Abstract
Poly(ADP-ribose) polymerase 3 (PARP-3) is a newly characterized PARP. In contrast to the two best-studied nuclear PARPs, PARP-1 and PARP-2, PARP-3 activity is apparently not stimulated by DNA damage. However, our previous work has demonstrated that PARP-3 interacts with several DNA damage response proteins, including Ku70/Ku80, DNA-PK, and PARP-1, suggesting that it contributes to the DNA damage response. Furthermore, a possible function for PARP-3 in the regulation of gene expression has been inferred from our observations that it associates with polycomb group proteins, which are responsible for epigenetic modifications leading to gene silencing. In this report, we extend our characterization of PARP-3 by revealing its distribution in the tissues and cell types of adult cynomolgous monkeys using a well-characterized PARP-3 polyclonal antibody. This study is the first to demonstrate that PARP-3 is genuinely expressed in most of the examined tissues. However, its expression is highly restricted to specific cell types of each tissue, indicating that PARP-3 expression is tightly regulated. One of the key findings of this study is that PARP-3 is highly expressed in the nuclei of epithelial cells forming the ducts of prostate, salivary glands, liver, and pancreas and in the neurons of terminal ganglia. (J Histochem Cytochem 57:675–685, 2009)
Keywords: poly(ADP-ribose) polymerase, immunohistochemistry, monkey, tissue distribution, ductal epithelia
Poly(ADP-ribose) polymerases (PARPs) constitute a family of enzymes that synthesize poly(ADP-ribose) on a variety of acceptor proteins in response to various cellular stresses. Poly(ADP-ribosyl)ation, which has profound effects on protein functions, regulates a variety of cellular processes, such as DNA damage repair, transcription, replication, and cell death (Rouleau et al. 2004; Hassa and Hottiger 2008). The best-characterized members of the PARP family are PARP-1 and PARP-2, two nuclear enzymes that are critically involved in the DNA damage response (Haince et al. 2008; Hassa and Hottiger 2008). PARP-3 is closely related to PARP-1 and PARP-2, based on amino acid sequence comparisons of the catalytic domain. Owing to alternative splicing of its mRNA, expression of the human PARP-3 gene produces two PARP-3 isoforms. The short isoform has an apparent molecular mass of 62 kDa, whereas the long isoform, which bears a seven-amino-acid extension at its N terminus, has an apparent molecular mass of 67 kDa (Augustin et al. 2003; Rouleau et al. 2007). The relative abundance of alternatively spliced mRNAs suggests that the 62-kDa isoform predominates in tissues and cell lines (Rouleau et al. 2007). However, the two isoforms share a similar, mostly nuclear, distribution and are involved in the same protein–protein interactions. Both isoforms interact with several proteins of the DNA damage response, including PARP-1, Ku70/80, and DNA-PK, suggesting that PARP-3 could contribute to this pathway (Rouleau et al. 2007; Mathivanan et al. 2008). In addition, a possible function of PARP-3 in the regulation of gene expression has been highlighted by the finding that PARP-3 colocalizes with polycomb group bodies and the trimethylated histone H3 K27 mark and coimmunoprecipitates with the polycomb group proteins EZH2 and SUZ12 (Rouleau et al. 2007). Polycomb group proteins play a crucial role in the maintenance of cell fate by epigenetic silencing of key sets of genes throughout the development and life of an organism. The fact that PARP-3 interacts with the methyltransferase EZH2 led to the suggestion that it could regulate gene silencing, possibly by its poly(ADP-ribose) polymerase activity. Taken together, these findings indicate that PARP-3 is probably involved in several cellular pathways.
Identification of tissues and cell types that express PARP-3 is an important step in the characterization of its physiological functions and could help in defining the best cell types with which to further investigate this protein. PARP-3 mRNA expression has been previously detected in nearly all examined human and mouse tissues (Johansson 1999; Urbanek et al. 2002). However, mRNA expression does not necessarily reflect protein expression levels. Furthermore, this type of analysis implies the examination of whole homogenized tissues and does not reveal the different cell types in which PARP-3 is expressed. The goal of this study was to establish the full scope of PARP-3 expression in tissues of the monkey, a mammal closely related to humans. We find that rather than being ubiquitously expressed, PARP-3 distribution is highly restricted to specific cell types in nearly all examined tissues. The results of our analysis indicate that the expression of PARP-3 is tightly regulated and differs from that of PARP-1 and PARP-2.
Materials and Methods
Cell Lines and Whole-cell Extracts
The human cell lines HeLa, SK-N-SH, A549, and M059K and the monkey cell line COS-7 were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and were cultured according to the instructions of the ATCC. COS-7 is a fibroblast-like cell line isolated from kidney, HeLa is an epithelial cell line isolated from an adenocarcinoma of the cervix, SK-N-SH is an epithelial cell line isolated from a neuroblastoma, A549 is an epithelial cell line established from a lung carcinoma, and M059K is a fibroblast cell line isolated from a glioblastoma. The human skin fibroblastic GM00637 cell line was obtained from the Coriell Institute for Medical Research (Camden, NJ), and the human lymphoblastoid C3ABR cells were kindly provided by Dr. M. Lavin (Queensland Institute for Medical Research, Australia). GM00637 and C3ABR were cultured as described (Haince et al. 2007). Cells at 80% confluence were washed twice in PBS and were harvested by scraping in Laemmli sample buffer (62 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 715 mM β-mercaptoethanol, 0.001% bromophenol blue) at a ratio of 10 × 106 cells/ml.
