Abstract
The accurate segregation of DNA is essential for the faithful inheritance of genetic information. Segregation of the prototypical P1 plasmid par system requires two proteins, ParA and ParB, and a centromere. When bound to ATP, ParA mediates segregation by interacting with centromere-bound ParB, but when bound to ADP, ParA fulfils a different function: DNA-binding transcription autoregulation. The structure of ParA is unknown as is how distinct nucleotides arbitrate its different functions. To address these questions, we carried out structural and biochemical studies. Crystal structures show that ParA consists of an elongated N-terminal α-helix, which unexpectedly mediates dimerization, a winged-HTH and a Walker-box containing C-domain. Biochemical data confirm that apoParA forms dimers at physiological concentrations. Comparisons of four apoParA structures reveal a strikingly flexible dimer interface that allows ParA to adopt multiple conformations. The ParA–ADP structure shows that ADP-binding activates DNA binding using a bipartite mechanism. First, it locks in one specific dimer conformation, and second, it induces the folding of two DNA-binding basic motifs that we show are critical for operator binding.
Keywords: ParA, partition NTPase, segregation, Walker box, winged-HTH
Introduction
Partition or segregation is the process whereby the genetic material is accurately moved and positioned to the daughter cells to ensure accurate genome transmission. In bacteria, low copy number plasmids represent excellent model systems to study partition from a structural standpoint because they require only three elements: a centromere-like DNA site, a partition NTPase and a centromere-binding protein (Hayes and Barillà, 2006; Schumacher, 2008). Plasmid partition (par) systems can be divided into two main types, types I and II, based on the kind of partition NTPase present (Gerdes et al, 2000). The type I systems contain partition ATPases called ParA with deviant Walker A-type folds and centromere-binding proteins called ParB. The type II systems use actin-like proteins called ParM, which can use either ATP or GTP and centromere-binding proteins called ParR (Gerdes et al, 2000; Popp et al, 2008). The type I par systems can be further divided into types Ia and Ib based on the size and function of the Par proteins. Specifically, the type Ia ParA and ParB proteins contain between 321–420 and 312–342 residues, respectively, whereas the type Ib ParA and ParB proteins are smaller, containing 192–308 and 46–131 residues, respectively. A notable distinction between the types Ia and Ib ParA proteins is the fact that the type Ia proteins also function as DNA-binding transcriptional regulators. More recently, type III systems that use tubulin-like GTPases and type IV systems that use a single protein for both centromere binding and partition with a predicted coiled-coil domain have been identified (Simpson et al, 2003; Larsen et al, 2007).
Plasmid partition is best understood for the type II systems, which use actin-like filaments formed by ParM to drive plasmid separation (Møller-Jensen et al, 2003; Popp et al, 2008; Salje and Löwe, 2008). How the type I par systems mediate plasmid separation is less clear. Data from other Walker-type proteins reveal that they undergo an ATP-induced dimerization (Lutkenhaus and Sundaramoorthy, 2003), suggesting that dimerization may be a pre-requisite for partition. However, it is difficult to envision how a simple monomer to dimer alteration could lead to the propulsion of replicated plasmid DNA to opposite cell poles. More recent data have suggested that the type I ParA proteins, δ, ParF, pB171ParA, IncC and SopA can form filaments on ATP binding, suggesting that they may function in a manner analogous to the type II ParM proteins (Barillà et al, 2005; Lim et al, 2005; Ebersbach et al, 2006; Bouet et al, 2007; Pratto et al, 2008; Batt et al, 2009). Although it is not clear in most of these cases how these polymers function in partition, the ability of Walker-type proteins to form such structures is not unprecedented as the Walker protein, MinD, forms filament-like structures on binding ATP and interacting with the membrane (Lutkenhaus and Sundaramoorthy, 2003).
The Escherichia coli P1 par system has served as a prototype in the study of type Ia partition, and consists of the partition ATPase, ParA (44 kDa), the centromere-binding protein ParB (38 kDa) and the centromere, parS, which together mediate segregation (Surtees and Funnell, 2003). The parS is one of the most complex centromeres and contains two different DNA-binding motifs, A-boxes and B-boxes, that are recognized by ParB (Funnell, 1988b, 1991; Funnell and Gagnier, 1994; Schumacher and Funnell, 2005; Schumacher et al, 2007). There are several par systems that are homologous to P1 par including the E. coli P7 plasmid and the par systems from the virulence plasmids, Salmonella typhimurium pSLT, Salmonella flexineri pWR501 and Yersinia pestis pMT1 (Cerin and Hackett, 1993; Hayes and Austin, 1993; Hu et al, 1998; Youngren et al, 2000). These systems encode homologous Par proteins and contain similar centromeres, suggesting that they all use the same partition mechanism. Studies on P1 segregation indicate that this mechanism involves several key steps. First, multiple ParB molecules bind cooperatively to the centromere to form the partition complex (Funnell and Gagnier, 1994; Hayes and Austin, 1994; Surtees and Funnell, 2003). In the last step of partition, ParA is recruited to the partition complex by interacting with the N-terminal domain of ParB. ParA then somehow drives the separation of paired, replicated plasmids (Davey and Funnell, 1994; Bouet and Funnell, 1999; Fung et al, 2001).
Notably, P1 ParA functions not only in segregation, but also as a transcription factor by binding to an extended DNA site within the par promoter to repress parAB transcription (Davis et al, 1992; Davey and Funnell, 1994, 1997). Autoregulation has important functional consequences as overexpression of either ParA or ParB interferes with partition (Abeles et al, 1985; Funnell, 1988a). Consistent with this extra function in transcription, ParA and other type Ia proteins contain an extra N-terminal of approximately 100 residues, not found in type Ib ParA homologues, that have been implicated in DNA binding (Radnedge et al, 1998). How ParA carries out the different functions of transcriptional regulation and DNA segregation is unclear. However, studies have shown that ParA switches between these two functional roles by binding distinct adenine nucleotides. Specifically, when bound to ADP, ParA is activated by >20-fold to bind its DNA site within the par promoter (Davey and Funnell, 1994). In contrast, ATP binding activates ParA for its role in partition to separate replicated plasmids (Davis et al, 1996; Bouet and Funnell, 1999; Fung et al, 2001). To gain insight into the molecular mechanisms by which these distinct adenine nucleotides toggle ParA between its DNA binding and partition states, we carried out structural and biochemical studies on P1 ParA and its close homologue, P7 ParA.
