Abstract
At specific times during bacterial growth, the transcription factor DksA and the unusual nucleotide regulator ppGpp work synergistically to inhibit some Escherichia coli promoters (e.g. rRNA promoters) and to stimulate others (e.g. promoters for amino-acid synthesis and transport). However, the mechanism of DksA action remains uncertain, in part because DksA does not function like conventional transcription factors. To gain insights into DksA function, we identified mutations in dksA that bypassed the requirement for ppGpp by selecting for growth of cells lacking ppGpp on minimal medium without amino acids. We show here that two substitutions in DksA, L15F and N88I, result in higher DksA activity both in vivo and in vitro, primarily by increasing the apparent affinity of DksA for RNA polymerase (RNAP). The mutant DksA proteins suggest potential roles for ppGpp in DksA function, identify potential surfaces on DksA crucial for RNAP binding, and provide tools for future studies to elucidate the mechanism of DksA action.
Keywords: DksA, ppGpp, RNA polymerase secondary channel, stringent response, transcription factor
Introduction
Rapid, global responses in gene expression following nutritional stresses are of central importance to all organisms. Studies of the nutritional (stringent) stress response in Escherichia coli have revealed a mechanism for promoter-specific modulation of transcription initiation by small molecules. This mechanism contrasts with regulation by conventional transcription factors that bind DNA to increase or decrease gene expression from specific promoters. Changes in the concentrations of ppGpp and NTPs regulate transcription by interacting with RNA polymerase (RNAP), which is reviewed in Haugen et al (2008) and [31]Potrykus and Cashel (2008). The response of RNAP to these small molecule effectors is amplified dramatically by the 151 amino-acid protein DksA, which binds in the enzyme's secondary channel (Paul et al, 2004; Perederina et al, 2004; Rutherford et al, 2007, 2009). DksA, in conjunction with ppGpp, increases the activities of some promoters needed for amino-acid biosynthesis and transport whereas decreasing the activities of rRNA, many tRNA, and other promoters coding for components of the protein synthesis machinery (Paul et al, 2004, 2005; Magnusson et al, 2005). In addition, DksA and ppGpp, either directly or indirectly, affect the expression of many products needed for resistance to nutritional stresses, as well as products needed for virulence, motility, and adhesion (Braeken et al, 2006; Magnusson et al, 2007; Aberg et al, 2008). DksA can affect transcription in the absence of ppGpp in vitro and at times when ppGpp is absent in vivo, but its effects are much greater when ppGpp and DksA work together (Paul et al, 2004, 2005; Magnusson et al, 2007).
ppGpp accumulates rapidly on nutrient deprivation and initiates the stringent response by modifying RNAP (Barker et al, 2001b; Potrykus and Cashel, 2008). At other times during growth, changes in NTP concentrations, rather than ppGpp concentrations, control responses to nutritional conditions (Murray et al, 2003). In either case, DksA is a critical component of these responses (Paul et al, 2004). DksA and ppGpp modulate transcription initiation by shifting the occupancy of specific intermediates on the pathway to open complex formation, perhaps by lowering the energy of the transition state between them (Haugen et al, 2008; Rutherford et al, 2009). DksA and ppGpp exert promoter-specific effects (positive, negative, or no effect) because of differences in the intrinsic kinetic properties of different promoters (Haugen et al, 2008). Although recognition of DksA's importance has improved our understanding of these nutritional responses, much remains to be determined about its mechanism of action and its interplay with ppGpp.
DksA does not follow the conventions of a typical transcriptional regulator. It does not bind DNA, and its concentration is relatively constant under the conditions that have been examined (changes in growth phase and growth rate resulting from changes in nutritional conditions) (Paul et al, 2004; Rutherford et al, 2007). DksA has some structural similarities to GreA and GreB (Perederina et al, 2004), transcription elongation factors that bind RNAP with their coiled coils protruding deep into the secondary channel towards the active site of the enzyme (Erie et al, 1993; Orlova et al, 1995; Stebbins et al, 1995; Opalka et al, 2003; Shaevitz et al, 2003; Borukhov et al, 2005). Like the Gre factors, DksA interacts with the RNAP secondary channel (Perederina et al, 2004; Rutherford et al, 2009), and a growing body of evidence indicates that these and other secondary channel binding factors may compete with one another (Potrykus et al, 2006; Rutherford et al, 2007; Lamour et al, 2008).
ppGpp and DksA display synergistic effects on transcription in vitro when their concentrations are non-saturating (Paul et al, 2004, 2005; Bernardo et al, 2006; Nakanishi et al, 2006; Sharma and Payne, 2006; Costanzo et al, 2008). However, the structural details of the RNAP–ppGpp and the RNAP–DksA interactions are not known (Vrentas et al, 2008; Rutherford et al, 2009). Thus, genetic approaches must be used to understand how these crucial factors function.
As ppGpp and DksA often work together to regulate transcription, cells lacking dksA share a wide variety of phenotypes with those deficient for ppGpp (Magnusson et al, 2007). In an attempt to help elucidate the mechanism of DksA action on RNAP, we screened for mutants in dksA that functioned in the absence of ppGpp. Two dksA mutations were identified that suppressed the inability of ΔrelA ΔspoT (ppGpp0) cells to grow on a medium lacking amino acids. These mutations share some similarities with mutations identified earlier in genes encoding RNAP subunits (Bartlett et al, 1998; Trautinger and Lloyd, 2002; Murphy and Cashel, 2003; Szalewska-Palasz et al, 2007; Rutherford et al, 2009) in that they bypass the requirements of ppGpp for growth without amino acids, including activation of certain promoters for amino-acid biosynthesis, and inhibition of rRNA promoters. Unlike the suppressor mutations that have been identified in the genes for the subunits of RNAP, most of which appear to function also by destabilizing RNAP-promoter complexes, the dksA mutations increase DksA function directly, at least in part by increasing the binding affinity of DksA for RNAP. Our study not only emphasizes the importance of secondary channel binding factors for modulation of RNAP activity in response to small molecule effectors, but it also identifies regions in DksA that affect its interactions with RNAP and suggests potential roles for ppGpp in the mechanism of DksA action.