PARP-3 Antibody
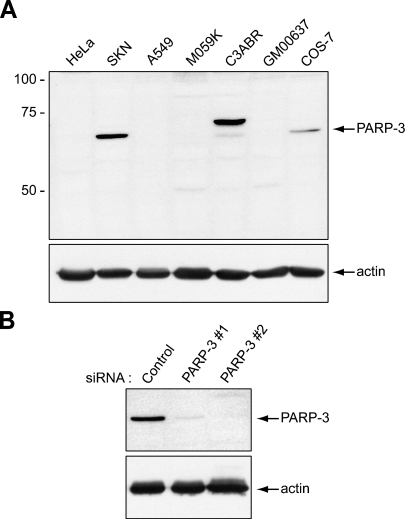
The PARP-3 antiserum, which was generated in our laboratory against a synthetic peptide corresponding to the human PARP-3 sequence, has been described (Rouleau et al. 2007). The sequence of this peptide (K24KGRQAGREEDPFRS38) is well conserved between human and monkey species. Only one mismatch (lower-case) is found in the predicted PARP-3 sequence of rhesus monkey (Macaca mulata: KKGRQgGREEDPFRS) and chimpanzee (Pan troglodytes: KKGwQAGREEDPFRS). Furthermore, the anti-PARP-3 antibody recognizes PARP-3 in COS-7 cells that were derived from the kidney of the African green monkey (Chlorocebus sabaeus) (Figure 1A).
Figure 1.
Validation of the specificity of the poly(ADP-ribose) polymerase 3 (PARP-3) antibody by Western blot analysis. (A) Top panel: The anti–PARP-3 antibody detects a single protein band of 62 kDa in the human cell line SK-N-SH and in the monkey cell line COS-7. In the C3ABR human cell line, both PARP-3 isoforms, of 67 kDa and 62 kDa, are detected (see text). The other cell lines do not express detectable levels of PARP-3. Lower panel: A duplicate blot was probed with an anti-actin antibody to show equal loading. (B) Top panel: In SK-N-SH cells transfected with small interfering RNAs (siRNAs) targeting PARP-3 (PARP-3#1 or PARP-3#2), levels of the 62-kDa protein detected with the PARP-3 antibody are drastically reduced. Levels of the 62-kDa protein are not affected by transfection with a control siRNA. Lower panel: A duplicate blot was probed with an anti-actin antibody to show equal loading.
Knock-down of PARP-3 Expression
SK-N-SH cells were seeded in 35-mm culture dishes and allowed to grow for ∼24 hr, or until they reached 60% confluence. Transfection of cells with a control Stealth small interfering RNA (siRNA) or with either of two PARP-3–specific Stealth siRNAs (Invitrogen Canada, Inc.; Burlington, Canada) was achieved with the HiPerfect transfection reagent (Qiagen, Inc.; Missisauga, Canada) according to the instructions of the manufacturer. The final concentration of the siRNA in the culture medium was 5 nM. The sequences of the siRNAs targeting PARP-3 are: #1: 5′CCCUACACUUCAACACAUCUGGAAA; and #2: 5′UGGACCGAGACUACCAGCUUCUCAA. Cells were harvested 48 hr after transfection as described above.
Western Blot Analysis
Total protein extracts corresponding to 200,000 cells were separated in each lane of an 8% SDS- polyacrylamide gel and transferred to polyvinylidene difluoride membranes (Millipore; Nepean, Canada). Membranes were blocked with PBSMT (PBS containing 0.1% Tween-20 and 5% non-fat dry milk) for 1 hr then incubated overnight at 4C with anti–PARP-3 diluted 1:5000 in PBSMT. Membranes were washed four times in PBST (PBS containing 0.1% Tween-20) then incubated with a goat anti-rabbit antibody (Jackson Immunoresearch Laboratories; West Grove, PA) diluted 1:5000 in PBSMT for 1 hr at room temperature. Membranes were washed eight times in PBST then revealed with the Chemiluminescence Plus reagents (Perkin Elmer Life Sciences; Shelton, CT). A duplicate blot was incubated with a monoclonal anti-actin antibody (clone JLA20; 1:10,000, Calbiochem, San Diego, CA) to demonstrate equal loading.
Monkey Tissues
We have used paraffin blocks of monkey tissues from a library of different adult (5–7-year-old) male and female monkeys sacrificed for previous studies. These animals were in good health, as verified by a complete veterinary examination, and were treated according to the guidelines and policies of the Canadian Council on Animal Care (http://www.ccac.ca/en/CCAC_Programs/Guidelines_Policies/gublurb.htm) and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The monkeys were sacrificed under isoflurane anesthesia and intracardiac perfusion with 10% neutral buffered formalin. Two consecutive serial sections were cut from each paraffin-embedded organ; the first was used for the immunostaining and the second as a control.