Results and discussion
apoParA structure: a novel Walker-box protein with an N-terminal α/winged-HTH region
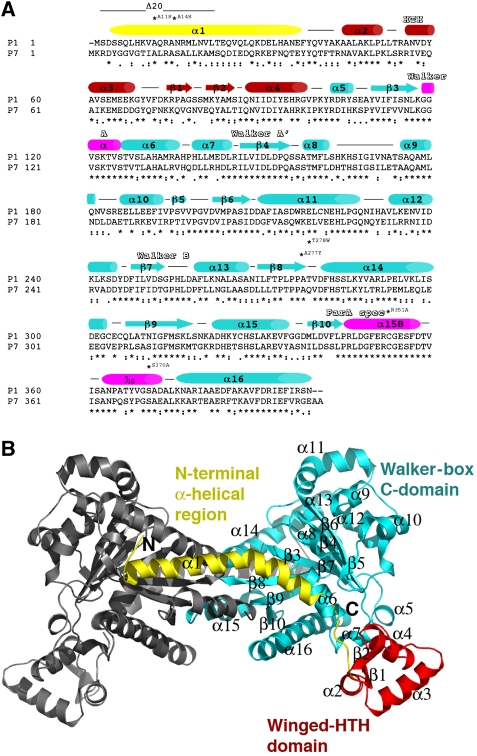
The P1 and P7 ParA proteins share 58% sequence identity and >75% sequence similarity, indicating that they contain the same fold (Figure 1A). Homology specificity scanning studies, which showed that functional regions of P1 and P7 ParA proteins can be freely interchanged to affect specificity in operator DNA binding or ParB interaction, are consistent with this supposition (Radnedge et al, 1998). To address ParA function, we first determined the apo structures of P1 and P7 ParA. Two crystal forms (C2221 and P6222) of P7 apoParA were obtained under different conditions. The P7 apoParA C2221 structure was solved by multiwavelength anomalous diffraction (MAD) and refined to an Rwork/Rfree of 25.4%/29.4% to 2.78 Å resolution (see Materials and methods; Table I). The structure contains two ParA subunits in the crystallographic asymmetric unit (ASU) and the structure includes residues 1–117, 126–162, 173–317, 321–343 and 373–399 of one subunit and residues 1–34, 39–117, 124–163, 173–317, 321–345 and 374–399 of the other subunit, 77 water molecules and 1 magnesium ion. The second P7 apoParA crystal form, P6222, was solved by molecular replacement and refined to an Rwork/Rfree of 25.2%/27.9% to 2.8 Å resolution. This structure contains one P7 ParA subunit in the ASU and includes residues 3–23, 38–117, 126–162, 169–345 and 373–399, 7 sulphate ions and 42 water molecules. Crystals of apo P1 ParA were obtained using 2 M sodium formate and 0.1 M acetate pH 4.6. The P1 apoParA structure was solved and refined to an Rwork/Rfree of 24.6%/28.1% to 2.92 Å resolution (Table I). The structure contains one subunit in the ASU and includes ParA residues 4–116, 123–158 and 172–398 and 19 water molecules.
Figure 1.
Sequence homology of P1 and P7 ParA proteins and overall structure. (A) Sequence alignment of the P1 and P7 ParA proteins. Secondary structural elements are indicated over the sequences and the three structural regions are coloured yellow (N-terminal α-helical region), red (winged-HTH region) and cyan (Walker-box C-domain). Regions that fold into helices on ADP binding are indicated by pink cylinders. The HTH, Walker A, Walker A′, Walker B and ParA-specific regions are labelled. Residues mutated in the study are also labelled. (B) Ribbon diagram of the P7 apoParA dimer. The molecule is coloured coded as in (A). This figure and Figures 2, 3A–C, 4A–C, 5 and 6A were made with PyMOL (Delano, 2002).
Table 1.
Selected crystallographic data for ParA structures
| Crystal | P1 apoParA | P7 apoParA | P7 apoParA | P1 ParA–ADP | P1 ParA–ADP |
| pdbID | 3EZ7 | 3EZF | 3EZ9 | 3EZ2 | 3EZ6 |
| Space group | I4122 | P6222 | C2221 | P212121 | P41212 |
| # Molecules/ASU | 1 | 1 | 2 | 2 | 2 |
| Resolution (Å) | 72.6–2.92 | 128.0–2.80 | 100.5–2.78 | 63.8–2.05 | 107.8–2.58 |
| Overall Rsym (%)a | 5.5 (37.4)b | 5.7 (44.1) | 4.8 (33.1) | 7.6 (48.7) | 8.9 (33.1) |
| Overall I/σ (I) | 9.4 (2.0) | 9.1 (1.8) | 10.1 (2.3) | 9.1 (2.1) | 7.0 (2.1) |
| #Total reflections | 73 545 | 32 572 | 92 696 | 237 236 | 221 119 |
| #Unique reflections | 14 700 | 11 570 | 28 792 | 70 677 | 35 970 |
| Refinement statistics | |||||
| Resolution (Å) | 72.6–2.92 | 128.0–2.80 | 100.5–2.78 | 63.8–2.05 | 107.8–2.58 |
| Rwork/Rfree (%)c | 24.6/28.1 | 25.2/27.9 | 25.4/29.4 | 19.0/23.0 | 18.5/24.0 |
| RMSD | |||||
| Bond angles (deg) | 1.59 | 1.68 | 2.21 | 1.56 | 1.36 |
| Bond lengths (Å) | 0.009 | 0.009 | 0.016 | 0.013 | 0.009 |
| Ramachandran analysis | |||||
| Most favoured (%/#) | 82.1/285 | 81.8/248 | 86.8/539 | 92.7/660 | 88.9/633 |
| Add. allowed (%/#) | 15.0/52 | 16.2/49 | 11.8/73 | 7.0/50 | 10.7/76 |
| Gen. allowed (%/#) | 2.9/10 | 1.7/5 | 1.3/8 | 0.3/2 | 0.4/3 |
| Disallowed (%/#) | 0/0 | 0.3/1 | 0.2/1 | 0.0/0 | 0.0/0 |
| aRsym=ΣΣ∣Ihkl−Ihkl(j)∣/ΣIhkl, where Ihkl(j) is observed intensity and Ihkl is the final average value of intensity. | |||||
| bValues in parentheses are for the highest resolution shell. | |||||
| cRwork=Σ∣∣Fo∣−∣Fc∣∣/Σ∣Fo∣ and Rfree=Σ∣∣Fo∣−∣Fc∣∣/Σ∣Fo∣, where all reflections belong to a test set of 5% randomly selected data. | |||||
The structures show that ParA contains a unique multidomain architecture that can be broken into three main regions: an extended N-terminal α-helix (residues approximately 1–43), a winged-HTH motif (residues 44–104, where residues 44–68, α2 and α3 comprise the HTH motif) and a large C-terminal domain (residues 105–399) that contains a deviant Walker A motif (Figure 1A and B; Supplementary Figure 1). The deviant Walker fold that is embedded in the C-terminal 300 residues of ParA shows the strongest structural similarity to that of the Thermus thermophilus Soj protein, which is a chromosomal type Ib ParA homologue (Leonard et al, 2005). The ParA Walker-box region and Soj can be maximally superimposed with a root mean square deviation (RMSD) of 2.47 Å for 199 corresponding Cα atoms. The ParA winged-HTH shows only weak homology to the excisionase protein from the conjugative transposon Tn916. Residues 10–64 of Tn916 can be superimposed onto ParA residues 37–95 (including multiple gaps and insertions) with an RMSD of 3.10 Å (Abbani et al, 2005).
