recognized risk factors for cancer of the larynx, and human papillomavirus (HPV) has been described as a cofactor for laryngeal carcinogenesis. However, the relative frequency of HPV genotypes in carcinoma of the larynx is still unknown,with several studies demonstrating variable frequencies ranging from 8% to 58.8% (García-Milián et al. 1998; Atula et al. 1999; Venuti et al. 2000; Almadori et al. 2001; Jacob et al. 2002).
Novel approaches are thus required to provide head and neck oncologists with a more-effective armamentarium against these challenging diseases. Ideally, reliable identification of high-risk patients will enable tailored therapeutic regimens and hopefully improve the predicted unfavorable prognosis for this subgroup (Plzák et al. 2004). Minimal residual disease and early recurrences and metastases should be detected using molecular markers (Almadori et al. 2005). Galectin-3 has been proposed as a biological marker of aggressiveness in several types of head and neck tumors (Saussez et al. 2008b). Galectin-3 expression in head and neck tumors is controversial. Whereas previous studies have demonstrated a reduced expression of galectin-3 in parathyroid carcinomas, galectin expression was found to be high in primary and metastatic tumors (Bergero et al. 2005). It has been reported recently that epigenetic changes of galectin DNA are responsible for differential expression in cancers (Ahmed et al. 2007). Therefore, galectin-3 has been considered to be a prognostic factor in different tumors, such as colorectal cancer, head and neck cancer, pancreatic cancer, and gastric cancer (Puglisi et al. 2004).
In view of the above considerations, the aim of the present study was to determine the expression of the galectin-3 immunoperoxidase in in situ laryngeal carcinomas and in invasive carcinomas with and without metastases in cervical lymph nodes. An additional objective was to compare quantitative analyses of galectin-3 by a computer-assisted system to quantitation by a pathologist.
Materials and Methods
Specimens
Between 1995 and 2004, 65 laryngeal cell carcinomas were selected from the archives of the Pathology Department, School of Medicine of Ribeirão Preto, University of São Paulo, Brazil. The study protocol was approved by the Brazilian Institutional Ethics Committee on Human Experimentation. The 65 malignant laryngeal neoplasias were stratified into three subgroups: 10 in situ carcinomas (LSCCS), 27 invasive carcinomas without metastases (LSCCWT), and 28 invasive carcinomas with cervical lymph node metastases (LSCCW). In addition, 28 cervical lymph nodes (LB) from the patients with LSCCW were also evaluated. Thin 5-μm sections were cut, placed on organosilane-pretreated slides, and subjected to immunohistochemical assays (galectin-3). Additionally, a 10-μm section was cut for DNA extraction and HPV typing. Clinicopathological information about the patients, such as age, sex, history of smoking, and history of alcohol consumption, was obtained from the patients' medical records.
Immunohistochemistry for Galectin-3
Immunohistochemistry was performed for the detection of galectin-3 protein using the avidin-biotin peroxidase complex method (Novocastra; Newcastle-upon-Tyne, United Kingdom). Paraffin-embedded sections (5-μm) were deparaffnized in xylene and then rehydrated through a graded alcohol series. For antigen retrieval, the sections were immersed in 10 mM sodium citrate (C6H5Na3O7) buffer, pH 6.0, and subjected to steam heating for 40 min at 95C. The sections were cooled at room temperature and immersed in one change of hydrogen peroxide in PBS (3%) for 20 min. The sections were rinsed in three consecutive 1-M standard PBS baths and incubated with horse serum for 30 min for nonspecific blockage. The sections were incubated with a primary monoclonal antibody raised against galectin-3 (clone 9c4, diluted 1:100; Novocastra). The slides were placed in a humidified chamber at room temperature overnight. After incubation with primary antibodies, the slides were rinsed in three changes of PBS, and immunoperoxidase staining was performed using a biotinylated secondary antibody and the streptavidin-peroxidase complex (Novocastra). In both steps, the sections were incubated for 30 min at room temperature according to the manufacturer's instructions. Between the stages of the above procedure, the sections were carefully rinsed with three changes of PBS. Subsequently, the sections were incubated in a solution containing 5 mg of diaminobenzidine (GIBCO; Gaithersburg, MD) dissolved in 5 ml PBS, and 100 μl of fresh of peroxidase solution (450 μl PBS, 50 μl hydrogen peroxide). The DAB reaction was blocked with one change of PBS, followed by rinses in distilled water. Next, the sections were counterstained with Harris' hematoxylin for 1 min, following several rinses with distilled water. The sections were dehydrated with three absolute alcohol changes, followed by two immersion changes in xylol, and mounted on Permount mounting medium (MERCK; Darmstadt, Germany).
Controls
Three controls were included for each section and for all proteins evaluated: positive, negative, and normal laryngeal tissue. The positive control was a section of human lymphoma (galectin-3). The negative control was the same human tissue used as positive control, in which the primary antibody was omitted from the assay and replaced with PBS. The normal laryngeal control was performed to obtain the basal levels of galectin-3 and the cut-off value by which the quantitative galectin-3 expression in laryngeal carcinomas was determined.