Results
Identification of novel mutations in dksA that function in the absence of ppGpp
As part of a genetic approach to identify residues in DksA crucial for its function, we sought mutations that increased its activity. To this end, we selected plasmid-encoded DksA variants that could compensate for the absence of ppGpp by suppressing the amino-acid auxotrophies of a ΔrelA ΔspoT (ppGpp0) strain (Xiao et al, 1991). A plasmid containing wild-type dksA expressed from a lac promoter was passaged through an mutD5 mutL mutator strain, and subsequently was introduced by transformation into a ΔrelA ΔspoT ΔdksA strain expressing Lac repressor (lacIq) from a plasmid (pJM101). The lacIq was provided on a separate plasmid, rather than on the mutagenized pDksA plasmid, to reduce the chance of inadvertant mutagenesis of lacIq and rescue of auxotrophy by overexpression of wild-type DksA (Magnusson et al, 2007; Blankschien et al, 2009). The transformation mixture was plated on minimal medium without IPTG, and multiple colonies appeared after 3 days of incubation. Of 29 initial isolates tested, 14 contained plasmids that continued to allow growth of a ppGpp0 strain on minimal medium. The dksA ORFs of these 14 plasmids were sequenced and revealed 2 independent mutations: an A to T transversion at dksA nucleotide 263, resulting in an N88I substitution in the coiled-coil domain, and a C to T transition at nucleotide 43, causing an L15F substitution in the globular domain (Figure 1A). The other sequenced dksA genes lacked mutations in dksA and were not examined further. One potential explanation for their effect on growth is that they contained plasmid-associated mutations leading to DksA overexpression.
Figure 1.
DksA substitutions rescue the amino-acid auxotrophies of ppGpp0 ΔdksA strains. (A) Models of DksA based on the crystal structure (Perederina et al, 2004) showing the positions of L15 and N88. Left, ribbon. Right, spacefill. The N- and C-termini are labelled N and C, respectively. (B) Plating efficiencies (cfu, colony forming units on M9-glu versus M9-glu-CAA) are provided on the Y-axis. Percentages depict means±standard deviations from three independent determinations. Light grey, −IPTG. Dark grey, +IPTG. pControl is plasmid pBA169 containing an IPTG-inducible pTrc promoter. The pDksA plasmids contain the wild-type dksA gene or variants fused to the pTrc promoter. The ppGpp0 indicates that the cells are deleted for relA and spoT, and ΔdksA indicates that the cells lack the chromosomal dksA gene. A colour version of this figure is available at The EMBO Journal Online.
Site-directed mutagenesis was performed on the initial wild-type DksA plasmid to verify that the identified mutations could suppress the auxotrophy. As expected, the reconstructed plasmids enabled growth of a ppGpp0 ΔdksA strain on minimal medium, both in the absence or presence of IPTG (Figure 1B). In either case, the strain containing the N88I variant had a plating efficiency in the absence of amino acids similar to that when plated on a medium with amino acids (89–108%), whereas a control strain containing a plasmid coding for wild-type DksA plated with approximately 400-fold lower efficiency on a medium without amino acids (0.24%). The uninduced L15F variant rescued growth slightly less efficiently than the N88I variant, plating 13% as well without IPTG and 75% as well with IPTG as on a medium with amino acids. We also combined N88I and L15F. In the absence of IPTG, the double mutant rescued growth in the absence of amino acids (97% plating efficiency), but surprisingly no growth was observed when DksA expression was induced with IPTG (see below).
Activation of the livJ promoter by N88I–DksA and L15F–DksA in vivo
The results described above suggested that single amino-acid substitutions in DksA could bypass the requirement for ppGpp to activate promoters for amino-acid biosynthesis and/or transport. Therefore, we examined the effects of the two DksA mutants described above (N88I and L15F) on PlivJ, a promoter that directs the transcription of a transporter of branched-chain amino acids shown earlier to be activated by ppGpp/DksA in vivo and in vitro (Paul et al, 2005).
Consistent with their effects on growth of a ppGpp0 ΔdksA strain on mimimal medium (Figure 1B), the plasmid-encoded DksA substitution mutants increased β-galactosidase activity from a livJ promoter–lacZ fusion in a ppGpp0 ΔdksA strain (Figure 2A). The N88I–L15F double mutant DksA increased livJ-promoter activity more than the N88I single mutant, and the L15F–DksA increased livJ-promoter activity to a lesser extent. In contrast, the plasmid encoding wild-type DksA had little or no effect on livJ-promoter activity, consistent with the fact that normal levels of chromosomally encoded wild-type DksA are insufficient to rescue the auxotrophy of ppGpp0 mutants. Under these conditions (i.e. in the absence of IPTG induction), ectopic wild-type DksA levels were ∼50% those of endogenous DksA levels (see western blot, Figure 2C, lanes 2–3).
Figure 2.
Substitutions in DksA increase transcription from the livJ promoter. (A, B, D), β-galactosidase activity from livJ promoter–lacZ fusions in LB medium with ampicillin at 32°C. The pDksA plasmids are variants of pBA169 containing wild-type or mutant dksA genes fused to the pTrc promoter; pControl is pBA169; pDksAAsp encodes D71N/D74N substitutions; pDksAAsp–N88I encodes the Asn substitutions as well as N88I. (A) PlivJ activity in the absence of IPTG in a ΔrelA ΔspoT ΔdksA host. (B) PlivJ activity after DksA induction (0.1 mM IPTG) in a ΔrelA ΔspoT host containing wild-type dksA. (C) Wild-type or mutant DksA levels. Protein extracts were separated by SDS–PAGE (15%) and analysed by western blotting using an anti-DksA antibody and quantified. DksA levels from the DksA plasmid-containing strains were ∼50% without IPTG and 5- to 10-fold higher with 0.1 mM IPTG than in the strain expressing DksA from its native promoter in the chromosome. (D) PlivJ activity after induction (0.1 mM IPTG) in a host wild-type for dksA, relA and spoT.
The effects of the DksA substitutions were also examined under inducing conditions (i.e. +IPTG) in strains containing chromosomally-encoded DksA, but lacking ppGpp (Figure 2B). Under conditions when the wild-type and mutant DksAs were overproduced approximately 5- to 10-fold (Figure 2C), induction of wild-type DksA resulted in a small increase in livJ-promoter activity relative to that observed in the same strain with a control plasmid lacking the dksA gene (Figure 2B). L15F–DksA increased livJ-promoter activity slightly more, and N88I–DksA strongly increased livJ-promoter activity. The effects of the DksA substitution mutants on livJ-promoter activity in the ppGpp0 strain paralled their effects on plating efficiency (Figure 1B).
To determine whether the presence of ppGpp affected the function of the mutant DksA proteins, the same set of experiments was also carried out in strains in which both ppGpp and chromosomally encoded DksA were present (Figure 2D). In these experiments, wild-type DksA (made from a plasmid and/or from the chromosome) resulted in higher levels of livJ-promoter activity than were observed in the strains lacking ppGpp (compare Figure 2B and D), consistent with earlier observations that transcription from the livJ promoter requires ppGpp even when DksA is present (Paul et al, 2005; Magnusson et al, 2007). As in the absence of ppGpp, livJ-promoter activity was increased more by N88I– and L15F–DksAs than by wild-type DksA. The effects of L15F– and N88I–DksAs were more similar when ppGpp was present (Figure 2D) than when ppGpp was missing (Figure 2B). This result suggests that L15F–DksA may be able to function with ppGpp better than N88I–DksA (see below). Finally, the large effects of the mutant DksAs observed in strains containing wild-type chromosomally encoded DksA (with or without ppGpp) indicate that the mutant DksAs are dominant to wild-type DksA (Figure 2B and D).