apoParA forms a conformationally flexible dimer
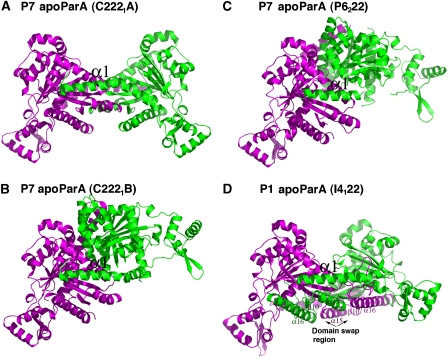
As expected, the P1 and P7 apoParA monomers are structurally homologous and can be superimposed with RMSDs of ∼1.0 Å for all corresponding Cα atoms. Unexpectedly, however, all the apoParA structures are dimeric (Figure 2A–D). The total buried surface area (BSA) for each dimer is significant: 4245 Å2 for C2221 dimer A, 4978 Å2 for C2221 dimer B and 5000 Å2 for P6222 dimer. Comparison of the three P7 apoParA dimers shows that although they are similarly arranged, they show a striking degree of variability in their interfaces. For example, the C2221 A dimer has a more open C-domain–C-domain′ interface than either the C2221 B or the P6222 dimers (where ′ indicates other subunit in the dimer). In fact, there is >90° difference in rotation of the two subunits in the C2221 A dimer compared with those of the other P7 apo dimers. Thus, these structures indicate that if apoParA forms a dimer, the dimer is conformationally flexible (Figure 2A–D).
Figure 2.
Four structures of apoParA reveal that ParA is a conformationally flexible dimer. (A) Structure of the P7 apoParA C2221 dimer A. One subunit is coloured green and the other magenta. The magenta subunit is shown in the same orientation in (B–D). The α1–C-domain′ interaction is the only conserved dimer interaction. (B) Structure of the P7 apoParA C2221 dimer B. (C) Structure of the P7 apoParA P6222 dimer. (D) Structure of the P1 apoParA-domain-swapped dimer. The swapped regions are labelled.
Although the interfaces are diverse, there is one interaction that is shared by all apoParA dimers, which is the insertion of α1 into a surface exposed hydrophobic crevice between α14′ and α15′ on the other subunit of the dimer (Figures 2A–D and 3A). The presence of this interface in the P1 apoParA structure is especially notable because this dimer is formed by domain swapping interactions that were likely stimulated by the low pH solution used for its crystallization (Figure 2D) (see Materials and methods). To date, multiple artefactual-domain-swapped oligomeric structures have been obtained under non-physiological low pH conditions. An important requirement for domain swapping is the presence of flexible, hinge-loop regions (Liu and Eisenberg, 2002). Notably, all the P7 apoParA structures contain a large number of disordered regions, including residues 118–125, 164–172, 318–325 and 344–372. The finding that the ParA-domain swap occurs within residues 318–398 is consistent with it being a flexible region in the apo protein. In the low pH, P1 apoParA structure, these C-domain residues rotate away from the subunit to which they are covalently attached by hinge residue 318 and swap into the C-domain of the other subunit in the dimer. As a result, α15–β10–α16, although identically located in the P1 structure as in the non-swapped apo structures, now belongs to the other subunit in the dimer (Figure 2D). Residues 318–398 become largely folded in the context of the swapped dimer, likely aiding in the stability of the swapped state. Indeed, factors influencing domain swapping include new interactions that are formed and strains that are relieved on swapping (Schymkowitz et al, 2001). Formation of the P1 apoParA-domain-swapped dimer also appears to support the notion that apoParA is a dimer, as to form the swapped dimer the two C-domains must be closely juxtaposed such that with a simple rotation of the flexible hinge region, residues in one subunit can substitute for the corresponding residues in the other subunit.
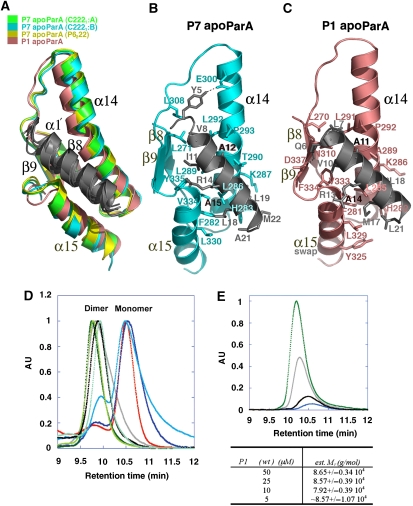
Figure 3.
The conserved ParA α1–C-domain′ dimer interface. (A) Superimposition of β8, α14, β9 and α15 of the P7 apoParA and the P1 apoParA structures, which results in a superimposition of α1′. α1 is coloured grey, whereas β8, α14, β9 and α15 are coloured green for the P7 apoParA (C2221 A dimer), blue for the P7 apoParA (C2221 B dimer), yellow for the P7 apoParA P6222 structure and salmon for the P1 apoParA structure. α15 of the P1 structure is from the other subunit due to domain swapping. (B) α1–C-domain′ contacts in the P7 apoParA structure. Shown as sticks are the residues mediating interactions. Residues Ala12 and Ala15 corresponding to Ala11 and Ala14 in P1 ParA are labelled in black. (C) α1–C-domain′ contacts in the P1 apoParA structure. (D) SEC-LS analysis of ParA oligomer state. For clarity, the portion of the chromatogram used for the analysis for the monomer and dimer states has been shown. Samples fall into two groups characterized by their molecular masses (mw) as calculated using ASTRA software; a dimeric state: P1 ParA, wt (jade; mw=8.86±0.02 104 g/mol), P7 ParA, wt (black; mw=8.73±0.07 104 g/mol), P1 ParA (T278W) (green; mw=1.00±0.01 105 g/mol), P1 ParA with C-terminal tag (light green; mw=9.16±0.82 104 g/mol) and ParA (A277Y) (grey; mw=1.00±0.01 105 g/mol); or monomeric state: ParA (Δnt20) (red; mw=4.99±0.03 104 g/mol), ParA (A14S) (blue; mw=5.14±0.11 104 g/mol) and ParA (A11S) (aqua; mw=6.27±0.05 104 g/mol). (E) SEC-LS carried out at the lower limit of detection showing ParA dimers. P1 ParA was measured at 50 μM (green), 25 μM (grey), 10 μM (black) and 5 μM (blue).
Role of α1 in ParA dimerization
The α1–C-domain′ dimer interface buries over 2800 Å2 of BSA, indicating that it alone could support ParA dimer formation. Moreover, the hydrophobic interactions in this interface are highly conserved between P1 and P7, despite the fact that the N-terminal domain is the region showing the lowest homology between P1 and P7 ParA (Figures 1A and 3B–C). In the P7 interface, the side chains of α1 residues Val8, Ile11, Ala12, Ala15, Leu19, Ala21 and Met22 make a series of hydrophobic contacts with α14 and α15 residues including the methylene carbons of Lys287, the side chains of Phe282, His283, Leu286, Leu 289, Thr290, Leu292, Pro293, Leu330, Val334 and Tyr335. Additional contacts with α1 are provided by residues from β8 and β9. The only polar contact in the interface is between Glu300 and Tyr5, whereas Arg14 makes a charge-helix dipole contact with the C-terminal end of α15 (Figure 3B). Similarly in the P1 interface, α1 residues Leu7, Val10, Ala11, Ala14, Met17, Leu18 and Leu21 interact with Phe281, His282, Leu285, Lys286, Ala289, Leu291, Pro292, Tyr325, Leu329, Val333, Phe334 and β8 residue Leu270. In the P1 interface, the analogous P7-helix capping interaction between the side chain of Arg13 and the negative C-terminal-helix dipole of α15 is observed. However, instead of the Tyr5-Glu300 interaction, a different stabilizing hydrogen bond is formed between α1 residue Gln6 and β9 residue Asp337 (Figure 3C). Thus, the apoParA dimer is an unusual dimer in that it is conformationally adaptable. This plasticity is imparted by the flexible linkage of the α1 arms to the rest of the protein (Figure 2A–D).