Immunohistochemistry
Immunostaining was performed using Zymed SP kits (San Francisco, CA). Paraffin sections (4-μm) were deparaffinized in toluene and gradually rehydrated through ethanol. Endogenous peroxidase activity was eliminated by preincubation of sections with 3% H2O2 in methanol for 20 min. A microwave antigen retrieval technique using citrate buffer was applied (Tacha and Chen 1994). Nonspecific binding sites were blocked using 10% goat serum for 30 min. Sections were incubated for 2 hr at room temperature with the PARP-3 antiserum (1:500), then with biotinylated anti-rabbit secondary antibody, and thereafter with streptavidin-peroxidase as described by the manufacturer. Under microscope monitoring, diaminobenzidine was used as the chromogen to visualize the biotin/streptavidin-peroxidase complex. Counterstaining was performed using #2 Gill's hematoxylin for 1 min. For the control slides, the PARP-3 antibody was preincubated with an excess of the antigenic peptide before its application on consecutive serial sections.
Results
Specificity of the Polyclonal Antibody Anti–PARP-3
To validate the specificity of the anti–PARP-3 antibody, whole-cell extracts from several cell lines of human and monkey origin were analyzed by Western blotting. The antibody detects a unique protein of the expected molecular mass for the short isoform (62 kDa) in human SK-N-SH and monkey COS-7 cells but not in the other cell lines, indicating that PARP-3 expression is beyond detection limits in many cell lines (Figure 1A). In the human lymphoblastoid C3ABR cell line, a predominant protein of ∼67 kDa is detected by the anti–PARP-3. This may be the longer PARP-3 isoform, whereas the 62-kDa isoform is only weakly detected (Rouleau et al. 2007). Levels of PARP-3 expression remain unchanged throughout the cell cycle and are not affected by the confluence of the cultured cells (not shown). We have confirmed the specificity of the antibody by verifying that preadsorption of the antibody with the immunogenic peptide interferes with the detection (not shown) and by knocking down PARP-3 expression in SK-N-SH cells using PARP-3–specific siRNAs (Figure 1B).
We have also established that the detection of PARP-3 with the anti–PARP-3 antibody using an immunohistochemical method is specific. The predominant nuclear distribution of PARP-3 detected in SK-N-SH cells is similar to that detected by immunofluorescence (Rouleau et al. 2007) and is abolished if the antibody is preadsorbed with an excess of the immunogenic peptide prior to cell labeling (Figures 2A and 2A′). Taken together, this analysis indicates that the antibody specifically recognizes PARP-3, a specificity that is further confirmed by a competition with the immunogenic peptide. Furthermore, we have previously shown that the antibody does not recognize the most closely related family members of PARP-3, PARP-1 and PARP-2 (Rouleau et al. 2007).
Figure 2.
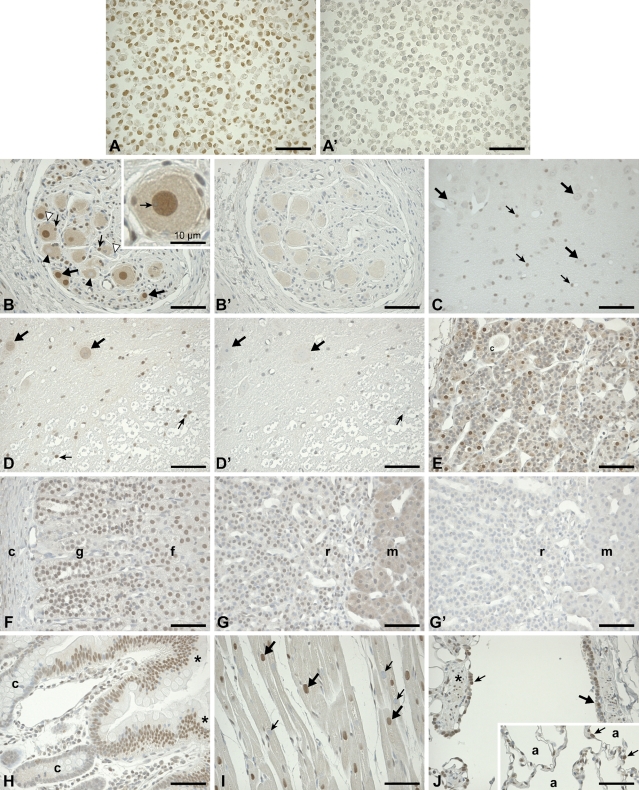
Expression of PARP-3 in normal monkey tissues. (A) Validation of the specificity of the PARP-3 antibody in paraffin section of the human cell line SK-N-SH. The nuclei of all cells are strongly labeled with the anti–PARP-3 antibody. (A′) Preincubation of the PARP-3 antibody with an excess of the immunogenic peptide abolishes staining of the consecutive SK-N-SH paraffin section, demonstrating the specificity of the antibody. (B) A strong nuclear staining is detected in many neurons of terminal ganglia (thick arrows) and satellite cells (thin arrows). However, a number of adjacent neurons and satellite cells are not labeled (black and white arrowheads, respectively). Nucleoli are not labeled, as indicated by the arrow in the inset. (B′) In the control adjacent section, no labeling is detected in the nuclei. (C) In the brain stem, several nuclei of neuroglial cells are labeled (thin arrows), whereas the neurons are not labeled (thick arrows). (D) In the gray matter of spinal cord, nuclei of some neurons are weakly labeled (thick arrows), whereas in the white matter, neuroglial cells are moderately labeled (thin arrows). (D′) No labeling is observed in the consecutive section of the spinal cord labeled with the preadsorbed antibody. Arrows indicate the same cells as in (D). (E) In the pituitary gland, a strong nuclear staining is detected in the pars tuberalis. The weak cytoplasmic staining is specific. Colloidal material (c) is seen within a cord of cells. (F) In the adrenal gland, nuclei of zona glomerulosa (g) and fasciculata (f) cells are moderately labeled. Cells of the capsule (c) are not labeled. (G) In cells of the zona reticularis (r), nuclei are weakly labeled. In the medulla (m), the cytoplasm of parenchymal cells is lightly but specifically labeled. (G′) In the adjacent control section, no staining could be detected. (H) In the duodenum, most of the absorptive epithelial cells lining the villi (asterisk) are labeled in the nucleus, whereas the glandular epithelia of the crypts (c) are not labeled. (I) The nuclei of several cardiac muscle cells are strongly labeled (thick arrows), whereas some of the nuclei of their neighbor cells are completely void of the labeling (thin arrows). (J) In the terminal and respiratory bronchioles of the lungs, some of the ciliated cells (thick arrow) and Clara cells (thin arrow) are moderately labeled. Nuclei of smooth-muscle cells (asterisks) are also labeled. In the alveolar walls (inset), nuclei of several septal cells (thin arrows) lining alveoli (a) are moderately labeled. Bar = 30 μM unless otherwise stated.