It was shown earlier that asparagine substitutions for the conserved aspartic-acid residues at the tip of the coiled coil (D71N/D74N) eliminated DksA function (Perederina et al, 2004). To address whether the stimulatory effect of N88I–DksA on livJ-promoter activity required a functional coiled-coil tip, we measured the effect of an N88I/D71N/D74N triple substitution on livJ-promoter activity. Induction of plasmids encoding either the D71N/D74N double substitution (labelled DksAAsp in Figure 2B and D) or the D71N/D74N/N88I triple substitution (labelled pDksAAsp–N88I) resulted in little or no activation of the livJ promoter. These results indicate that function of the N88I variant requires at least one of the coiled-coil tip aspartate residues and suggests that pN88I–DksA functions through a mechanism similar to wild-type DksA.
In summary, our results show that single amino-acid changes in DksA can increase DksA function in vivo, thereby bypassing the requirement for ppGpp.
DksA variants require ppGpp for transcription activation in vitro
We next tested whether the DksA variants increased the activity of the livJ promoter more than wild-type DksA in vitro and whether the effects of the mutant proteins were dependent on ppGpp (Figure 3). N-terminal hexahistidine (His6)-tagged versions of the DksA variants were constructed and purified (Supplementary Figure S1). In the absence of ppGpp, wild-type DksA alone (at a concentration of 2 μM) did not increase transcription from the livJ promoter or from either of two other amino-acid biosynthetic promoters, hisG or thrABC (Figure 3A–C, compare lanes 1 and 3 in each panel), consistent with the results reported earlier (Paul et al, 2005). Each of the purified mutant DksA proteins (N88I, L15F, or the N88I–L15F double mutant) stimulated transcription from the three promoters only very slightly in vitro (⩽two-fold; Figure 3A–C, lanes 1 versus lanes 5, 7, 9). Furthermore, no concentration-dependent increases in the effects of the wild-type or mutant DksA proteins were observed in vitro in the absence of ppGpp (Supplementary Figure S2A and S2B).
Figure 3.
The DksA variants require ppGpp to stimulate amino-acid promoter activity in vitro. Multiple-round in vitro transcription from a plasmid containing the (A) livJ promoter (livJ sequence endpoints –60/+13; pRLG4416), (B) hisG promoter (endpoints −60/+1; pRLG4413), and (C) thrABC promoter (endpoints −72/+16; pRLG5073). Reactions were carried out with 2 μM wild-type or mutant DksA in transcription buffer containing 165 mM NaCl, 10 nM RNAP, and 50 ng supercoiled plasmid template DNA as described in Materials and methods and separated on polyacrylamide gels. The results from a single representative experiment are shown, but similar results were obtained for each promoter in at least three independent experiments. Transcription reactions with either no DksA, wild-type DksA, L15F–DksA, N88I–DksA, or L15F–N88I–DksA as indicated were performed in pairs, with the lane from the reaction at the right in each pair also containing 100 μM ppGpp. The transcript resulting from the amino-acid promoter is indicated by an arrow. RNA-I is a plasmid-encoded transcript. The fold increase in transcript from the reaction containing DksA (wild-type or mutant), relative to that without DksA (lane 1), is indicated beneath the gel lanes. In all cases, significant increases in transcription were observed only when both DksA and ppGpp were included in the transcription reaction.
In contrast, relatively large increases in transcription were observed when 100 μM ppGpp and either the wild-type or mutant DksAs were both present (Figure 3A–C, lanes 1 versus lanes 4, 6, 8, 10). These results indicate that the wild-type and mutant DksA proteins can activate transcription synergistically with ppGpp in vitro. Activation by the mutant DksAs in the presence of ppGpp appeared to be slightly greater than with wild-type DksA on some of the promoters (Figure 3; see also Supplementary Table S1).
The very small effects of the DksA mutants on transcription from the amino-acid promoters in the absence of ppGpp in vitro contrasts with the much larger effects of DksA on livJ-promoter activity in vivo in the absence of ppGpp (Figure 2). There are at least two potential explanations for the observed difference in the requirements for transcription activation by the mutant DksAs in vivo versus in vitro: (a) a factor other than ppGpp may be needed for the DksA variants to increase transcription, but may be missing in the reaction containing only purified components, or (b) most of the increased expression from the livJ promoter in vivo could result from indirect effects of the DksA variants, for example from stronger direct inhibition of rRNA expression, leading to release of more RNAP to activate livJ (see Discussion). Therefore, effects of the DksA variants on rRNA transcription were examined next.
Potent inhibition of rrnB P1 transcription by the dksA mutants in vivo
Inhibition of rRNA transcription by ppGpp and DksA has been examined in detail earlier (Paul et al, 2004; Potrykus et al, 2006; Rutherford et al, 2007, 2009) and reviewed in Haugen et al (2008). To help address whether effects of the mutant DksA proteins on rRNA expression were indirectly responsible for the observed suppression of the ppGpp0 auxotrophies, we measured transcription from an rrnB P1 promoter–lacZ fusion in a ΔrelA ΔspoT (ppGpp0) ΔdksA background (Figure 4). rrnB P1-promoter activity was reduced by the plasmid encoding wild-type DksA, reduced more by L15F–DksA, inhibited still further by N88I–DksA, and almost eliminated by the L15F–N88I double substitution (Figure 4A).
Figure 4.
DksA mutants inhibit rrnB P1 more strongly than wild-type DksA in the absence of ppGpp in vivo. (A) β-Galactosidase activity from rrnB P1–lacZ promoter fusions in LB medium with ampicillin at 32°C plotted at increasing culture optical density. (A) ΔrelA ΔspoT ΔdksA strain without induction of pDksA plasmids. (B) ΔrelA ΔspoT ΔdksA strain with IPTG induction of pDksA plasmids. (C) ΔrelA ΔspoT strain with induction of pDksA plasmids. The DksA variants are described in the text and in the legend to Figure 2.
As expected, induction of the wild-type and mutant DksA constructs with IPTG exacerbated the inhibition of rrnB P1-promoter activity in the ppGpp0 ΔdksA strains (Figure 4B). Wild-type DksA, L15F–DksA, and N88I–DksA decreased rrnB P1 activity even more than without induction. Effects of the induced double mutant on rrnB P1-promoter activity could not be measured because the strain failed to grow (Figure 1B). Aspartate to asparagine substitutions introduced into the coiled-coil tip of either wild-type DksA (pDksAAsp) or into N88I–DksA (pDksAAsp–N88I) eliminated their inhibitory effects on rrnB P1 activity (Figure 4B), just as for stimulation of livJ-promoter activity (Figure 2B), suggesting that the mechanism of inhibition by N88I–DksA is similar to that for wild-type DksA. Inhibitory effects of L15F– and N88I–DksAs were also observed in a host strain devoid of ppGpp, but with its native dksA gene, indicating that the mutant dksA alleles are dominant to wild-type dksA (Figure 4C).