Image Acquisition and Galectin-3 Image Analysis Quantification
Positive cytoplasms were automatically quantified by a computer-assisted system (Image-Pro Plus; MediaCybernetics, Bethesda, MD) consisting of a microscope, a digital camera, and a software package. A mean of 10 random microscope fields were selected to analyze 1000 cytoplasm fields per biopsy in all patient sections. The image acquisition of the sections of 65 patients stratified into in situ laryngeal carcinoma, laryngeal invasive squamous cell carcinoma (with and without metastasis), and cervical lymph node biopsies was performed on an electron photomicrograph, and the image was then processed and analyzed by the software. For each slide, the digitized image segmentation was controlled interactively by the red/green/blue color filter in the software program. The automatic cytoplasm count was determined and expressed as percentage.
Qualitative Immunolabeling Evaluation
All scoring and qualitative interpretation of immunohistochemical results were carried out by an experienced pathologist and classified as negative, discrete, moderate, or intense immunolabeling. In the present study, we considered the expression of galectin-3 to be low when laryngeal biopsies were not immunolabeled or showed discrete immunostaining. Galectin-3 expression was classified as high when the pathologist observed moderate or intense immunolabeling.
HPV Detection and Typing
Genomic DNA was obtained from two sections of paraffin blocks (10 μm each), according to the protocol proposed by Bettini et al. (2003), modified, and amplified by PCR using the GP5+ and GP6+ primers (Manos et al. 1989), which amplify a 150-bp DNA fragment and which were used for generic HPV amplification. Amplification was performed together with a set of primers for a beta-globin (PCO3 and PCO4) gene (PCO3+ CTT CTG ACA CAA CTG TGT TCA CTA GC and PCO4+ TCA CCA CAA CTT CAT CCA CGT TCA CC) as an internal control of amplification. The generic HPV-positive samples were amplified with specific primers for HPV16, HPV18, HPV31, HPV33, HPV6, and HPV11 as described by Walboomers et al. (1999). The amplification procedure for HPV detection and typing was carried out using a final volume of 25 μl. The reaction mixture included 20 ng of genomic DNA, 0.20 mM deoxynucleoside triphosphate (Pharmacia; Uppsala, Sweden), 0.6 μM of each primer (IDT; Coralville, IA), 1.25 U Taq polymerase (Invitrogen; São Paulo, Brazil), 3 mM MgCl2, and 1× buffer (Invitrogen). The cycling conditions for almost all generic and HPV types consisted of an initial denaturation step at 94C for 5 min, followed by 35 cycles at 94C for 1 min, at 55C for 1 min, and at 72C for 1 min, with a final extension cycle of 72C for 10 min. An annealing temperature of 60C was used for HPV11 amplification. PCR products were separated on 10% non-denaturing polyacrylamide gels followed by a silver staining procedure, as described by Sanguinetti et al. (1994).
HPV Controls
The HPV-positive control and beta-globin control were cervical samples collected by cytologic cytobrushing. As a negative control, all PCR reagents were added to an Eppendorf tube containing no DNA sample. For HPV6 and HPV11 typing, the PCR controls were HP-positive DNA collected from cervical samples. For HPV16 and HPV18 controls, DNA was extracted from SiHa and HeLa cell lines, respectively, whereas HPV31 and HPV33 were from patient samples. All controls (HPV16, -18, -31, and -33) were kindly provided by Dr. Luisa Lina Villa, Ludwig Institute for Cancer Research, Sao Paulo, Brazil.
Statistical Analysis
The distributions of three clinical parameters (gender, tobacco, and alcohol exposure) were compared between the different subgroups of laryngeal malignant neoplasias (LSCCS, LSCCWT, and LSCCW) by means of the two-sided Fisher's exact test for 2 × 2 contingency tables, with the aid of GraphPad InStat software (San Diego, CA), which was also used to estimate the odds ratio and its 95% confidence interval. Age, reported as arithmetic mean and standard deviation, was also compared between groups by means of the two-sided Student's unpaired t-test.
Qualitative differences in galectin-3 expression between groups determined according to clinical parameters were evaluated by means of Fisher's exact test (in case of 2 × 2 tables) using the GraphPad InStat software or an exact test that uses the Metropolis algorithm to obtain an unbiased estimate of the exact p value and its standard error (in more-complex RxC tables) using the RXC software (http://www.marksgeneticsoftware.net/rxc.htm).
The standard survival time analysis was performed using Kaplan-Meier curves, and differences between curves were evaluated by the Gehan's Wilcoxon test, using the Statistica 8.0 software (StatSoft; Tulsa, OK). Overall survival was computed from the date of surgery to documented date of the follow-up or death, whereas disease-free survival was considered to cover the period from the date of surgery to the date of recurrence.