apoParA dimerizes at physiologically relevant concentrations
The possibility that apoParA may be a dimer in vivo has important consequences for its cellular functions. Indeed, if apoParA is already a dimer in vivo, then mechanisms other than a monomer to dimer switch must be considered for its roles in partition and transcription. The crystal structures of ParA were obtained at concentrations of 70–100 μM; however, the physiological concentration of ParA has been measured to be ∼1 μM (BE Funnell, unpublished data). Thus, to examine the oligomeric state of ParA in solution at close to biologically relevant concentrations, we performed exclusion chromatography resolved-light scattering (SEC-LS) studies. SEC-LS showed that both P1 and P7 apoParA proteins have apparent masses of ∼90 kDa, consistent with a dimer. Moreover, sample concentrations as low as 5 μM (roughly the detectable limit for ParA using SEC-MALS) still showed only the dimeric species (Figure 3D). Finally, glutaraldehyde cross-linking of apo P1 ParA revealed that the protein is dimeric at 500 nM (Supplementary Figure 2).
These data support that apoParA is a dimer. We next tested whether the dimer observed in the crystal structures is that found in solution using mutagenesis and SEC-LS. Two categories of mutations were made: those within the α1–C-domain′ interface and those within the C-domain–C-domain′ interface. Although the α1–C-domain′ interface is conserved in all apoParA structures and predicted to be essential in dimer formation, the flexible C-domain–C-domain′ interface is not. Consistent with this hypothesis, the removal of the N-terminal 20 residues of P1 ParA (Δ20 ParA) completely abrogated dimerization. The α1 residues Ala11 and Ala14 (Ala12 and Ala15 in P7 ParA) make interactions in the α1–C-domain′ interface that are dependent on the small size of the alanine side chains; Ala14 is buried in the interface, whereas Ala11 is more surface exposed. Mutation of Ala14 to serine eliminated apoParA dimerization, whereas an A11S mutation caused a near abrogation of dimerization (Figure 3D). In contrast, substitutions of C-domain residues found in the P1 apoParA C-domain–C-domain′ interface, Ala277 and Thr278, to the much larger residues tyrosine and tryptophan, had essentially no effect on oligomerization. This is consistent with the C-domain–C-domain′ interface being flexible. In fact, as both Ala277 and Thr278 are in the C-domain–C-domain′ interface in the P1 apoParA structure, neither of the corresponding residues in P7 ParA (Ala278 and Gln279) are involved in C-domain–C-domain′ contacts in the P7 apoParA C2221 A dimer and only Gln279 participates in weak C-domain–C-domain′ contacts in the P7 apoParA C2221 B and P7 apoParA P6222 dimers. Thus, the combined data indicate that the α1–C-domain′ interaction is necessary and sufficient for apoParA dimerization.
ParA–ADP structures
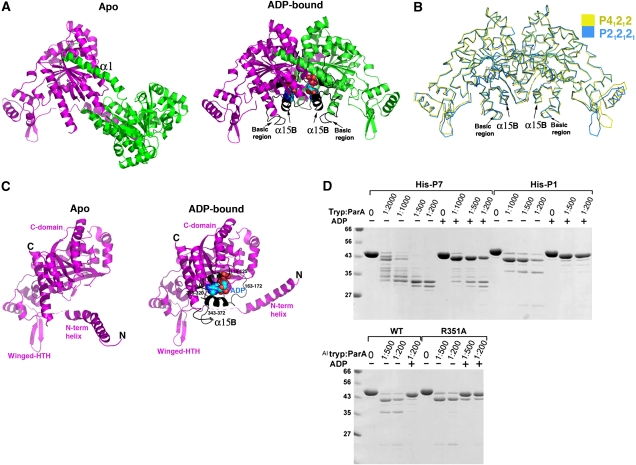
P7 and P1 ParA function as DNA-binding proteins to autoregulate par transcription (Hayes et al, 1994). Studies on P1 ParA have shown that it recognizes a 40 nucleotide imperfect inverted repeat between the –10 box and ribosome-binding site of the P1 par promoter (Hayes et al, 1994). However, the entire protected site includes approximately 150 nucleotides that is centred over the inverted repeat (Davey and Funnell, 1994). ADP binding stimulates DNA binding by ParA by >20-fold. To gain insight into how ADP activates ParA to bind DNA, we determined crystal structures of the P1 ParA–ADP complex. Two crystal forms (P212121 and P41212) were grown at ADP concentrations of 5 and 1 mM, respectively, and both were obtained at pH 7.0. The P212121 crystal form was solved and refined to an Rwork/Rfree of 19.0%/23.0% to 2.05 Å resolution (Table I). The structure contains two ParA molecules in the ASU and contains residues 5–29, 37–398 of one subunit, residues 5–398 of the second subunit and includes 426 water molecules, 2 glycerol molecules, 1 HEPES molecule, 2 magnesium ions and 4 ADP molecules. The final P41212 crystal has an Rwork/Rfree of 18.5%/24.0% to 2.58 Å resolution and also contains two subunits in the ASU. The model includes residues 5–29 and 36–398 of one subunit, 5–398 of the second subunit, 267 water molecules, 2 magnesium ions and 2 ADP molecules (Figure 4A and B).
Figure 4.
ParA–ADP structure: conformational locking and local refolding. (A) Comparison of apoParA C2221 A and ParA–ADP dimers. One subunit is coloured magenta and one green. The magenta subunits are shown in the same orientation for reference. The ADPs are shown as CPK. The regions that become folded on ADP binding are coloured black. (B) Superimposition of all corresponding Cα atoms of the two ParA–ADP dimers, space group P212121 (cyan) and P41212 (yellow), showing that, unlike the apo structures, ADP-binding locks in one dimer conformation. (C) Comparison of the apo ParA and ParA–ADP monomer structures. The monomers are shown in the same orientation and the regions that fold on ADP binding are coloured black and labelled in the ParA–ADP monomer. (D) Partial proteolysis assays. Each reaction mixture (10 μl) contained 3 μg of the indicated ParA and trypsin as indicated. ADP, when present, was at 2 mM. The mixtures were analysed by electrophoresis on 12% SDS–polyacrylamide gels. The trypsin:parA ratio (w/w) and the presence of ADP are indicated above each lane.