We also measured effects of the mutant DksAs on rrnB P1 activity in a ΔdksA strain able to synthesize ppGpp (Figure 5). The results were similar to those observed in the strains lacking ppGpp (Figure 4). Plasmid-encoded wild-type DksA reduced rrnB P1 activity even in the absence of induction, L15F–DksA inhibited transcription more, N88I–DksA decreased transcription even more, and the N88I/L15F double substitution virtually eliminated expression (Figure 5A). Induction of the plasmid-encoded DksAs with IPTG reduced transcription further (Figure 5B), and as in Figures 2B and 4B, inhibition required the acidic residue(s) at the tip of the coiled coil in DksA. Not surprisingly, induction of the mutant DksA proteins resulted in growth defects correlating with the effects on rRNA expression (Figure 5C), and inhibition of rRNA-promoter activity was observed even when the wild-type dksA locus on the bacterial chromosome was intact (Figure 5D). Thus, effects of the induced DksA variants on rrnB P1 activity were dominant.
Figure 5.
DksA mutants inhibit rrnB P1 more strongly than wild-type DksA in the presence of ppGpp in vivo. β-Galactosidase activity from strains containing an rrnB P1–lacZ promoter fusion grown in LB medium with ampicillin at 32°C, plotted at increasing culture optical density. (A) ΔdksA background without induction of pDksA plasmids. (B) ΔdksA background with IPTG induction of pDksA plasmids. (C) Growth curves in the ΔdksA background with IPTG induction of pDksA plasmids. Semilog plot of OD600 versus time after dilution from stationary phase. Doubling times of strains containing the plasmid encoding N88I–L15F–DksA, ∼330 min; N88I–DksA, ∼91 min; L15F–DksA, ∼42 min; wild-type DksA, ∼33 min. For comparison, a strain containing the induced pBA169 control plasmid doubled in ∼32 min. (D) β-Galactosidase activity from an rrnB P1–lacZ promoter fusion in LB medium plotted at increasing culture optical density (wild-type dksA background, with IPTG induction). The DksA variants are described in the text and in the legend to Figure 2.
In summary, the L15F–, N88I–, and L15F–N88I–DksAs display dramatic effects on rrnB P1–lacZ expression in ppGpp0 strains (and when ppGpp levels are relatively low during steady-state growth in strains able to synthesize ppGpp). These results support the model that the DksA mutants, by themselves, mimic the effects of wild-type DksA and ppGpp together, and that this is sufficient to support growth of ppGpp0 strains in the absence of amino acids.
DksA variants do not require ppGpp to inhibit rrnB P1 in vitro
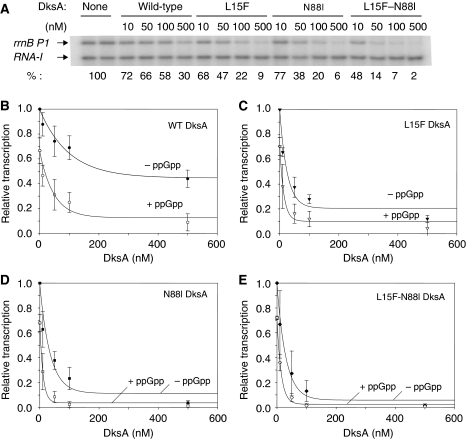
To determine whether the DksA variants could inhibit RNAP directly, independently of ppGpp or other cofactors, we examined their effects on rrnB P1 in vitro. In the experiments shown in Figure 6, solution conditions were chosen in which wild-type DksA inhibited single-round transcription only moderately, so that larger effects of the mutant proteins, if they occurred, could be detected. Transcription from an rrnB P1 promoter on a supercoiled plasmid template was carried out at a series of DksA concentrations, either in the absence or the presence of ppGpp, and after resolving transcripts on acrylamide gels, the relative amounts of the rrnB P1 transcript were quantified. A representative transcription gel is shown in Figure 6A. In the absence of ppGpp, wild-type and the mutant DksA proteins inhibited rrnB P1-promoter activity in a concentration-dependent manner (Figure 6B–E). Consistent with their relative effects in vivo, N88I–L15F–DksA and N88I–DksA were the most effective inhibitors (∼90% inhibition at the highest DksA concentrations), and L15F–DksA reduced transcription by ∼70%, compared with wild-type DksA, which decreased transcription ∼50% under these conditions (Figure 6B–E). The DksA concentration required for half-maximal inhibition of transcription, ∼80 nM for wild-type DksA, was consistent with earlier observations (Paul et al, 2004, 2005; Rutherford et al, 2007, 2009), and was significantly higher than the half-maximal concentration required for the mutant DksAs (⩽50 nM). These results suggested that the substitutions might alter the binding constants of RNAP for DksA (see below).
Figure 6.
The DksA mutants inhibit rrnB P1 more strongly than wild-type DksA in vitro, and inhibition is increased further by ppGpp. (A) Representative transcription gel illustrating effects of DksAs on the rrnB P1 promoter in vitro. Single-round transcription reactions (see Materials and methods for details) contained the indicated concentration of wild-type or mutant DksA, 50 mM NaCl transcription buffer, and supercoiled plasmid DNA with an rrnB P1 promoter (endpoints −61/+1; pRLG5944). Percent transcription relative to that without DksA is indicated below each gel lane. (B–E) Effects of DksA (filled symbols) or DksA plus ppGpp (open symbols) on transcription from rrnB P1 in vitro. In each panel, transcription from rrnB P1 in the presence of DksA protein (wild-type or variant) is plotted on the Y-axis relative to that without DksA (or DksA+100 μM ppGpp). Error bars indicate variation in effects of ppGpp and DksA from multiple experiments.
We next examined the effects of 100 μM ppGpp in combination with each of the DksA variants. As the mutant proteins virtually eliminated transcription at high DksA concentrations, effects of ppGpp on transcription were most apparent at lower DksA concentrations, where they worked together to reduce rrnB P1-promoter activity dramatically. Thus, although the mutant proteins compensated for the absence of ppGpp, they were still capable of responding to ppGpp. This implies that changes in ppGpp concentration could affect transcription in vivo even for the mutant DksA proteins. Nevertheless, 50 nM N88I– or N88I–L15F–DksA alone inhibited transcription from rrnB P1 at least as strongly as the wild-type DksA and ppGpp together.
In summary, the mutant proteins inhibit rrnB P1 more strongly than wild-type DksA in vitro, mirroring their stronger effects on rrnB P1-promoter activity in vivo. This suggests that the increased potency of the DksA mutants on rRNA transcription might be sufficient to explain their ability to compensate for the absence of ppGpp in vivo.