Distribution of PARP-3 in Tissues of Cynomolgous Monkey
PARP-3 is not a ubiquitously expressed protein. It is, rather, specifically expressed in a subset of cells in each of the tissues examined. This distribution is summarized in Table 1. In the majority of monkey tissues surveyed in which PARP-3 is detected, this enzyme is preferentially nuclear but excluded from the nucleolus. This is best seen in high-magnification views of neurons of the terminal ganglia (Figure 2B). However, specific cytoplasmic labeling is also detected in some cells of the pituitary and adrenal glands (Figures 2E and 2G). The cellular localization of PARP-3 is in agreement with a previous assessment of its distribution by immunofluorescence in human and monkey cell lines (Rouleau et al. 2007).
Table 1.
PARP-3 expression levels in various tissues of adult cynomolgous monkeys
| Tissuea | Histological localizationb | Proportion of cellsc | Immunostaining intensityd |
|---|---|---|---|
| Nervous system | |||
| Cerebrum | Neuroglial cells | Majority | ++ |
| Neurons | Some | ++ | |
| Spinal cord | Neuroglial cells | Majority | ++ |
| Neurons | Some | + | |
| Terminal ganglia | Neurons | Some | +++ |
| Satellite cells | Majority | ++ | |
| Peripheral nerve | Schwann cells | Majority | ++ |
| Heart | Myocardial cells | Majority | +++ |
| Smooth muscles | Muscle cells | Some | ++ |
| Skeletal muscles | Muscle cells | All | − |
| Skin | Epidermal cells | Majority | + |
| Sebaceous glands | Majority | ++ | |
| Apocrine sweat glands | Majority | ++ | |
| Hair follicles | Some | + | |
| Mammary gland | Secretory and ductal cells | Majority | + |
| Salivary glands | Acinar cells | All | − |
| Intercalated duct cells | Majority | + | |
| Striated duct cells | Majority | ++ | |
| Excretory duct cells | Majority | +++ | |
| Gastrointestinal tract | |||
| Stomach | Parietal cells | Majority | + |
| Duodenum | Absorptive epithelial cells | Majority | ++ |
| Glandular epithelia | All | − | |
| Colon | Goblet and columnar epithelial cells | Majority | + |
| Liver | Epithelial cells, small bile ducts | Majority | + |
| Epithelial cells, large bile ducts | Majority | +++ | |
| Hepatocytes | All | − | |
| Pancreas | Acinar cells | All | − |
| Centroacinar cells | Majority | ++ | |
| Ductal cells | Majority | +++ | |
| Langherans cells | Some | ++ | |
| Lung | Ciliated and Clara cells | Some | ++ |
| Septal cells | Some | ++ | |
| Alveolar epithelial cells | Majority | + | |
| Kidney | Podocytes | Majority | +++ |
| Collecting duct cells | Majority | ++ | |
| Proximal convoluted tubule cells | Majority | ++ | |
| Distal convoluted tubule cells | All | − | |
| Spleen | |||
| White pulp (germinal center and marginal zone) | Reticular cells | Majority | ++ |
| Lymphocytes | All | − | |
| Red pulp | Endothelial cells of venous sinuses | Majority | +++ |
| Macrophages | Majority | ++ | |
| Thymus | Epithelial reticular cells | Majority | ++ |
| Lymphocytes | All | − | |
| Endocrine glands | |||
| Adrenal gland | Zona glomerulosa | Majority | ++ |
| Zona fasciculata | Majority | ++ | |
| Zona reticularis | Some | + | |
| Medulla | Majority | ++C | |
| Pituitary gland | Pars tuberalis - basophils | Majority | +++ |
| Pars distalis - basophils | Majority | +++ | |
| Pars intermedia | Majority | + | |
| Female reproductive system | |||
| Ovary | Oocytes | All | − |
| Primordial follicular cells | Majority | + | |
| Granulosa lutein cells | Majority | +++ | |
| Theca lutein cells | All | − | |
| Oviduct | Epithelial cells | Majority | ++ |
| Vagina | Stratified squamous epithelium | All | − |
| Deep epithelium | Some | + | |
| Male reproductive system | |||
| Prostate | Cranial and caudal lobe cells | Majority | + |
| Ductal cells | Majority | +++ | |
| Epididymis | Head and corpus epithelial cells | Majority | ++ |
| Tail epithelial cells | Majority | + | |
| Testis | Leydig's cells | Majority | ++ |
| Sertoli cells | Majority | + | |
| Spermatocytes | All | − | |
| Seminal vesicle | Epithelial cells | All | − |
| Stromal cells | Majority | + | |
Tissues that are not mentioned were not available.