For quantitative analysis, because the galectin-3 expression data did not meet the normality assumption for parametric tests, non-parametric analyses were conducted. The Kruskal-Wallis non-parametric test followed by Dunn's multiple comparison posttest was applied to compare the mean expression levels of galectin-3 among the three subgroups of laryngeal malignant neoplasias (LSCCS, LSCCWT, and LSCCW). The mean expression levels of galectin-3 between the invasive carcinomas with metastasis biopsies (LSCCW) and cervical lymph node biopsies (LB) rising from such laryngeal metastatic lesions were compared using the Wilcoxon matched-pairs signed-ranks test. The correlation analysis between LSCCW and LB was calculated using the Spearman rank correlation test. P values were two-sided and the level of significance was set at 5% (p=0.05).
As mentioned above, the intensity of the staining pattern of galectin-3 in each sample was classified as low or high immunostaining intensity. To compare the distribution of these two types of intensity among the different subgroups of laryngeal malignant neoplasias (in situ carcinoma, invasive carcinomas without metastasis, and invasive carcinomas with metastasis), the non-parametric two-sided Fisher's exact test for 2 × 2 contingency tables was employed. All data were analyzed using GraphPad Instat software.
Results
Laryngeal Carcinomas and Clinical Parameters
The clinical parameters of the patients with in situ laryngeal carcinoma and invasive laryngeal carcinoma with and without metastasis demonstrated no significant association with age, sex, or tobacco consumption (Tables 1 and 2). However, the patients were predominantly males exposed to tobacco and alcohol.
Table 1.
Clinical parameters of the 65 patients with laryngeal carcinoma stratified according to age, sex, and tobacco and alcohol exposure
| Age
|
Sex
|
Tobacco exposure
|
Alcohol exposure
|
||||
|---|---|---|---|---|---|---|---|
| Mean ± SE | Male | Female | Yes | No | Yes | No | |
| LSCCS (n=10) | 59.2 ± 2.56 | 6 | 4 | 6 | 4 | 3 | 7 |
| LSCCWT (n=27) | 59.18 ± 1.47 | 18 | 9 | 23 | 3 | 15 | 12 |
| LSCCW (n=28) | 56.07 ± 1.78 | 23 | 5 | 28 | 0 | 20 | 8 |
LSCCS, in situ laryngeal carcinoma; LSCCWT, invasive laryngeal carcinoma without metastases; LSCCW, invasive laryngeal carcinoma with metastases.
Table 2.
Statistical analysis of clinical parameters of the 65 patients with laryngeal carcinoma
| Comparisons | p valuea | p valueb and OR valueb | p valueb and OR valueb | p valueb and OR valueb |
|---|---|---|---|---|
| LSCCWT vs LSCCS | p=0.9945; | p=0.7158; OR = 1.333 (0.2983–5.960) | p=0.6881; OR = 5.333 (0.9321–30.517) | p=0.2691; OR = 2.917 (06.182–13.760) |
| LSCCWT vs LSCCW | p=0.1869 | p=0.2270; OR = 2.300 (0.6553–8.0722) | p=0.1115; OR = 8.143 (0.4003–165.6501) | p=0.2695; OR = 2.000 (0.6541–6.1153) |
Unpaired t-test.
Fisher's exact test.
LSCCS, in situ laryngeal carcinoma; LSCCWT, invasive laryngeal carcinoma without metastases; LSCCW, invasive laryngeal carcinoma with metastases; OR, odds ratio.
Galectin-3 and Clinicopathological Features
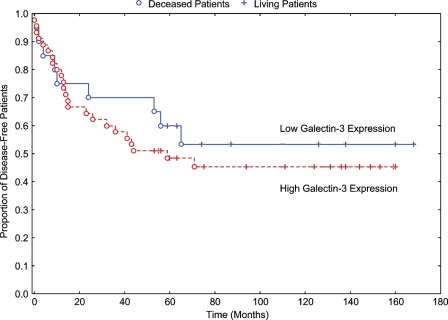
The relationship between galectin-3 expression and clinicopathological features (histological grade, TNM stage, and recurrence) does not show any statistical difference between the qualitative expression of galectin-3 and the laryngeal carcinomas studied (Kaplan-Meier curve for cumulative disease-free rate of patients with laryngeal carcinomas). To the contrary, galectin-3 expression was positively associated with tumor site (p=0.0003 ± 0.001) when analyzed in LSCCWT, summarized in Table 3. We have not found statistical significance in the results of survival disease-free (p=0.5284), as summarized in Figure 1. There was no significant difference between galectin-3 low expression and galectin-3 high expression in all groups of laryngeal carcinomas (LSCCS, LSCCWT, and LSCCW) studied.
Table 3.