DksA variants display stronger effects than wild-type DksA on RNAP-promoter complex lifetime
We next explored the mechanism responsible for the potency of the mutant DksA proteins. It was shown earlier that DksA decreased the lifetimes of complexes formed by RNAP at all promoters tested, and it was proposed that its effects on transcription (i.e. whether it decreases, increases, or has no effect) depends on the intrinsic kinetic properties of the promoter (Paul et al, 2004, 2005; Rutherford et al, 2007, 2009). Therefore, we measured the effects of the mutant DksAs on the lifetimes of lacUV5-promoter complexes. This promoter makes a complex with a lifetime between that of rRNA and amino-acid biosynthetic promoters, and thus, it was suitable for detection of decreases or increases in complex lifetime.
After formation of RNAP-promoter complexes in vitro in the presence or absence of wild-type or mutant DksA, heparin was added (as a competitor to sequester-free RNAP), and the fraction of complexes remaining with time was assayed by transcription (Figure 7A; see Materials and methods) (Paul et al, 2004). Consistent with their relative effects on growth, transcription in vivo, and rrnB P1-promoter activity in vitro, the N88I–L15F and N88I mutant DksA proteins decreased complex lifetime the most, followed by the L15F mutant and wild-type DksA (Figure 7B). Thus, the effects of the DksA variants on RNAP-promoter complex lifetime support the model that the mutants function by a mechanism analogous to, but are more effective than, wild-type DksA, and that this improved function alleviates the requirement for ppGpp.
Figure 7.
DksA mutants decrease the half-lives of promoter complexes more than wild-type DksA. (A) Representative gel images and (B) plots depicting fraction of lacUV5-promoter complexes remaining at various times after competitor addition using transcription as a readout (see Materials and methods for details). RNA-I is a plasmid-encoded transcript.
DksA variants bind more tightly to RNAP than wild-type DksA
To alter RNAP function, DksA binds in the secondary channel of RNAP and reduces promoter DNA interactions by an allosteric mechanism (Rutherford et al, 2009). In theory, the increased function of the mutant DksA proteins could result from improved binding to RNAP and/or more effective transmission of effects of binding to the sections of RNAP that interact with the promoter. As an estimate of the relative binding affinities of the wild-type and mutant DksA proteins for RNAP holoenzyme, we utilized an assay in which Fe2+ was substituted for Mg2+ at the RNAP active site, resulting in production of hydroxyl radicals that cleave the coiled-coil tip of DksA (Perederina et al, 2004) (Figure 8A and D). Apparent binding constants for RNAP with the wild-type and mutant DksA proteins were determined by measurement of the fraction of 32P-labelled, N-terminally heart muscle kinase (HMK)-tagged DksA cleaved at different RNAP concentrations, normalized to the fraction cleaved at saturating RNAP concentration (see Materials and methods; CWL and RLG, unpublished).
Figure 8.
The L15F, N88I, and L15F–N88I substitutions increase the affinity of DksA for RNAP holoenzyme. The binding affinity of 32P-labelled HMK-His6-tagged DksA (wild-type, L15F, N88I, or L15F–N88I) was measured quantitatively using an RNAP-dependent localized iron cleavage assay (CWL and RLG, unpublished). In panels A–D, the normalized fraction of DksA (wild-type or mutant) cleaved is plotted versus the concentration of RNAP holoenzyme in the reaction. Curves were generated from at least six separate experiments. Inset: representative gel images in which the lanes from left to right contain 0, 4, 8, 16, 32, 64, or 128 nM RNAP holoenzyme. (E) Binding curves are displayed together for comparison. WT (solid line), L15F (long dashes), N88I (short dashes), and L15F–N88I DksA (filled circles). Apparent binding constants are shown in the inset.
Apparent binding affinities of the wild-type and mutant DksA proteins for RNAP are compared in Figure 8E. We determined that N88I–DksA and N88I–L15F–DksA bind to RNAP about five-fold more tightly and L15F–DksA binds about two-fold more tightly than wild-type DksA. These results suggest that much (or all) of the increase in DksA function derives from increased affinity of the mutant DksA proteins for RNAP.
Discussion
Mutations in dksA that bypass a requirement for ppGpp
We identified two mutations in dksA that compensated for the multiple amino-acid auxotrophies of ppGpp-deficient (ppGpp0) cells, and we analysed their effects on an rRNA promoter and on promoters for amino-acid biosynthesis and transport in vivo and in vitro. The mutant proteins functioned as ‘super DksAs', affecting gene expression in vivo more than wild-type DksA. Even in the absence of ppGpp, the super DksAs displayed potent inhibitory effects on the rRNA promoter in vitro, at least as strong as the inhibitory effects of wild-type DksA and ppGpp together.
These results are reminiscent of recent observations for TraR, a protein like the DksA mutants described here that can affect transcription independently of ppGpp (Blankschien et al, 2009). TraR has sequence similarity to one of the α-helices in the DksA coiled–coil, but contains only one α-helix, in contrast to the two present in the DksA coiled coil.
DksA concentrations in vivo remain relatively constant under all conditions that have been tested (Paul et al, 2004; Rutherford et al, 2007). Thus, even though the super DksAs by themselves can dramatically inhibit rRNA transcription, they alone cannot provide the graded response afforded by DksA and ppGpp together, adjusting rRNA-promoter activity to changes in the nutritional status of the cell. In fact, expression of the super DksAs strongly inhibited cell growth in the ppGpp0 strain (Figure 4). Although DksA still functions and has important effects on cell physiology during stationary phase and outgrowth from stationary phase, times when ppGpp concentrations are very low or absent (Paul et al, 2004), changes in the concentrations of NTPs, which work in conjunction with DksA, are probably needed to modulate rRNA-promoter activity at these times (Murray et al, 2003).
Both DksA and ppGpp are normally required for growth on minimal medium. The super DksA mutants restore growth in the absence of ppGpp and activate at least one promoter implicated in amino-acid biosynthesis/transport. However, we were unable to detect activation of amino-acid promoters by the super DksAs in the absence of ppGpp in vitro. Therefore, we suggest that in the absence of ppGpp, the DksA mutants might suppress the amino-acid requirements indirectly. As rRNA transcription accounts for the majority of all transcription in moderate to fast growing E. coli (Bremer and Dennis, 1996), by directly inhibiting rRNA promoters more strongly than wild-type DksA, the super DksAs could increase the availability of RNAP for binding to the weak amino-acid promoters, thereby suppressing the requirement for amino acids in the ppGpp0 strain (Zhou and Jin, 1998; Barker et al, 2001a).
The effect of the super dksA alleles on amino-acid promoter(s) in vivo suggests an explanation for the growth defects of ppGpp0 strains on media lacking amino acids. However, ppGpp0 and ΔdksA strains are extremely pleiotropic (Paul et al, 2004; Durfee et al, 2008; Traxler et al, 2008) and have defects not only in gene expression, but also in DNA replication and repair (Trautinger and Lloyd, 2002) and on cellular motility (Magnusson et al, 2007) (see also Supplementary Figure S3). Therefore, we feel that caution should be exercised in assuming that the effects of the super DksA mutants on amino-acid promoters are fully responsible for suppressing the growth defects of ΔdksA strains.