ADP binding to ParA leads to local and global conformational changes
Both ParA–ADP structures are dimeric and contain the same α1–C-domain′ interaction observed in the apo dimers (Figure 4A). However, unlike the flexible dimer observed in apoParA, superimposition of all the Cα atoms of the two ParA–ADP dimer structures, which results in an RMSD of 1.0 Å, shows that the ADP-bound ParA dimers adopt the same configuration (Figure 4B). Only residues in the flexible wing from the winged-HTH show slight differences, and, similar to other winged-HTH proteins, likely become stabilized on DNA binding. Therefore, ADP, which binds to a specific set of residues from both ParA subunits in the dimer, appears to lock in one specific dimer state. Moreover, comparison of the apoParA and ParA–ADP structures revealed that ADP binding also leads to a striking, large-scale folding of four regions of ParA: residues 118–125, residues 164–173 and residues 318–320; 344–375. These regions either directly interact with ADP or are near residues that interact with ADP. The result of this folding is the formation of a new α-helix (α15B; residues 346–355 and 364–367) and several additional turns of α-helix at the N-terminus of α6 (Figures 1A and 4A, C). Such extensive conformational changes on ADP binding are unusual for Walker-box proteins, which tend to be well folded in the absence of nucleotide. However, the finding that ADP binding leads to ∼4% increase in ParA helix explains earlier circular dichroism data showing ∼5% increase in ParA helical content on adenine nucleotide binding (Davey and Funnell, 1994).
Limited proteolysis experiments showing that apoParA has multiple flexible regions that become stabilized on ADP binding are consistent with the structures (Figure 4D). Although the P7 apo protein appears to be more flexible than the P1 protein, the trypsinolysis pattern is very similar between the two. N-terminal sequencing of the fragments was used to identify proteolysis sites. These data show that the initial cut site corresponds to Arg16. After this cleavage, the N-terminal region of α1 likely remains bound to the other subunit of the dimer, but the C-domain becomes more exposed. These data, in combination with the observation that P1 R351A is lacking a cleavage, indicate that the residue after Arg351 in P1 apoParA is cleaved after proteolysis in α1 (Figure 4D). The P1 ParA structure shows that the region from 159 to 171 would also become exposed, and indeed, the residue after Lys164 is cleaved closely after or at the same time as that after Arg351. ADP binding leads to marked protection against all these proteolysis events as expected from our structural data (Figure 4D).
Walker-box motifs and a ParA-specific motif interact with ADP
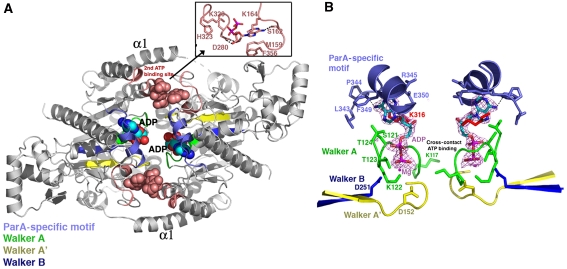
Like other deviant Walker-box proteins, ParA contains three conserved structural regions: the Walker A, Walker A′ (switch 1) and Walker B (switch 2) motifs. The Walker A motif, which is a glycine-rich region, corresponds to ParA residues 116–124, whereas the Walker A′ and Walker B motifs correspond to residues 147–155 and 246–251 (Figure 5A and B) (Lutkenhaus and Sundaramoorthy, 2003). The ParA Walker A motif is mostly disordered in the apo structures. However, on ADP binding, these residues fold up to encase the ADP phosphate groups, allowing the amide nitrogens to hydrogen bond with the β phosphate (Figure 5B). The side chain of Lys122 also contacts the β phosphate moiety. The importance of Lys122 in adenine nucleotide binding is underscored by the finding that the K122Q and K122E mutant proteins show reduced or no ATPase activity and altered repressor and partition function (Fung et al, 2001). Lys117 of the Walker A motif makes no contacts with ADP in the ParA–ADP structure. However, in structures of other Walker-box proteins bound to ATP, the corresponding residue makes contacts with the γ phosphate of the ATP molecule bound in the other subunit of the dimer, favouring the formation of an ATP sandwich dimer. As ParA is already a dimer, such cross-contacts, if present, would likely play a different role, such as stabilizing a specific dimer state that is active for partition. However, the structure of a ParA–ATP complex is needed to address this issue.
Figure 5.
ADP-binding interactions: Walker motifs and a second ADP-binding pocket. (A) Overall structure of the P212121 ParA–ADP structure. The α1 helices and the Walker motifs, A, A′ and B are labelled. The Walker motifs are coloured green (Walker A), yellow (Walker A′) and dark blue (Walker B). The ParA-specific motif is cyan. The second ADP-binding site is coloured salmon and the ADP is shown as CPK. Inset to the upper right is a close-up view of the interactions between the two ParA subunits and the second (non-primary)-bound ADP molecule. (B) Close-up of the ‘primary' ADP-binding site and a 2.05 Å resolution Fo–Fc map (magenta mesh), calculated after omitting the ADP, and contoured at 4.5σ. The nucleotide-binding motifs are coloured as in (A).
Asp152 of the Walker A′ motif and Asp251 of the Walker B motif make water-mediated contacts with a magnesium ion bound in the pocket (Figure 5B). These acidic residues are also critical for ParA function as their mutation leads to ParA proteins with impaired ATPase activity, DNA binding and partition functions (Fung et al, 2001). Notably, ParA also contains a motif, which we term the ParA-specific motif, composed of residues 343–350 that becomes ordered on ADP binding and is responsible for mediating most of the contacts with the ADP adenine (Figure 5B). The carbonyl of Pro344 in this motif makes a key hydrogen bond to the adenine N6 moiety. This interaction explains ParA's discrimination against guanine nucleotides (Davey and Funnell, 1994). Residues Lys316, Leu343, Arg345 and Phe349 also engage in stacking interactions to the adenine group.
The ParA–ADP structure obtained with 5 mM ADP contains a second-bound ADP molecule, which is <10 Å from the primary ADP site and located in a surface exposed cleft between both subunits of the ParA dimer (Figure 5A; Supplementary Figure 3). There are numerous contacts with this ADP from each ParA subunit in the dimer. Residues Lys164, Lys320′ and His323′ contact ADP phosphate groups, whereas Asp280′ interacts with both ribose hydroxyls. The ADP adenine is contacted by the side chains of Ser162, Lys164, Met159 and Phe356 (Figure 5A). Given that the in vivo concentration of ADP (∼100 μM) is far lower than the 5 mM used to crystallize the complex, it appears unlikely that this binding site is important in vivo. Moreover, the residues that contact this ADP are not conserved between the P1 and P7 ParA proteins. Interestingly, however, recent data showed that the ParA protein, IncC, from the broad-host-range plasmid RK2 also has a second ADP-binding site (Batt et al, 2009). The affinity of this ADP-binding site was approximately 1000-fold lower than that of the ‘primary' ADP-binding site in IncC (54 μM). The possible physiological relevance, if any, of a second ADP-binding event in type Ia ParA proteins, therefore, remains to be tested.