Quantitative immunoexpression of galectin-3 in laryngeal carcinoma and clinicopathological data
| LSCCS (n=10)
|
LSCCWT (n=27)
|
LSCCW (n=28)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| LE | HE | p value | LE | HE | p value | LE | HE | p value | |
| Tumor site | |||||||||
| Glottic area | 3 | 0 | p=0.6699 ± 0.0013a | 1 | 5 | p=0.0003 ± 0.001a | 0 | 9 | p=0.1669 ± 0.0012a |
| Supraglottic area | 3 | 2 | 1 | 5 | 2 | 8 | |||
| Subglottic area | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Larynxc | 1 | 0 | 0 | 10 | 1 | 5 | |||
| More than twod | 1 | 0 | 4 | 0 | 0 | 2 | |||
| Histological grade | |||||||||
| Well differentiated | – | 1 | 10 | p=0.2179 ± 0.0010a | 1 | 12 | p=1.0000b | ||
| Moderately differentiated | 3 | 12 | 1 | 14 | |||||
| Poorly differentiated | 1 | 0 | 0 | 0 | |||||
| TNM stage | |||||||||
| T1-T2 | 2 | 1 | p=0.3000b | 2 | 7 | p=1.0000a | 0 | 1 | p=1.0000b |
| T3-T4 | 7 | 4 | 14 | 4 | 23 | ||||
| N0 | 0 | 1 | p=1.0000b | 3 | 17 | p=0.2896a | 2 | 4 | p=0.1915b |
| N1-N3 | 1 | 8 | 3 | 4 | 2 | 20 | |||
| M0 | 6 | 1 | p=1.0000b | 3 | 4 | p=0.3278a | 0 | 0 | – |
| MX | 2 | 1 | 4 | 16 | 4 | 24 | |||
| Tumor treatment | |||||||||
| Total laryngectomy | 1 | 0 | p=1.0000 ± 0.0000a | 3 | 15 | p=0.7029 ± 0.0014a | 2 | 15 | p=0.2733 ± 0.0012a |
| Partial laryngectomy | 1 | 0 | 1 | 4 | 1 | 0 | |||
| Supraglottic laryngectomy | 0 | 0 | 0 | 2 | 0 | 1 | |||
| Partial pharyngolaryngectomy | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Total pharyngolaryngectomy | 0 | 0 | 1 | 1 | 1 | 8 | |||
| Other treatmentse | 6 | 2 | 0 | 0 | 0 | 0 | |||
| Recurrence | |||||||||
| Local recurrence | 1 | 2 | p=0.6024 ± 0.0010a | 1 | 1 | p=0.3078 ± 0.0014a | 1 | 2 | p=0.5660 ± 0.0014a |
| Distant recurrence | 3 | 1 | 1 | 4 | 3 | 2 | |||
| No recurrence | 4 | 2 | 22 | 2 | 3 | 20 | |||
RXC software (http://www.marksgeneticsoftware.net/rxc.htm).
Fisher's exact test.
The whole larynx.
Supraglottic and subglottic.
Laser CO2; without surgery.
LE, low expression; HE, high expression.
Figure 1.
Kaplan-Meier curve for cumulative disease-free rate of patients with laryngeal carcinomas. There was no statistical difference between the curves (p=0.5284).
HPV Detection, Typing, and Correlation With Expression of Galectin-3
Among 65 patients, 55 (84.6%) were positive to beta-globin (internal control) and 10 (15.4%) patients were beta-globin negative, indicating that the DNA from paraffin sections was degradated. Therefore, they were excluded from HPV evaluation. Thus, in the present study, 7 (12.7%) of 55 patients were HPV positive, and HPV DNA of low-risk types was observed in 1 (14.3%), whereas high-risk types were detected in 6 (85.7%). Forty-eight (87.3%) of 55 patients were HPV negative (Table 4). Qualitatively, 5 out of 7 HPV+ patients (71.4%) presented high expression levels of galectin-3, whereas only 37 of 55 HPV− patients (67.2%) presented high expression levels of galectin-3; however, such figures are not statistically significant (p=0.6079; Fisher's exact test).The same happened when we analyzed quantitatively the expression of galectin-3 and HPV+/− patients (p=0.5034; Mann-Whitney test).
Table 4.
HPV DNA detection (GP5+/GP6+) and typing (specific primers) in 55 patients with laryngeal carcinoma stratified according to histological diagnosis
| HPV positive n (%) | HPV negative n (%) | HPV6 n (%) | HPV18 n (%) | HPV31 n (%) | HPV two types n (%) | |
|---|---|---|---|---|---|---|
| LSCCS | 1 (14.3) | 9 (16.4) | — | 1 (14.3) | — | 1 (14.3)a |
| LSCCWT | 2 (28.6) | 25 (45.5) | 1 (14.3) | — | — | 1 (14.3)b |
| LSCCW | 2 (28.6) | 26 (47.3) | — | — | 1 (14.3) | 2 (28.6)c |
| Lymph nodes | 2 (28.6) | 26 (47.3) | — | — | — | |
| Total | 7 (10.8) | 48 (87.3) | 1 (14.3) | 1 (14.3) | 1 (14.3) | 4 (57.1) |
HPV31 and -33.
HPV6 and -16.
First patient HPV16 and -31 and second patient HPV16 and -33.
In 10 (15.4%) patients, the beta-globin gene was not amplified, indicating DNA degradation, and these patients were not considered to be HPV negative or positive. HPV, human papillomavirus.