Structural and mechanistic considerations derived from analysis of the DksA variants
Wild-type DksA and ppGpp together inhibit the activities of some promoters and stimulate the activities of others. However, how DksA and ppGpp work together is not understood. In theory, several mechanisms for the apparent synergy are possible: (1) ppGpp could increase an intrinsic activity of DksA, (2) ppGpp and DksA could cooperatively induce a common conformational change in RNAP that neither could perform alone, or (3) ppGpp and DksA could induce different conformational changes in RNAP that together result in larger effects on promoter activity than those accomplished by either factor alone. The super DksA mutants could provide insights into how ppGpp affects the DksA–RNAP complex.
The super DksA mutants described here provide one mechanism for compensating for the absence of ppGpp. These DksA mutants appear to be more prone, than the wild-type protein, to adopt an ‘active conformation', in this case one that binds better to RNAP than wild-type DksA. The super DksAs likely destabilize promoter-RNAP interactions in the vicinity of the transcription start site the same way as wild-type DksA does, but the mutants likely do so more efficiently because they occupy the RNAP secondary channel longer and/or because a greater fraction of the RNAP population contains bound DksA at any one time. Although the super DksAs compensated to some degree for the absence of ppGpp, they did not make RNAP insensitive to further effects of ppGpp. The super DksA proteins are likely to prove useful as reagents for future biochemical and structural studies on the allosteric effects of DksA.
Substitutions in the β, β', and σ subunits of RNAP have also been identified that compensate for the absence of ppGpp (Bartlett et al, 1998; Trautinger and Lloyd, 2002; Murphy and Cashel, 2003; Szalewska-Palasz et al, 2007; Rutherford et al, 2009). Like these RNAP mutants, the super DksAs reduce the stability of the RNAP-promoter complex, altering the kinetics of transcription initiation. The fact that increasing the apparent affinity of DksA for RNAP mimics effects of ppGpp on transcription suggests one potential model for ppGpp function: increasing binding of DksA to RNAP. This model is currently under investigation. Although most of the RNAP mutants identified to date that bypass the DksA/ppGpp requirement for growth on minimal medium do not alter RNAP–DksA interactions directly (Rutherford et al, 2009), our results suggest that it may also be possible to identify ppGpp0 suppressors in RNAP with increased affinity for DksA.
The structure of DksA has been determined (Perederina et al, 2004). The 151 amino-acid protein folds into two domains: a domain containing two antiparallel α-helices (coiled coil) and a globular domain with a C4 Zn2+ finger motif (Figure 1A). The overall size of DksA, the structural similarity of its coiled-coil domain to that in the Gre factors, the presence of two highly conserved acidic residues at its coiled-coil tip, and the cleavage of the coiled-coil tip by hydroxyl radicals generated by Fe2+ at the RNAP active site strongly suggest that DksA occupies the RNAP secondary channel, with its coiled-coil tip extending within ∼10 Å of the active site (Figure 8) (Perederina et al, 2004; Rutherford et al, 2009). The orientation of DksA in the secondary channel and the residues in DksA responsible for RNAP binding remain to be defined. However, our data suggest that at least two parts of DksA play roles in RNAP binding: the section of the globular domain containing L15 and the section of the coiled-coil containing N88. We suspect that residues N88 and L15 themselves do not make direct contacts to RNAP, as both are only ∼15% conserved in DksA homologues (Liu et al, 2006). Interestingly, the α-helix present in TraR corresponds to the one containing N88, where we obtained the substitution greatly affecting RNAP binding.
Although the two super DksA substitutions each affected RNAP binding and displayed additive effects on DksA function in some assays, we did not observe additive effects of the two substitutions on RNAP binding (Figure 8). Thus, at least one of the substitutions might also cause a conformational change subsequent to RNAP binding.
Finally, it was proposed earlier that ppGpp binds deep inside the secondary channel near the RNAP active site (Artsimovitch et al, 2004), and that DksA facilitates this interaction, enhancing effects of ppGpp by chelation of a ppGpp-bound Mg2+ using its highly conserved D71 and D74 aspartic-acid residues at the coiled-coil tip (Perederina et al, 2004). Although quite attractive, we consider this model unlikely, as ppGpp does not appear to function by binding to the site identified in the ppGpp/T. thermophilus RNAP co-crystal (Vrentas et al, 2008). Thus, the role of the conserved aspartates at the coiled-coil tip of DksA remains to be defined.
Conclusion
Small proteins that bind in the RNAP secondary channel are encoded not only by bacteria, but also by self-transmissible plasmids and bacteriophages (Blankschien et al, 2009), and it is clear that they play crucial roles in microbial gene expression. Our identification of DksA mutants with increased activity on RNAP, thereby bypassing a requirement for ppGpp in transcription initiation, is a step on the road towards understanding the mechanism of DksA action and the function of its cofactors.
Materials and methods
Media and bacterial growth
Cells were grown at 32°C in LB medium or M9-supplemented as indicated with sodium citrate (5–20 mM), ampicillin (50 μg/ml), kanamycin (30 μg/ml), chloramphenicol (12.5 μg/ml), tetracycline (3.33 μg/ml when medium contained sodium citrate and 10 μg/ml when without), glucose (glu) (0.1%), casamino acids (CAA) (0.3%), and IPTG (0.1 mM). M9-medium was always supplemented with FeCl2 (10 μM) and thiamine (2 μg/ml).
Bacterial strains, plasmids, and primers
Transduction with P1vir and other standard genetic methods were performed as described by Miller (1992). Site-directed mutagenesis, plasmid construction, and transformation were performed as described by Sambrook and Russell (2001). Strains and plasmids are listed in Supplementary Table S2 and primers are in Supplementary Table S3 (Supplementary data). The promoter-lacZ fusions have been described earlier (Paul et al, 2005) and were carried on a lambda prophage on the bacterial chromosome. Unless otherwise stated, all strains are derivatives of MG1655. Antibiotic resistance, PCR, DNA sequencing, and/or phenotypic assays were performed to verify constructions.
Selection and identification of ΔrelA ΔspoT ΔdksA suppressor mutations
Plasmid pBA169, a pTrc99A (Amann et al, 1988) derivative containing wild-type dksA, was transformed into a mutD mutL mutator strain. Colonies (approximately 1000) were harvested, pooled, plasmids were purified, transformed into a ΔrelA ΔspoT ΔdksA strain containing pJM101 (lacIq), and plated on minimal M9-glu medium without IPTG or amino acids. Colonies appeared after approximately 3 days of incubation at 32°C. A total of 29 isolates were streaked on minimal medium, and the plasmids were purified and transformed into a ΔrelA ΔspoT ΔdksA strain; 14 plasmids from this collection grew on minimal medium without IPTG or amino acids. The dksA ORFs were sequenced, and two mutants were examined in detail (see text).