Cells that are not mentioned were not labeled.
In many tissues, only a fraction of the listed cell types were labeled. Proportions of cells with the indicated staining intensity are given.
+: weak; ++: moderate; +++: strong; −: no staining detected. Staining was detected in the nucleus unless specified as ++C, indicating cytoplasmic staining.
PARP-3, poly(ADP-ribose) polymerase 3.
Central and Peripheral Nervous Systems
PARP-3 expression varies widely among the different cell types and tissues of the nervous system. In the central nervous system, a moderate reaction was detected in the nuclei of neuroglial cells but not in neurons (Figure 2C). Similarly, nuclei of neuroglial cells of the spinal cord are moderately stained, whereas only a few neurons are labeled (Figures 2D and 2D′). In contrast, many of the large, spherical nuclei of neurons of terminal ganglia in various tissues, such as vaginal, pancreatic, and gastrointestinal tract (Meissner's plexus), are strongly labeled (Figures 2B and 2B′). A careful examination of these large, labeled nuclei reveals that the nucleolus is not labeled (see inset in Figure 2B). Many satellite cell nuclei in terminal ganglia are also strongly labeled. However, it is interesting to note that some of the terminal ganglia neurons and satellite cells are not labeled, although they appear to be similar in nature to those intensely labeled (Figure 2B). The specific detection of PARP-3 is demonstrated by the absence of nuclear staining on consecutive sections incubated with the same antibody preadsorbed with an excess of the immunogenic peptide (Figures 2B′ and 2D′). Overall, nuclei of neuronal cells of the peripheral nervous system express more PARP-3 than those of the central nervous system, whereas neuroglial and satellite cells throughout tissues of the nervous system express significant levels of PARP-3.
Pituitary and Adrenal Glands
In the pars distalis and tuberalis regions of the pituitary gland, ∼30% of the nuclei were strongly labeled, whereas cytoplasmic staining was detected in several cells (Figure 2E). After examination of the control serial section, this cytoplasmic staining was found to be specific. In the adrenal gland, a moderate PARP-3 nuclear staining is detected in the cortex (zona glomerulosa ∼ zona fasciculata > reticularis) (Figures 2F and 2G). In the medulla, a moderate but specific signal is detected in the cytoplasm of these cells, whereas the staining is not detected in the nuclei (Figure 2G). The cytoplasmic staining detected in both the pituitary and adrenal glands appears specific, because it is efficiently eliminated by a preadsorption of the antibody with the immunogenic peptide (cf. Figures 2G and 2G′).
Gastrointestinal Tract
In the duodenum, nuclei of columnar cells lining the villi are moderately labeled, whereas cells of the intestinal gland and lamina propria are not labeled (Figure 2H). In the colon (not shown), some of the goblet and columnar epithelial cell nuclei are weakly labeled and, in particular, the upper part of the tubular glands. Some lamina propria cell nuclei are also weakly labeled.
Muscles
In the heart, most but not all cardiac muscle cell nuclei are strongly labeled (Figure 2I). As observed in the terminal ganglia neurons, some nuclei of the cardiac muscle cells are not labeled, in contrast to the heavily decorated neighboring cells. The reason for this variability among the same cell kind of apparently similar nature is obscure. The cytoplasmic staining detected in cardiac cells is nonspecific, because it is not abolished by a preincubation of the antibody with an excess of the immunogenic peptide. No labeling could be detected in the elongated nuclei of skeletal muscle cells (not shown).
Lungs
In the bronchioles of the lungs, some of the ciliated cells, as well as the non-ciliated Clara cells, are moderately to strongly labeled (Figure 2J). The cytoplasm of the pseudostratified squamous epithelial cells is significantly and specifically labeled, because this staining is nearly eliminated when the antibody reaction is blocked by the immunogenic peptide (not shown). In the alveolar walls, some septal cells are moderately labeled.
Pancreas and Salivary Glands
In the pancreas, several nuclei of the islets of Langerhans cells are moderately labeled (Figure 3A). In the exocrine portion of the pancreas, most of the nuclei of epithelial cells lining intralobular, collecting, and large ducts are strongly labeled with the anti–PARP-3 antibody, whereas acinar cells are not labeled (Figure 3B). Centroacinar cells are moderately labeled (Figure 3A). This staining pattern is similar to that of the salivary glands in which nuclei of intercalated duct cells are moderately labeled, and the staining intensity increases in the nuclei of striated duct cells and of collecting ducts (Figure 3C). Furthermore, the acinar cells of the salivary glands are not labeled.