Immunohistochemical Expression of Galectin-3 in Laryngeal Carcinomas
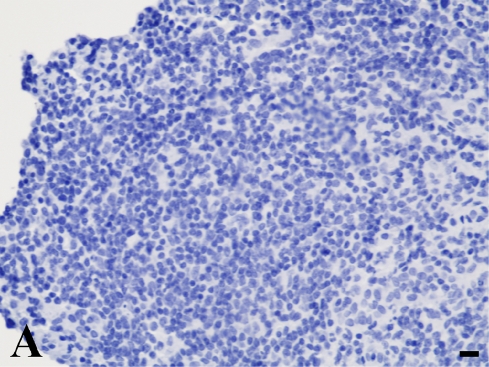
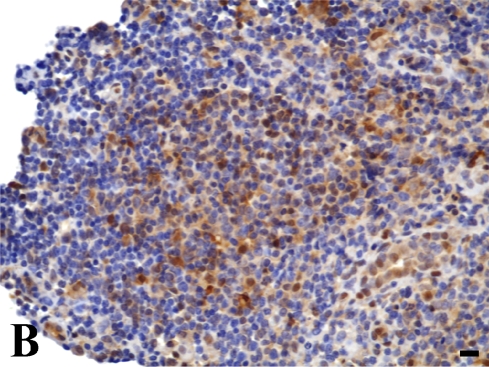
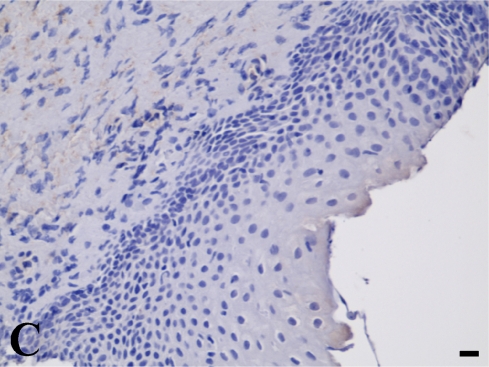
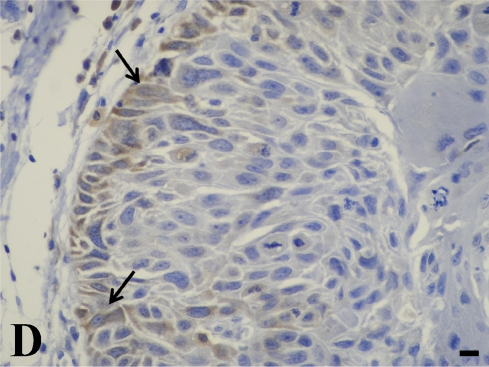
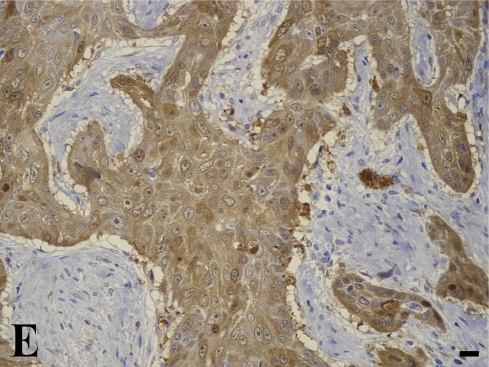
In the quantitative evaluation of galectin-3 (Figures 2D and 2E; Table 5), we observed a significantly higher expression in invasive laryngeal carcinoma, independent of the presence or absence of metastases or in their lymph nodes (LSCCWT, LSCCW, and LB), when compared with in situ laryngeal carcinoma (LSCCS). Normal laryngeal biopsies (Figure 2C) and negative controls (Figure 1B) did not present any immunolabeling in squamous cells and in cylindric cells (Figure 1C), whereas the positive control (non-Hodgkin's lymphoma) showed specific immunostaining (Figure 2A). In this study, we observed only immunolabeling of galectin-3 in the cytoplasm of tumor cells.
Figure 2.
Galectin-3 expression determined by immunoperoxidase staining. (A) Positive control. (B) Negative control (non-Hodgkin's lymphoma). (C) Normal larynx negative for galectin-3. (D) Low expression of galectin-3 in in situ laryngeal carcinoma, with a few immunolabeled cells and weak staining intensity (arrows). (E) High expression of galectin-3 in invasive laryngeal carcinoma without metastases, showing diffuse and high-intensity immunolabeling. Bar = 100 μm.
Table 5.
Quantitative immunoexpression of galectin-3 in laryngeal carcinoma stratified according to histological diagnosis
| LSCCS (n=10) | 36.0 ± 10.30 |
| LSCCWT (n=27) | 70.5 ± 3.90 |
| LSCCW (n=28) | 66.3 ± 5.16 |
| Lymph nodes (n=28) | 61.5 ± 4.50 |
Analysis of variance (Kruskal Wallis Test): LSCCS vs LSCCWT, p<0.05; LSCCS vs LSCCW, p<0.05; LSCCWT vs LSCCW, p>0.05. Spearman correlation: LSCCW vs LB, p<0.0001, r = 1.000. Wilcoxon matched-pairs signed-ranks test: p=0.1085. LB, cervical lymph nodes. Data are reported as mean ± SEM.