Plating efficiencies of auxotrophic strains
Overnight cultures grown in LB were serially diluted in 10 mM MgSO4 and plated on M9-glu and M9-glu-CAA supplemented with ampicillin and IPTG (0.1 mM). Plating efficiencies were obtained from colony counts after incubation for 4 days at 32°C.
β-Galactosidase activity assays
Overnight cultures were diluted 1/100 and grown with shaking at 32°C in LB supplemented with ampicillin, and when appropriate, IPTG (0.1 mM) to express DksA. Aliquots (0.5 ml) were assayed essentially as described earlier (Miller, 1992), except that samples were centrifuged to pellet cell debris before determination of OD420. The β-galactosidase activity was determined for at least two independent experiments and is represented as OD420/ml culture as a function of the OD600 of the culture. Polynomial (third order) regression lines were determined using Microsoft Excel.
Motility assay
Cells were grown overnight in LB media containing ampicillin, inoculated with a toothpick onto low percentage agar (0.375%) LB plates, incubated at room temperature for ∼24 h, and halos were measured (Magnusson et al, 2007).
Measurements of DksA levels
Western blots were performed as described (Sambrook and Russell, 2001). Briefly, uninduced or induced cultures were grown in LB medium to an OD600 of 0.4, 1 ml aliquots were centrifuged and resuspended in 100 μl of SDS loading buffer, and 10 μl samples were analysed on 15% polyacrylamide gels by western blotting and coomassie blue staining. Blots utilized antibodies against DksA (primary; 1:2500 dilution of chicken polyclonal IgY, courtesy of M. Cashel, National Institutes of Health) and goat anti-chicken IgY-TR (secondary) (1:400 dilution, Santa Cruz Biotechnology). The polyvinylidene fluoride membrane was scanned and quantified with a Typhoon Trio imager (GE).
Protein purification
N-terminally His6-tagged DksA was over-expressed from a phage T7 promoter in plasmid pRLG7067, a pET28a derivative encoding His6–DksA, in a BL21(DE3) variant in which the greB and dksA genes were both inactivated (Rutherford et al, 2007). DksA was purified by nickel–agarose affinity chromatography as described (Paul et al, 2004) (see Supplementary Figure S1). For producing 32P-labelled proteins for the Fe2+-cleavage experiments (Figure 8 and see below), HMK and His6 mutant DksAs labelled at the N-terminus were constructed and over-expressed from E. coli BL21(DE3) (Novagen) as described for wild-type DksA (Rutherford et al, 2009), using plasmid pRLG8150 for wild-type DksA, pRLG9137 for L15F–DksA, pRLG9148 for N88I–DksA, and pRLG9149 for L15F–N88I–DksA. RNAP holoenzyme was purified by standard procedures (Burgess and Jendrisak, 1975; Gaal et al, 2001). Protein concentrations were determined with the Bradford assay reagent (Bio-Rad), using bovine serum albumin (BSA) as a standard.
In vitro transcription
Single and multiple-round in vitro transcription were performed as described (Paul et al, 2004) with 50 ng supercoiled plasmid and 10 nM RNAP at 30°C in transcription buffer (40 mM Tris–HCl (pH 7.9), 10 mM MgCl2, 1 mM DTT, and 0.1 mg/ml of BSA) and the salt concentrations indicated in the figure legends. Template DNA was combined with transcription buffer and NTPs (500 μM ATP, 200 μM GTP, 200 μM CTP, 10 μM UTP, and 1.0 μCi of [α-32P] UTP, final concentrations), transcription was initiated by the addition of RNAP, and the reaction was terminated after 10 min by the addition of an equal volume of stop solution. Transcripts were separated on 6% polyacrylamide gels containing 7 M urea. The amount of transcription was quantified by phosphorimaging using Image-Quant software and normalized to transcription by wild-type RNAP in the absence of factors. ppGpp was obtained from TriLink Inc and used at the concentrations indicated in the figure legends. Curves were fit using SigmaPlot.
RNAP-promoter complex stability assay
Complex lifetime was measured on supercoiled templates using a transcription-based assay (Barker et al, 2001b). The 10 nM RNAP was incubated with 50 ng supercoiled plasmid pRLG3422 containing the lacUV5 promoter (lac promoter endpoints −46 to +1) in transcription buffer (40 mM Tris–HCl (pH 7.9), 10 mM MgCl2, 1 mM DTT, and 0.1 mg/ml BSA) containing 200 mM NaCl, wild-type DksA, mutant DksA, or storage buffer (no factor) at 30°C for 10 min. Transcription reactions were initiated with NTPs (500 μM ATP, 200 μM GTP, 200 μM CTP, 10 μM UTP, and 1.0 μCi of [α-32P] UTP, final concentrations) at times after the addition of heparin (100 μg/ml). Reactions were stopped and the RNA products were separated by electrophoresis and quantified as described above. The amount of transcription relative to that at the time of competitor addition was plotted as a function of time.
Measurement of DksA binding using RNAP-dependent localized iron cleavage
Mg2+ in the active centre of RNAP was replaced with Fe2+, and cleavage of DksA by hydroxyl radicals was used as a quantitative estimate of binding to RNAP (Zaychikov et al, 1996; Perederina et al, 2004; Rutherford et al, 2009) (CWL and RLG, unpublished). In this assay, the apparent binding constants of the wild-type and mutant DksA proteins for RNAP were determined by measurement of the fraction of 32P-labelled N-terminally HMK-tagged DksA that was cleaved at different RNAP concentrations, normalized to the fraction cleaved at saturating RNAP concentration (CWL and RLG, unpublished). The concentration of DksA was held constant and was always much lower than the RNAP concentration. The concentration of RNAP where half-maximal cleavage of DksA was obtained was a measure of the apparent binding affinity of DksA for RNAP. We note that the binding constants would have been the same even if the position of the cleavage was different from that in the wild-type protein or if the fraction cleaved was lower for these mutants than for the wild-type protein (although neither was the case for these particular mutants).
Briefly, 10 μg of purified HMK-His6-tagged DksA (wild-type, L15F, N88I, or L15F/N88I) was 32P-labelled in a 30-μl reaction and purified using a G-50 QuickSpin column (GE Healthcare) pre-equilibrated with buffer A (20 mM NaHEPES pH 7.5 and 20 mM NaCl). After purification, the DksA concentration was re-measured using the protein dye reagent assay (Bio-Rad). DksA–RNAP complexes were formed by incubation of 1–10 nM DksA and at least three-fold molar excess of RNAP (4–400 nM) in a 10-μl reaction in buffer A for 15 min at 30°C. In the example shown in Figure 8, DksA was 1 nM and RNAP was 4–128 nM (Figure 8). The 1 μl of freshly prepared 500 μM (NH4)2 Fe(SO4)2 and 1 μl of 100 mM DTT were added together to the DksA–RNAP complex and incubated for 10 min at 30°C. The reaction was quenched with 12 μl 2 × LDS loading buffer (Invitrogen) containing glycerol. Products were analysed on 4–12% NuPAGE Bis–Tris gels in MES buffer (Invitrogen), and the dried gels were analysed by phosphorimaging. Binding curves were generated by calculating the fraction of cleaved DksA/cleaved plus uncleaved DksA at each RNAP concentration, normalized to the plateau value (maximum cleavage) at different RNAP holoenzyme concentrations. Binding curves, apparent KDs, and standard errors were generated using SigmaPlot.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure Legends
Supplementary Table S1
Supplementary Table S2
Supplementary Material
Review Process File
Acknowledgments
We thank A Choudhary, D Satory, A Gordon, and J Wang for helpful comments and/or excellent technical help and A Farewell, M Cashel, and S Rosenberg for strains and/or reagents. This work was funded by HFSPO grant RGY0060/2006 to CH, National Institutes of Health (NIH) grant R37GM37048 to RLG, a postdoctoral fellowship from the Korean Research Foundation to J-H L, and a Biotechnology predoctoral fellowship from the NIH to CWL.