Figure 3.
Expression of PARP-3 in glandular tissues and reproductive organs of male and female monkeys. (A) In the pancreas, several nuclei of cells in the islets of Langerhans are moderately labeled with the anti–PARP-3 (arrows), whereas acinar cells are not labeled (arrowhead). Centroacinar cells (small arrowheads) forming an intercalated duct are labeled. (B) Most nuclei of the pancreatic duct cells are labeled, as shown in one of the large collecting ducts. (C) In the salivary glands, most of the nuclei of intercalated duct cells (thin arrow) as well as the nuclei of the larger striated duct (thick arrow) and excretory duct (asterisk) cells are labeled. Acinar cells (arrowheads) are not labeled. (D) In the liver, nuclei of epithelial cells lining the bile ducts (arrows) are labeled, whereas nuclei of hepatocytes (arrowheads) are devoid of staining. The light staining seen in the cytoplasm of hepatocytes is not specific, because it persisted in the corresponding control section (not shown). (E) In renal corpuscles of the kidney, nuclei of several podocytes (arrows), as well as nuclei of some endothelial cells, are labeled. Most of the nuclei of the epithelial cells lining collecting ducts (CT) and proximal convoluted tubules (pt) are labeled, whereas no immunostaining reaction could be detected in cells of the distal convoluted tubules (dc). (F) In the thymus, nuclei of several epithelial reticular supporting cells (thick arrows) and of Hassall's corpuscle cells (thin arrows) are labeled. Lymphocytes (arrowheads) are not labeled. (G) In the epidermis of the skin, the nuclei of some basal cells are weakly to moderately labeled (arrows), whereas the upper epidermal cell nuclei are less labeled. (H) Many nuclei of sebaceous gland cells (arrowheads) are labeled. (I) In the testis, the nuclei of Leydig cells (thick arrow) and some of the nuclei of blood vessels (thin arrow) of the interstitial tissue are labeled. In the seminiferous tubules (st), none of the spermatogenic cells are labeled (white arrowheads), whereas most of Sertoli cell nuclei are weakly labeled (black arrowheads). (J) In the prostate, the majority of epithelial cell nuclei of the cranial lobe (arrows) are weakly labeled, whereas (K) most nuclei of cells lining the large prostatic ducts (arrows) as well as of many stromal cells (arrowheads) are strongly labeled. (L) In the corpus of the epididymis, most nuclei of ductal epithelial cells are moderately labeled. (M) In the ovary, some nuclei of follicular cells of a primordial follicle are labeled (thick arrow), whereas the nucleus of the oocyte is not labeled (thin arrow). The weak staining of the germinal epithelium (asterisks) is not specific, because labeling persisted in the control section (not shown). (N) The nuclei of granulosa lutein cells of the corpus luteum show strong labeling (thick arrows) but not the nuclei of theca lutein cells (thin arrows). (O) In the villi of the oviduct, most of the nuclei of epithelial lining cells are moderately labeled but not the nuclei of connective tissue cells (ct). Bar = 30 μM.
Liver and Kidney
In the liver, as in the pancreas and in the salivary glands, nuclei of intercalated duct cells are weakly labeled, whereas those lining larger ducts are strongly decorated by the anti–PARP-3 antibody (Figure 3D). In contrast, nuclei of hepatocytes are not labeled, and the weak cytoplasmic staining is not specific (Figure 3D). In renal corpuscles of the kidney, nuclei of several podocytes, as well as of some endothelial and mesangial cells and of macula densa cells, are moderately to strongly labeled (Figure 3E). Nuclei of collecting ducts and proximal convoluted tubule cells are moderately labeled, whereas no immunostaining reaction could be detected in distal convoluted tubule cells.
Thymus
In the thymus, lymphocytes are devoid of staining, whereas the nuclei of the supporting epithelial reticular cells dispersed throughout the organ as well as in the Hassall's corpuscle are moderately labeled (Figure 3F).
Skin
In the skin, a weak to moderate staining is observed in the majority of nuclei of epidermal basal cells (Figure 3G). Nuclei of apocrine sweat gland cells and basal cells of sebaceous glands (Figure 3H) are moderately labeled. Some nuclei of hair follicle cells are lightly stained.
Male Reproductive Organs
In the testis, only the nuclei of Leydig cells, Sertoli cells and some nuclei of the endothelial cells lining blood vessels are labeled (Figure 3I). Spermatocytes are devoid of staining. In the prostate, the majority of epithelial cell nuclei of the cranial lobe are weakly labeled (Figure 3J). In contrast, nuclei of epithelial cells lining larger prostatic ducts as well as some of the stromal cells are strongly labeled (Figure 3K), whereas those lining the smaller ducts have a weaker labeling intensity. In the corpus and the head of the epididymis, some basal cells and most of the epithelial cell nuclei are moderately labeled (Figure 3L), whereas the labeling is weak in the tail cells (not shown). In the seminal vesicle, nuclei of the lining epithelial cells are not labeled, whereas most of the stromal cells are weakly labeled (not shown).