Interestingly, the qualitative evaluation performed by an experienced pathologist also demonstrated a significantly higher intensity of galectin-3 cytoplasmatic expression in invasive carcinomas (LSCCWT, LSCCW, and LB) and a low intensity in LSCCS (Figures 2D and 2E; Table 6). Thus, both quantitative and qualitative galectin-3 expression demonstrated that this protein could be considered a biological marker for invasive laryngeal carcinoma, although it is not related to metastasis.
Table 6.
Expression of galectin-3 classified according to immunolabeling intensity in laryngeal carcinoma stratified according to histological diagnosis
| Low expression n (%) | High expression n (%) | |
|---|---|---|
| LSCCS (n=10) | 8 (80) | 2 (20) |
| LSCCWT (n=27) | 6 (22.2) | 21 (77.7) |
| LSCCW (n=28) | 4 (14.28) | 24 (84.71) |
| LB (n=28) | 2 (7.14) | 26 (92.85) |
Fisher's Exact Test: LSCCS vs LSCCWT, p=0.0023; LSCCS vs LSCCW, p=0.0004; LSCCWT vs LSCCW, p=0.5027; LSCCW vs LB, p>0.6695.
Discussion
Among the 65 patients with laryngeal carcinoma, we detected a close association between alcohol and tobacco exposure, although no differences were found between in situ and invasive laryngeal lesions. Epidemiological studies have consistently shown that alcohol drinking and tobacco smoking increase the risk of laryngeal cancer (Altieri et al. 2005). It is a well-accepted fact that heavy drinkers tend to be heavy smokers. In the present study, the patients were predominatly Caucasian males with a mean age of 59 years. There is ample evidence suggesting that susceptibility to the effects of both alcohol and tobacco is likely to differ in terms of both gender and race (Zang and Wynder 2001). A case control study on laryngeal cancer in Sao Paulo, Brazil found an adjusted odds ratio of 7.5 for heavy smokers and of 3.7 for heavy drinkers (Sartor 2003). The population of the present study was from the interior of the state of Sao Paulo and, although we did not determine the number of cigarettes smoked or the amount of alcohol consumed, we detected an odds ratio of 8.1 for alcohol exposure in patients with invasive laryngeal carcinoma. In addition, the odds ratio for tobacco exposure (2.0 for invasive laryngeal carcinoma) was also quite similar to that observed in the previous study (Sartor 2003).
The larynx is part of the head and neck, and owing to its various clinical and molecular particularities, it has been classified separately from the oral cavity and pharynx (Jemal et al. 2004). In addition, molecular markers could be expressed differentially in the larynx when compared with other head and neck sites. Previous studies have demonstrated a close association between a high expression of galectin-3 and the aggressiveness of head and neck tumors (Gillenwater et al. 1996; Saussez et al. 2008a,b). The results obtained from analysis of clinicopathological data (tumor site, histological grade, TNM stage, survival, and recurrence) of 65 patients with laryngeal carcinoma did not show any relationship with expression of galectin-3, probably because the number of cases studied was too small. Some authors also did not find a relationship with some of clinicopathological data, such as tumor site and TNM stage, but found some relationship when the correlation was between expression of galectin-3 and survival, recurrence, histological grade, and tumor keratinization (Piantelli et al. 2002; Plzák et al. 2004; Saussez et al. 2006).
Although the relationship between anogenital cancer and HPV has been clearly demonstrated, the role of HPV in laryngeal carcinomas is unclear, and contradictory results have been reported thus far (Lindeberg and Krogdahl 1999; Jacob et al. 2002; Morshed et al. 2008). Our findings demonstrated that only 7 of 55 patients were HPV positive. This result supports the view that the role of HPV is more important in head and neck tumors than in the larynx (Koskinen et al. 2007). The reported range of HPV frequency in these tumors is 8% to 58.8%, and the reason for this incredible range could only be explained by false-positive results in many of these reports (Lindeberg and Krogdahl 1999). Although different sample types (fresh-frozen, paraffin-embedded) may influence the result, convincing data exist as to a low prevalence of HPV in laryngeal carcinomas (Lindeberg and Krogdahl 1999; Koskinen et al. 2007). Nevertheless, tumors from HPV-infected patients were high-risk types (n=4), and although their frequency was low, a close association was observed between those types and invasive laryngeal carcinoma.