Folding and conformational locking: a mechanism for ParA DNA-binding activation
Our structures reveal that ADP binding has significant global and local structural consequences. We next asked how these changes might activate the protein for DNA binding by modelling the ParA–ADP–DNA complex. Although ParA functions by cooperatively coating an ∼150 bp region of the promoter, mutagenesis data indicate that the ∼40 bp imperfect inverted repeat contains the recognition site for ParA (Hayes et al, 1994). Thus, each ParA HTH element was docked in successive DNA major grooves of a 40-bp DNA duplex, as earlier homology scanning studies with P1 and P7 proteins revealed that residues 22–66, which corresponds to the ParA HTH, are essential for operator-specific DNA binding (Radnedge et al, 1998). The HTHs could not be fit into successive major grooves unless the DNA was bent (Figure 6A). After the DNA was modelled to allow HTH insertion into successive major grooves and the wings into minor grooves, an interesting result emerged, which is that two basic regions of ParA (herein termed motif 1 and motif 2), composed of residues 351–354 and 365–380, come into contact distance with the phosphate backbone of central region of the DNA (Figure 6A). Remarkably, these regions only fold on ADP binding. Residues 351–354 corresponds to the N-terminus of α15B (motif 1), whereas residues 365–380 form a long loop and the N-terminus of α16 (motif 2) (Figure 6A). In addition to direct contacts from basic residues, the dipoles of these helices are also positioned to interact with the phosphate backbone. This finding suggested that a key role of ADP-induced folding might be the creation of new DNA-binding motifs. Interestingly, recent studies have revealed the importance of basic residues in DNA binding by partition ATPases. Studies on Soj showed that ATP binding leads to the creation of a nucleotide sandwich dimer in which the basic residues are positioned to contact the DNA phosphate backbone, whereas a basic residue in SopA was shown to be critical for non-specific DNA binding and partition (Hester and Lutkenhaus, 2007; Castaing et al, 2008).
Figure 6.
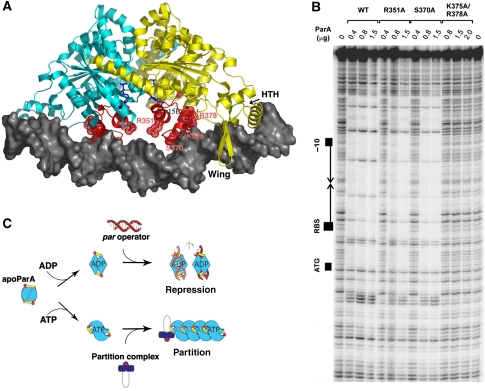
ADP-mediated folding of basic regions/ATP-induced polymer formation. (A) ParA–ADP–DNA model predicting the role of basic regions (coloured red) that fold and are stabilized on ADP binding in DNA binding. The ADP molecules are shown as blue sticks. (B) DNase I protection of parOP site. Shown are footprint experiments for wild type P1 ParA and ParA proteins in which basic motif residues, R351, S370, K375 and R378 were mutated to alanines. (C) Schematic of the proposed ADP/ATP switching mechanism mediating the dual roles of ParA. ParA is coloured according to Figure 1A. The dimer interface is shown to morph in between multiple conformations indicative of the plasticity of the apo dimer. ADP-binding locks in a specific dimer state active for DNA binding, whereas ATP binding is predicted to stabilize a different dimeric state that stimulates filament formation leading to plasmid separation.
To test the importance of the basic loop regions in P1 ParA, we mutated residues Arg351, Ser370, Lys375 and Arg378 (Figure 6A). These residues are conserved in the P7 and P1 proteins, as would be expected, if they play key roles in DNA binding by both proteins. DNase I protection assays revealed that the S370A and R351A mutant proteins bound the 150-bp parOP operator region with 2–2.5- and 5-fold reduced affinity relative to wild type, respectively, whereas the K375A/R378A double mutation essentially abrogated DNA binding (Figure 6A). These residues likely make direct contacts with DNA, but also may reconfigure the DNA site to permit the docking of the HTH and wing elements, which would explain the severity of their mutations. Thus, our combined data indicate that ADP binding activates specific DNA binding by ParA using a bipartite mechanism. First, it leads to large-scale folding of basic regions that play key roles in DNA binding, and second, it locks in a specific dimeric state that is optimal for docking its four DNA-binding elements, the HTH, the wing and two basic regions, onto the DNA.
ATP stimulates polymerization of P1 ParA
To be active for partition, ParA must bind ATP. Addition of ATP-Mg2+ caused marked precipitation of P1 and P7 ParA proteins and closer examination of the precipitate revealed that it consisted of string-like formations, suggesting that ParA–ATP might form polymers. Subsequent EM studies revealed that the proteins form filaments, which are of probable importance in partition (Supplementary Figure 4; Supplementary data). Indeed, ATP-dependent polymer formation has earlier been observed for the type I proteins Soj, Streptococcus pyogenes δ, pB171ParA, TP228 ParF, F plasmid SopA and RK2 IncC (Barillà et al, 2005; Lim et al, 2005; Ebersbach et al, 2006; Bouet et al, 2007; Hatano et al, 2007; Hester and Lutkenhaus, 2007; Batt et al, 2009). The P7 and P1 ParA structures are the first obtained for the type Ia partition ATPase. Structures have been solved of the type Ib NTPases S. pyogenes pSM19035 δ and T. thermophilus Soj. Interestingly, the dimer configurations of Soj and δ differ. In addition, unlike Soj, which is a monomer in its apo form, δ appears to be dimeric. Thus, although δ, Soj and the larger type Ia ParA proteins display distinct conformational properties, all appear to form polymers in the presence of ATP and likely use a similar mechanism of DNA segregation. However, the details behind the ATP-induced switch differ in each case.
In conclusion, our studies have shown that the type Ia ParA proteins form dimers in their apo state and have revealed the structural architecture of these proteins. The flexibility imparted by the α1–C-domain′ dimer interface of ParA permits binding of ADP or ATP to the Walker-box motif (Figure 6C). As observed for other Walker-type proteins, we find that ATP binding appears to stimulate ParA polymerization. Although ATP binding activates partition, ADP binding stimulates the transcription regulation function of ParA. Our structural and biochemical studies show that ADP stabilizes a specific dimer state of ParA and leads to large-scale folding of several regions of ParA, including two basic motifs, which are critical for DNA binding.
Materials and methods
Purification and crystallization of P7 and P1 apoParA proteins
The genes encoding P1 ParA containing a K122Q mutation was subcloned into pET15b, which added an N-terminal hexa-his tag for purification and a C-terminal linker connected to the first 28 residues of P1 ParB. The protein was crystallized in the presence of 100 μM AMP-PCP and 1 mM MgCl2 in the hopes of revealing the ParB–ParA interaction. However, our subsequent data show that AMP-PCP does not functionally substitute for ATP, which is required for ParB binding. The protein was expressed in BL21(DE3) and was purified in one step using Ni-NTA chromatography. The P1 apoParA protein was concentrated to 3 mg/ml and crystallized using 2 M sodium formate, 0.1 M sodium acetate pH 4.6 as a crystallization solution. AMP-PCP was not observed in the final structure and thus, the ParB fragment was also disordered. An artificial P7 parA gene, codon optimized for E. coli expression, was designed and purchased from Genscript Corporation (Piscatway, NJ, USA); Web: www.genscript.com. This gene was subcloned into the pET15b vector such that the N-terminal hexa-his tag was expressed for purification. Two P7 apoParA crystal forms (C2221 and P6222) were obtained at a protein concentration of 5 mg/ml. The C2221 crystals were grown using 20% PEG 3000, 15% ethylene glycol and 0.1 M HEPES pH 7.5, whereas the P6222 crystal form was grown using 2 M sodium/potassium phosphate pH 6.8. All data were collected at ALS and processed with MOSFLM (Table I).