Female Reproductive Organs
In the ovary, primordial follicular cells are weakly labeled, whereas the nuclei of the oocytes are not labeled (Figure 3M). In the corpus luteum, the strong labeling of nuclei of many granulosa lutein cells contrasts with the unlabeled theca lutein cells (Figure 3N). In the villi of the oviduct, many of the nuclei of epithelial lining cells are moderately labeled (Figure 3O). Cells of the connective tissue are not labeled.
Discussion
This study is the first to examine the tissue distribution of PARP-3 protein expression in mammals. We provide the full scope of PARP-3 expression in very well preserved monkey tissues. We find that PARP-3 is expressed at least at a low level in all tissues, a finding that agrees with the published analyses of PARP-3 mRNA expression by Northern blot of human and mouse tissue extracts (Johansson 1999; Urbanek et al. 2002). Distribution of protein expression correlates relatively well with that of the mRNA, with the exception of the skeletal muscle, where we did not detect any significant PARP-3 protein expression. Highest expressions of PARP-3 mRNA were detected in the spleen, kidney, liver, and skeletal muscles, whereas lowest levels were in the thymus and brain. However, our histochemical analysis revealed that in each tissue examined, PARP-3 expression is restricted to a subset of cell types rather than ubiquitously expressed in all cell types. For instance, PARP-3 expression is limited to Leydig's and Sertoli cells of the testis, to neuroglial cells in the brain and spinal cord, to ductal cells of glands such as the pancreas, liver, and salivary glands, and to epithelioreticular cells of the thymus. Remarkably, PARP-3 expression in the same tissue varies among the same kind of adjacent cells, as seen in the heart (Figure 2I) and in neurons (Figure 2B). Thus, although some nuclei are strongly labeled, no labeling is detected in adjacent cells. The reason for this variability in expression levels is unknown but suggests that PARP-3 expression is tightly regulated.
An important finding from this work is the observation that PARP-3 is highly expressed in the ductal epithelia of several secretory tissues, including pancreas, salivary glands, liver, and prostate. Interestingly, we observed that the expression of PARP-3 is at a lower level in small ducts and increases to a maximum in epithelial cells of the largest collecting ducts. In contrast, PARP-3 is not expressed in the glandular epithelia of these secretory tissues.
We noticed that levels of PARP-3 expression are generally higher in differentiated cells than in immature or proliferating cells. For example, PARP-3 expression is low to undetected in the crypt cells and glandular cells of the small intestine and colon, respectively, but is moderately to strongly expressed in many cells of the villi and surface epithelial cells. Little to no expression is detected in the proliferating spermatogenic cells, maturing follicles, and oocytes. One exception would be the skin epidermis, in which basal cells are weakly labeled but more intensely labeled than the well-differentiated keratinocytes. In contrast, neurons of terminal ganglia and ductal epithelial cells of several secretory tissues are examples of well-differentiated cells expressing high levels of PARP-3. This indicates that PARP-3 expression is generally favored in differentiated cells. Although PARP-3 function as a tumor suppressor has not been studied, the PARP-3 gene is located in the 3p21.3 area of chromosome 3, a region that suffers alterations in several malignant tumors, including lung, breast, and neuroblastoma (Lerman and Minna 2000; Hesson et al. 2007; Nair et al. 2007).
PARP-3 subcellular expression is largely restricted to the nucleus. Expression is weak in the cytoplasm and below detection in the nucleolus. However, it is worth noting that the cytoplasmic staining detected in some cells of the pituitary and adrenal glands is specific, because it is efficiently eliminated by a preadsorption of the antibody with the immunogenic peptide. This predominant nuclear expression of PARP-3 is similar to that detected by immunofluorescence staining of human and monkey cell lines (Rouleau et al. 2007). Remarkably, few cell lines express PARP-3 at levels detectable by immunoblot or histochemical analysis (Figure 1A). We suspect that PARP-3 expression is relatively low in actively proliferating cells, such as laboratory cell lines, a hypothesis supported by the cellular distribution of PARP-3 reported here.
The functions of PARP-3 remain little studied. Amino acid sequence analysis indicates that PARP-3 is most closely related to PARP-1 and PARP-2, two nuclear PARPs involved in the DNA damage response. Indeed, we have previously identified that PARP-3 is associated with several proteins of the DNA damage response, including PARP-1 and DNA-PK (Rouleau et al. 2007; Mathivanan et al. 2008). These findings support a possible function of PARP-3 in the sensing and repair of DNA strand breaks, and are consistent with the nuclear accumulation of PARP-3. However, the tissue and cellular distribution of PARP-3 is relatively distinct from that of PARP-1 and PARP-2. Although a thorough survey of the distribution of these two proteins has not been carried out in human or monkey tissues, a few studies have addressed their expression in rodent tissues. In adult rodent tissues, expression of PARP-1 and PARP-2 predominates in the testis, and particularly in spermatogenic cells (Concha et al. 1989; Menegazzi et al. 1991; Schreiber et al. 2002). This markedly contrasts with the low expression of PARP-3 in the testis, which is restricted to Leydig's and Sertoli cells. A significant enrichment of PARP-1 and PARP-2 mRNA has also been detected in the thymus of adult mice, especially in the subcapsular region where immature lymphocytes proliferate (Schreiber et al. 2002). This distribution is again in marked contrast to that of PARP-3, which is not expressed in proliferating and mature lymphocytes. This indicates that PARP-3 has a distribution and most likely functions distinct from those of PARP-1 and PARP-2, at least in testis and thymus.