Galectin-3 is a multifunctional effector and the only chimera-type member of the galectin family of endogenous lectins, which share specificity with beta-galactosides and have a jellyroll-like folding pattern (Wang et al. 2004). This protein acts in the modulation of cell–cell, cell–extracellular matrix interactions and in the regulation of proliferation and apoptosis/anoikis. Thus, galectin-3 is involved in various biological phenomena, including cell growth, adhesion, differentiation, angiogenesis, and apoptosis (Saussez et al. 2008b). Galectin-3 exerts different functions in a variety of neoplastic cell types, playing a role in tumorigenesis and metastasis (Puglisi et al. 2004), modifying the levels of migration of cancer cells (Saussez et al. 2008b). However, to our knowledge, the present study was the first to assess both the quantitative and qualitative expression of galectin-3 in invasive laryngeal carcinomas. High intensity and number of cells expressing galectin-3 were found, indicating a close relationship between overexpression of galectin-3 and invasive laryngeal cancer. Galectin-3 expression seems to be positively associated with tumor keratinization and histological grade, and a significant correlation was found between tumor galectin-3 positivity and a longer metastasis-free and overall survival of patients with laryngeal carcinoma (Piantelli et al. 2002; Almadori et al. 2005).
Semi-quantitative evaluation and manual cell counts are the procedures commonly used to assess positive labeling of molecular markers in tissue sections. In the present study, no difference was found between the qualitative assessment provided by the pathologist's readings and image analysis of the quantitative immunolabeling of galectin-3. Although the imaging method has several advantages, such as speed of analysis, consistency, and automation (Loukas et al. 2003; Teresa et al. 2007), evaluation by the pathologist is a useful tool guaranteeing a robust immunohistochemical evaluation.
We conclude that galectin-3 was overexpressed in invasive laryngeal carcinoma, representing an important biological marker of larynx cancer aggressiveness. Moreover, both quantitative and qualitative determination of galectin-3 expression proved to be a good methodological strategy for the differentiation of in situ from invasive laryngeal cancer.
Acknowledgments
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council of Scientific and Technological Development), a Brazilian research funding organization.
We thank Ana Maria Rocha for the excellent technical assistance.
References
- Ahmed H, Banerjee PP, Vasta GR (2007) Differential expression of galectins in normal, benign and malignant prostate epithelial cells: silencing of galectin-3 expression in prostate cancer by its promoter methylation. Biochem Biophys Res Commun 358:241–246 [DOI] [PubMed] [Google Scholar]
- Almadori G, Bussu F, Cadoni G, Galli J, Paludetti G, Maurizi M (2005) Molecular markers in laryngeal squamous cell carcinoma: towards an integrated clinicobiological approach. Eur J Cancer 41:683–693 [DOI] [PubMed] [Google Scholar]
- Almadori G, Cadoni G, Cattani P, Galli J, Bussu F, Ferrandina G, Scambia G, et al. (2001) Human papillomavirus infection and epidermal growth factor receptor expression in primary laryngeal squamous cell carcinoma. Clin Cancer Res 7:3988–3993 [PubMed] [Google Scholar]
- Altieri A, Garavello W, Bosetti C, Gallus S, La Vecchia C (2005) Alcohol consumption and risk of laryngeal cancer. Oral Oncol 41:956–965 [DOI] [PubMed] [Google Scholar]
- Atula S, Grenman R, Kujari H, Syrjäne S (1999) Detection of human papillomavirus (HPV) in laryngeal carcinoma cell lines provides evidence for a heterogeneic cell population. Eur J Cancer 35:825–832 [DOI] [PubMed] [Google Scholar]
- Bergero N, De Pompa R, Sacerdote C, Gasparri G, Volante M, Bussolati G, Papotti M (2005) Galectin-3 expression in parathyroid carcinoma: immunohistochemical study of 26 cases. Hum Pathol 36:908–914 [DOI] [PubMed] [Google Scholar]
- Bettini J de S, Soares EG, Duarte G, Simões RT, Simões AL (2003) PCR diagnosis of HPV in cervical biopsies of CIN and invasive neoplasia formely diagnosed as HPV negative. Acta Cytol 47:545–549 [DOI] [PubMed] [Google Scholar]
- García-Milián R, Hernández H, Panadé L, Rodríguez C, González N, Valenzuela C, Araña MDJ, et al. (1998) Detection and typing of human papillomavirus DNA in benign and malignant tumours of larymgeal epithelium. Acta Otolaryngol 118:754–758 [DOI] [PubMed] [Google Scholar]
- Gillenwater A, Xu XC, el-Naggar AK, Clayman GL, Lotan R (1996) Expression of galectins in head and neck squamous cell carcinoma. Head Neck 18:422–432 [DOI] [PubMed] [Google Scholar]