Structure determination of P1 and P7 apoParA
The P1 apoParA and C2221 P7 apoParA structures were solved by MAD using crystals grown with selenomethionine-substituted protein. The selenium sites were located using SOLVE (Terwilliger and Berendzen, 1999). Model building was carried out using O and refinement with CNS (Jones et al, 1991; Brünger et al, 1998). The final P1 apoParA structure contains one molecule per ASU and has Rwork/Rfree values of 24.6%/28.1% to 2.92 Å resolution. The final C2221 P7 apoParA structure contains two molecules per ASU and was refined to a final Rwork/Rfree of 25.4%/29.4% to 2.78 Å resolution. The P6222 P7 apoParA structure was solved by molecular replacement using one subunit of the C2221 structure in which the N-terminal 50 residues were removed as a search model in MolRep. After initial refinement, clear density was observed for residues 3–23 and 38–50 at the N-terminus. These residues and solvent molecules were subsequently added using O and the structure refined to a final Rwork/Rfree of 25.2%/27.9% to 2.80 Å resolution (Jones et al, 1991) (Table I).
Crystallization and structure determination of P1 ParA–ADP complexes
Purified P1 ParA protein (with only an N-terminal hexa-his tag) at 5 mg/ml was mixed with a range of ADP concentrations (1–5 mM) and 5 mM MgCl2. Crystals were obtained using 10% PEG 6000, 0.1 M HEPES pH 7.0. Two crystal forms, P212121 (orthorhombic) and P41212 (tetragonal), were obtained. The orthorhombic crystal form was grown using 5 mM ADP, whereas the tetragonal form was obtained using 1 mM ADP. The orthorhombic crystal form was solved first by using molecular replacement. There are two subunits in the ASU, and both were found using MolRep. After extensive rebuilding and refinement, the N-terminal regions (removed in the initial search model) and residues 317–374, 118–125 and 164–175 were located. Clear density was also observed for four ADP molecules. The final model has Rwork/Rfree values of 19.0%/23.0% to 2.05 Å (Brünger et al, 1998). The tetragonal crystal structure was solved using one subunit from the orthorhombic structure as a search model. To eliminate bias, the N-terminal α1 region (1–40) and regions disordered in apoParA were removed. There are two subunits in the ASU and two clear solutions to the rotation and translation functions were found in MolRep. The structure has been refined to an Rwork/Rfree of 18.5%/24.0% to 2.58 Å resolution (Table I).
DNase I protection assays
DNase I protection assays were performed as described by Fung et al, 2001. Each assay (vol=25 μl) contained the amount of ParA indicated in Figure 6B and 2 mM ADP. The DNA substrate was a 218-bp DNA fragment that includes the par regulatory region and the start of the parA gene. The −10 promoter signal, the inverted repeat sequence, the ribosome-binding site and the start codon (ATG) for the parA gene are indicated in Figure 6B.
Chromatography resolved-light scattering
For SEC-LS, samples were analysed using a Wyatt technologies SEC-LS instrument consisting of a Shodex size exclusion column coupled to a light scattering detector, Dawn-Heleos, and a refractive index detector, OptilabrEX. Samples (5–50 μM) were injected onto the column equilibrated with 25 mM Tris–HCl pH 7.5, 250 mM NaCl and 2 mM DTT at 1 ml/min. Data collected at 0.5-s intervals were analysed using ASTRA software to arrive at an apparent molecular mass and mass distribution for the samples.
Partial proteolysis assays
Each reaction mixture (10 μl) contained 3 μg of the indicated ParA protein in 25 mM Hepes-KOH pH 7.5, 150 mM KCl, 25 mM NaCl, 5 mM MgCl2, 5% glycerol, 0.05 mM Na2EDTA, 0.5 mM DTT and trypsin as indicated. ADP, when present, was at 2 mM. The mixtures were incubated for 2 h at room temperature, and then analysed by electrophoresis on 12% SDS-polyacrylamide gels. In Figure 4D, the tryp:ParA ratio (w/w) and the presence of ADP are indicated above each lane.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Review Process File
Acknowledgments
This work was supported by the Burroughs Wellcome Career Development Award 992863, NIH grant GM074815 (to MAS) and Canadian Institutes of Health grant MOP-37997 (to BEF). Coordinates for the P1 apoParA, P7 apoParA (C2221), P7 apoParA (P6222), P1 ParA–ADP (P212121) and P1 ParA–ADP (P41212) structures have been deposited with the Protein Data Bank under the Accession codes 3EZ7, 3EZ9, 3EZF, 3EZ2 and 3EZ6, respectively.
References
- Abbani M, Iwahara M, Clubb RT (2005) The structure of the excisionase (Xis) protein from conjugative transposon Tn916 provides insight into the regulation of heterobivalent tyrosine recombinases. J Mol Biol 347: 11–25 [DOI] [PubMed] [Google Scholar]
- Abeles AL, Friedman SA, Austin SJ (1985) Partition of unit-copy miniplasmids to daughter cells III. The DNA sequence and functional organization of the P1 partition region. J Mol Biol 185: 261–272 [DOI] [PubMed] [Google Scholar]
- Barillà D, Rosenberg MF, Nobbmann U, Hayes F (2005) Bacterial DNA segregation dynamics mediated by the polymerizing protein ParF. EMBO J 24: 1453–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt SM, Bingle LEH, Dafforn TR, Thomas CM (2009) Bacterial genome partitioning: N-terminal domain of IncC protein encoded by broad-host-range plasmid RK2 modulates oligomerization and DNA binding. J Mol Biol 385: 1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet J-Y, Funnell BE (1999) P1 ParA interacts with the P1 partition complex at parS and an ATP–ADP switch controls ParA activities. EMBO J 18: 1415–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouet J-Y, Ah-Seng Y, Benmeradi N, Lane D (2007) Polymerization of SopA partition ATPase: regulation by DNA binding and SopB. Mol Microbiol 64: 468–481 [DOI] [PubMed] [Google Scholar]
- Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Crosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54: 905–921 [DOI] [PubMed] [Google Scholar]
- Castaing JP, Bouet JY, Lane D (2008) F plasmid partition depends on interactions of SopA with non-specific DNA. Mol Microbiol 70: 1000–1011 [DOI] [PubMed] [Google Scholar]
- Cerin H, Hackett J (1993) The parVP region of the Salmonella typhimurium virulence plasmid pSLT contains 4 loci required for incompatibility and partition. Plasmid 30: 30–38 [DOI] [PubMed] [Google Scholar]
- Davey MJ, Funnell BE (1994) The P1 plasmid partition protein ParA. J Biol Chem 269: 29908–29913 [PubMed] [Google Scholar]
- Davey MJ, Funnell BE (1997) Modulation of the P1 plasmid partition protein ParA by ATP, ADP and P1 ParB. J Biol Chem 272: 15286–15292 [DOI] [PubMed] [Google Scholar]
- Davis MA, Martin KA, Austin SJ (1992) Biochemical activities of the ParA partition protein of the P1 plasmid. Mol Microbiol 6: 1141–1147 [DOI] [PubMed] [Google Scholar]