We have previously observed that PARP-3 is a component of polycomb group bodies and is associated with the polycomb proteins EZH2 and SUZ12, which are involved in the epigenetic regulation of transcription (Sparmann and van Lohuizen 2006; Rouleau et al. 2007). The nuclear accumulation of PARP-3 is consistent with a regulatory role in transcription. Polycomb group proteins are transcriptional repressors that regulate the proper spatial and temporal expression of developmental genes. The preferential expression of PARP-3 in well-differentiated cells suggests that PARP-3 could be required for the repression in mature cells of genes expressed only during early developmental programs.
In conclusion, PARP-3 is expressed in a specific subset of cells in each of the examined tissues. Highest expression of PARP-3 is detected in the ductal epithelia of secretory tissues and neurons of terminal ganglia.
Acknowledgments
We are grateful to the Cancer Research Society, Inc. for their financial support.
We thank Maxime Tardif for the synthesis of the PARP-3 immunogenic peptide, and the infography group of the CHUL research center for the preparation of figures.
References
- Augustin A, Spenlehauer C, Dumond H, Menissier-De Murcia J, Piel M, Schmit AC, Apiou F, et al. (2003) PARP-3 localizes preferentially to the daughter centriole and interferes with the G1/S cell cycle progression. J Cell Sci 116:1551–1562 [DOI] [PubMed] [Google Scholar]
- Concha II, Figueroa J, Concha MI, Ueda K, Burzio LO (1989) Intracellular distribution of poly(ADP-ribose) synthetase in rat spermatogenic cells. Exp Cell Res 180:353–366 [DOI] [PubMed] [Google Scholar]
- Haince JF, Kozlov S, Dawson VL, Dawson TM, Hendzel MJ, Lavin MF, Poirier GG (2007) Ataxia telangiectasia mutated (ATM) signaling network is modulated by a novel poly(ADP-ribose)-dependent pathway in the early response to DNA-damaging agents. J Biol Chem 282:16441–16453 [DOI] [PubMed] [Google Scholar]
- Haince JF, McDonald D, Rodrigue A, Dery U, Masson JY, Hendzel MJ, Poirier GG (2008) PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J Biol Chem 283:1197–1208 [DOI] [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO (2008) The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front Biosci 13:3046–3082 [DOI] [PubMed] [Google Scholar]
- Hesson LB, Cooper WN, Latif F (2007) Evaluation of the 3p21.3 tumour-suppressor gene cluster. Oncogene 26:7283–7301 [DOI] [PubMed] [Google Scholar]
- Johansson M (1999) A human poly(ADP-ribose) polymerase gene family (ADPRTL): cDNA cloning of two novel poly(ADP-ribose) polymerase homologues. Genomics 57:442–445 [DOI] [PubMed] [Google Scholar]
- Lerman MI, Minna JD (2000) The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res 60:6116–6133 [PubMed] [Google Scholar]
- Mathivanan S, Ahmed M, Ahn NG, Alexandre H, Amanchy R, Andrews PC, Bader JS, et al. (2008) Human Proteinpedia enables sharing of human protein data. Nat Biotechnol 26:164–167 [DOI] [PubMed] [Google Scholar]
- Menegazzi M, Grassi-Zucconi G, Carcerero De Prati A, Ogura T, Poltronieri P, Nyunoya H, Shiratori-Nyunoya Y, et al. (1991) Differential expression of poly(ADP-ribose) polymerase and DNA polymerase beta in rat tissues. Exp Cell Res 197:66–74 [DOI] [PubMed] [Google Scholar]
- Nair PN, McArdle L, Cornell J, Cohn SL, Stallings RL (2007) High-resolution analysis of 3p deletion in neuroblastoma and differential methylation of the SEMA3B tumor suppressor gene. Cancer Genet Cytogenet 174:100–110 [DOI] [PubMed] [Google Scholar]
- Rouleau M, Aubin RA, Poirier GG (2004) Poly(ADP-ribosyl)ated chromatin domains: access granted. J Cell Sci 117:815–825 [DOI] [PubMed] [Google Scholar]
- Rouleau M, McDonald D, Gagne P, Ouellet ME, Droit A, Hunter JM, Dutertre S, et al. (2007) PARP-3 associates with polycomb group bodies and with components of the DNA damage repair machinery. J Cell Biochem 100:385–401 [DOI] [PubMed] [Google Scholar]
- Schreiber V, Ame JC, Dolle P, Schultz I, Rinaldi B, Fraulob V, Menissier-de Murcia J, et al. (2002) Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem 277:23028–23036 [DOI] [PubMed] [Google Scholar]
- Sparmann A, van Lohuizen M (2006) Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 6:846–856 [DOI] [PubMed] [Google Scholar]
- Tacha DE, Chen T (1994) Modified antigen retrieval procedure: calibration technique for microwave ovens. J Histotechnol 17:365–366 [Google Scholar]
- Urbanek P, Paces J, Kralova J, Dvorak M, Paces V (2002) Cloning and expression of PARP-3 (Adprt3) and U3–55k, two genes closely linked on mouse chromosome 9. Folia Biol (Praha) 48:182–191 [PubMed] [Google Scholar]