- Hunter KD, Parkinson EK, Harrison PR (2005) Profiling early head and neck cancer. Natl Rev 5:127–135 [DOI] [PubMed] [Google Scholar]
- Jacob EL, Sreevidya S, Chacko E, Pillai MA (2002) Cellular manifestations of human papillomavirus infection in laryngeal tissues. J Surg Oncol 79:142–150 [DOI] [PubMed] [Google Scholar]
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, et al. (2004) Cancer statistics, 2004. CA Cancer J Clin 54:8–29 [DOI] [PubMed] [Google Scholar]
- Koskinen WJ, Brøndbo K, Mellin Dahlstrand H, Luostarinen T, Hakulinen T, Leivo I, Molijn A, et al. (2007) Alcohol, smoking and human papillomavirus in laryngeal carcinoma: a Nordic prospective multicenter study. J Cancer Res Clin Oncol 133:673–678 [DOI] [PubMed] [Google Scholar]
- Lindeberg H, Krogdahl A (1999) Laryngeal cancer and human papillomavirus: HPV is absent in the majority of laryngeal carcinomas. Cancer Lett 146:9–13 [DOI] [PubMed] [Google Scholar]
- Loukas CG, Wilson GD, Vojnovic B, Linney A (2003) An image analysis-based approach for automated counting of cancer cell nuclei in tissue sections. Cytometry A 55:30–42 [DOI] [PubMed] [Google Scholar]
- Manos MM, Ting Y, Wright DK, Lewis AJ, Broker TR, Wolinsky SM (1989) The use of polymerase chain reaction amplification for the detection of genital humam papillomaviruses. In Furth M, Greaves M, eds. Molecular Diagnostics of Human Cancer: Cancer Cells, vol. 7. New York, Cold Spring Harbor Laboratory, 209–214
- Morshed K, Polz-Dacewicz M, Szymanski M, Polz D (2008) Short-fragment PCR assay for highly sensitive broad-spectrum detection of human papillomaviruses in laryngeal squamous cell carcinoma and normal mucosa: clinico-pathological evaluation. Eur Arch Otorhinolaryngol 265(suppl 1):89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantelli M, Iacobelli S, Almadori G, Iezzi M, Tinari N, Natoli C, Cadoni G, et al. (2002) Lack of expression of galectin-3 is associated with a poor outcome in node-negative patients with laryngeal squamous-cell carcinoma. J Clin Oncol 18:3850–3856 [DOI] [PubMed] [Google Scholar]
- Plzák J, Betka J, Smetana K Jr, Chovanec M, Kaltner H, André S, Kodet R, et al. (2004) Galectin-3—an emerging prognostic indicator in advanced head and neck carcinoma. Eur J Cancer 15:2324–2330 [DOI] [PubMed] [Google Scholar]
- Puglisi F, Minisini AM, Barbone F, Intersimone D, Aprile G, Puppin C, Damante G, et al. (2004) Galectin-3 expression in non-small cell lung carcinoma. Cancer Lett 2:233–239 [DOI] [PubMed] [Google Scholar]
- Sanguinetti CJ, Dias Neto E, Simpson AJ (1994) Rapid silver staining and recovery of PCR products separated on polyacrylamide gels. Biotechniques 5:914–921 [PubMed] [Google Scholar]
- Sartor SG (2003) Risco Ocupacionais para Câncer de Laringe: Um Estudo Caso-Controle. PhD Dissertation. Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil
- Saussez S, Decaestecker C, Lorfevre F, Chevalier D, Mortuaire G, Kaltner H, Andre S, et al. (2008a) Increased expression and altered intracellular distribution of adhesion/growth-regulatory lectins galectins-1 and -7 during tumour progression in hypopharyngeal and laryngeal squamous cell carcinomas. Histopathology 52:483–493 [DOI] [PubMed] [Google Scholar]
- Saussez S, Decaestecker C, Lorfevre F, Cucu DR, Mortuaire G, Chevalier D, Wacreniez A, et al. (2006) High level of galectin-1 expression is a negative prognostic predictor of recurrence in laryngeal squamous cell cacinomas. Int J Oncol 30:1109–1117 [PubMed] [Google Scholar]
- Saussez S, Lorfevre F, Lequeux T, Laurent G, Chantrain G, Vertongen F, Toubeau G, et al. (2008b) The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol 44:86–93 [DOI] [PubMed] [Google Scholar]
- Teresa DB, Neves KA, Neto CB, Fregonezi PA, de Oliveira MR, Zuanon JA, Donadi EA, et al. (2007) Computer-assisted analysis of cell proliferation markers in oral lesions. Acta Histochem 5:377–387 [DOI] [PubMed] [Google Scholar]
- Venuti A, Manni V, Morello R, De Marco F, Marzetti F, Marcante ML (2000) Physical state and expression of human papillomavirus in laryngeal carcinoma and surrounding normal mucosa. J Med Virol 60:396–402 [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, et al. (1999) Human papillomavirus is a necessary cause of invasive cancer worldwide. J Pathol 189:12–19 [DOI] [PubMed] [Google Scholar]
- Wang JL, Gray RM, Haudek KC, Patterson RJ (2004) Nucleocytoplasmic lectins. Biochim Biophys Acta 1673:75–93 [DOI] [PubMed] [Google Scholar]
- Wünsch-Filho V (2004) The epidemiology of laryngeal cancer in Brazil. Sao Paulo Med J 122:188–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang EA, Wynder EL (2001) Reevaluation of the confounding effect of cigarette smoking on the relationship between alcohol use and lung cancer risk, with larynx cancer used as a positive control. Prev Med 4:359–370 [DOI] [PubMed] [Google Scholar]