- Davis MA, Radnedge L, Martin KA, Hayes F, Youngren B, Austin SJ (1996) The P1 ParA protein and its ATPase activity play a direct role in the segregation of plasmid copies to daughter cells. Mol Microbiol 21: 1029–1036 [DOI] [PubMed] [Google Scholar]
- Delano WL (2002) The PyMOL Molecular Graphics System. San Carlos, California: DeLano Scientific [Google Scholar]
- Ebersbach G, Ringgaard S, Møller-Jensen J, Wang Q, Sherratt DJ, Gerdes K (2006) Regular cellular distribution of plasmids by oscillating and filament-forming ParR ATPase of plasmid pB171. Mol Microbiol 61: 1428–1442 [DOI] [PubMed] [Google Scholar]
- Fung E, Bouet J-Y, Funnell BE (2001) Probing the ATP binding site of P1 ParA: partition and repression have different requirements for ATP binding and hydrolysis. EMBO J 17: 4901–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell BE (1988a) Mini-P1 plasmid partitioning: excess ParB protein destabilizes plasmids containing the centromere parS. J Bacteriol 170: 954–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell BE (1988b) Participation of Escherichia coli host integration factor in the P1 plasmid partition system. Proc Natl Acad Sci USA 85: 6657–6661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell BE (1991) The P1 partition complex at parS: the influence of Escherichia coli integration host factor and of substrate topology. J Biol Chem 266: 14328–14337 [PubMed] [Google Scholar]
- Funnell BE, Gagnier L (1994) P1 plasmid partition: binding of P1 ParB protein and Escherichia coli integration host factor to altered parS sites. Biochimie 76: 924–932 [DOI] [PubMed] [Google Scholar]
- Gerdes K, Møller-Jensen J, Bugge Jensen R (2000) Plasmid and chromosome partitioning: surprises from phylogeny. Mol Microbiol 37: 455–466 [DOI] [PubMed] [Google Scholar]
- Hatano T, Yamaichi Y, Niki H (2007) Oscillating focus of SopA associated with filamentous structure guides partitioning of F plasmid. Mol Microbiol 64: 1198–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes F, Austin S (1993) Specificity determinants of the P1 and P7 plasmid centromere analogs. Proc Natl Acad Sci USA 90: 9228–9232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes F, Radnedge L, Davis MA, Austin SJ (1994) The homologous operons for P1 and P7 plasmid partition are autoregulated from dissimilar operator sites. Mol Microbiol 11: 249–260 [DOI] [PubMed] [Google Scholar]
- Hayes F, Austin S (1994) Topological scanning of the P1 plasmid partition site. J Mol Biol 243: 190–198 [DOI] [PubMed] [Google Scholar]
- Hayes F, Barillà D (2006) Assembling the bacterial segrosome. Trends Biochem Sci 31: 247–250 [DOI] [PubMed] [Google Scholar]
- Hester CM, Lutkenhaus J (2007) Soj (ParA) DNA binding is mediated by conserved arginines and is essential for plasmid segregation. Proc Natl Acad Sci USA 104: 20326–20331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Elliot J, McCready P, Skowronski E, Garnes J, Kobabyashi A, Brubaker RR, Garcia E (1998) Structural organization of the virulence-associated plasmids of Yersinia pestis. J Bacteriol 180: 5192–5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Zou J-Y, Cowan SW, Kjeldgaard M (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 47: 110–119 [DOI] [PubMed] [Google Scholar]
- Larsen RA, Cusumano C, Fujioka A, Lim-Fong G, Patterson P, Pogliano J (2007) Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis. Genes Dev 21: 1340–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard TA, Butler PJ, Löwe J (2005) Bacterial chromosome segregation: structure and DNA binding of the Soj dimer—a conserved biological switch. EMBO J 24: 270–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GE, Dermna AI, Pogliano J (2005) Bacterial DNA segregation by dynamic SopA polymers. Proc Natl Acad Sci USA 102: 17658–17663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Eisenberg D (2002) 3D domain swapping: as domains continue to swap. Protein Sci 11: 1285–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J, Sundaramoorthy M (2003) MinD and role of the deviant Walker A motif, dimerization and membrane binding in oscillation. Mol Microbiol 48: 295–303 [DOI] [PubMed] [Google Scholar]
- Møller-Jensen J, Borch J, Dam M, Jensen RB, Roepstorff P, Gerdes K (2003) Bacterial mitosis: ParM of plasmid R1 moves plasmid DNA by an actin-like insertional polymerization mechanism. Mol Cell 12: 1477–1487 [DOI] [PubMed] [Google Scholar]
- Pratto F, Cicek A, Weihofen WA, Lurz R, Saenger W, Alonso JC (2008) Streptococcus pyogenes pSM19035 requires dynamic assembly of ATP-bound ParA and ParB on parS DNA during plasmid segregation. Nucleic Acids Res 36: 3676–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp D, Narita A, Oda T, Fujisawa T, Matsuo H, Nitanai Y, Iwasa M, Maeda K, Onishi H, Maeda Y (2008) Molecular structure of the ParM polymer and the mechanism leading to its nucleotide-driven dynamic instability. EMBO J 27: 570–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnedge L, Youngren B, Davis M, Austin S (1998) Probing the structure of complex macromolecular interactions by homolog specificity scanning: the P1 and P7 plasmid partition systems. EMBO J 17: 6076–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salje J, Löwe J (2008) Bacterial actin: architecture of the ParMRC plasmid DNA partitioning complex. EMBO J 27: 2230–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Funnell BE (2005) Structures of ParB bound to DNA reveal mechanism of partition complex formation. Nature 438: 516–519 [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Mansoor A, Funnell BE (2007) Structure of a four-way bridged ParB-DNA complex; implications for segrosome assembly. J Biol Chem 282: 10456–10464 [DOI] [PubMed] [Google Scholar]
- Schumacher MA (2008) Structural biology of plasmid partition: uncovering the molecular mechanisms of DNA segregation. Biochem J 412: 1–18 [DOI] [PubMed] [Google Scholar]
- Schymkowitz JW, Rousseau F, Wilkinson HR, Friedler A, Itzhaki LS (2001) Observation of signal transduction in three-dimensional domain swapping. Nat Struct Mol Biol 8: 888–892 [DOI] [PubMed] [Google Scholar]
- Simpson AE, Skurray RA, Firth N (2003) A single gene on the staphylococcal multiresistance plasmid pSK1 encodes a novel partitioning system. J Bacteriol 185: 2143–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees JA, Funnell BE (2003) Plasmid and chromosome traffic control: how ParA and ParB drive partition. Curr Top Dev Biol 56: 145–180 [DOI] [PubMed] [Google Scholar]
- Terwilliger TC, Berendzen J (1999) Automated MAD and MIR structure solution. Acta Crystallogr D Biol Crystallogr 55: 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren B, Radnedge L, Hu P, Garcia E, Austin S (2000) A plasmid partition system of the P1–P7par family from the pMT1 virulence plasmid of Yersinia pestis. J Bacteriol 182: 3924–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Review Process File