References
- Aberg A, Shingler V, Balsalobre C (2008) Regulation of the fimB promoter: a case of differential regulation by ppGpp and DksA in vivo. Mol Microbiol 67: 1223–1241 [DOI] [PubMed] [Google Scholar]
- Amann E, Ochs B, Abel KJ (1988) Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69: 301–315 [DOI] [PubMed] [Google Scholar]
- Artsimovitch I, Patlan V, Sekine S, Vassylyeva MN, Hosaka T, Ochi K, Yokoyama S, Vassylyev DG (2004) Structural basis for transcription regulation by alarmone ppGpp. Cell 117: 299–310 [DOI] [PubMed] [Google Scholar]
- Barker MM, Gaal T, Gourse RL (2001a) Mechanism of regulation of transcription initiation by ppGpp. II. Models for positive control based on properties of RNAP mutants and competition for RNAP. J Mol Biol 305: 689–702 [DOI] [PubMed] [Google Scholar]
- Barker MM, Gaal T, Josaitis CA, Gourse RL (2001b) Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol 305: 673–688 [DOI] [PubMed] [Google Scholar]
- Bartlett MS, Gaal T, Ross W, Gourse RL (1998) RNA polymerase mutants that destabilize RNA polymerase-promoter complexes alter NTP-sensing by rrnB P1 promoters. J Mol Biol 279: 331–345 [DOI] [PubMed] [Google Scholar]
- Bernardo LM, Johansson LU, Solera D, Skarfstad E, Shingler V (2006) The guanosine tetraphosphate (ppGpp) alarmone, DksA and promoter affinity for RNA polymerase in regulation of sigma-dependent transcription. Mol Microbiol 60: 749–764 [DOI] [PubMed] [Google Scholar]
- Blankschien MD, Potrykus K, Grace E, Choudhary A, Vinella D, Cashel M, Herman C (2009) TraR, a homolog of a RNAP secondary channel interactor, modulates transcription. PLoS Genetics 5: e1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borukhov S, Lee J, Laptenko O (2005) Bacterial transcription elongation factors: new insights into molecular mechanism of action. Mol Microbiol 55: 1315–1324 [DOI] [PubMed] [Google Scholar]
- Braeken K, Moris M, Daniels R, Vanderleyden J, Michiels J (2006) New horizons for (p)ppGpp in bacterial and plant physiology. Trends Microbiol 14: 45–54 [DOI] [PubMed] [Google Scholar]
- Bremer H, Dennis P (1996) Modulation of chemical composition and other parameters of the cell by growth rate. In: Escherichia coli and Salmonella: Cellular and Molecular Biology, Neidhardt FC, Curtiss R, 3rd, Ingraham EC, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (eds) Vol. 2, pp 1553–1569. Washington, District of Columbia: American Society for Microbiology [Google Scholar]
- Burgess RR, Jendrisak JJ (1975) A procedure for the rapid, large-scale purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry 14: 4634–4638 [DOI] [PubMed] [Google Scholar]
- Costanzo A, Nicoloff H, Barchinger SE, Banta AB, Gourse RL, Ades SE (2008) ppGpp and DksA likely regulate the activity of the extracytoplasmic stress factor sigmaE in Escherichia coli by both direct and indirect mechanisms. Mol Microbiol 67: 619–632 [DOI] [PubMed] [Google Scholar]
- Durfee T, Hansen AM, Zhi H, Blattner FR, Jin DJ (2008) Transcription profiling of the stringent response in Escherichia coli. J Bacteriol 190: 1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erie DA, Hajiseyedjavadi O, Young MC, von Hippel PH (1993) Multiple RNA polymerase conformations and GreA: control of the fidelity of transcription. Science 262: 867–873 [DOI] [PubMed] [Google Scholar]
- Gaal T, Ross W, Estrem ST, Nguyen LH, Burgess RR, Gourse RL (2001) Promoter recognition and discrimination by EsigmaS RNA polymerase. Mol Microbiol 42: 939–954 [DOI] [PubMed] [Google Scholar]
- Haugen SP, Ross W, Gourse RL (2008) Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat Rev Microbiol 6: 507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour V, Rutherford ST, Kuznedelov K, Ramagopal UA, Gourse RL, Severinov K, Darst SA (2008) Crystal structure of Escherichia coli Rnk, a new RNA polymerase-interacting protein. J Mol Biol 383: 367–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XI, Korde N, Jakob U, Leichert LI (2006) CoSMoS: conserved sequence motif search in the proteome. BMC Bioinformatics 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson LU, Farewell A, Nystrom T (2005) ppGpp: a global regulator in Escherichia coli. Trends Microbiol 13: 236–242 [DOI] [PubMed] [Google Scholar]
- Magnusson LU, Gummesson B, Joksimovic P, Farewell A, Nystrom T (2007) Identical, independent, and opposing roles of ppGpp and DksA in Escherichia coli. J Bacteriol 189: 5193–5202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH (1992) A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press
- Murphy H, Cashel M (2003) Isolation of RNA polymerase suppressors of a (p)ppGpp deficiency. Methods Enzymol 371: 596–601 [DOI] [PubMed] [Google Scholar]
- Murray HD, Schneider DA, Gourse RL (2003) Control of rRNA expression by small molecules is dynamic and nonredundant. Mol Cell 12: 125–134 [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Abe H, Ogura Y, Hayashi T, Tashiro K, Kuhara S, Sugimoto N, Tobe T (2006) ppGpp with DksA controls gene expression in the locus of enterocyte effacement (LEE) pathogenicity island of enterohaemorrhagic Escherichia coli through activation of two virulence regulatory genes. Mol Microbiol 61: 194–205 [DOI] [PubMed] [Google Scholar]
- Opalka N, Chlenov M, Chacon P, Rice WJ, Wriggers W, Darst SA (2003) Structure and function of the transcription elongation factor GreB bound to bacterial RNA polymerase. Cell 114: 335–345 [DOI] [PubMed] [Google Scholar]
- Orlova M, Newlands J, Das A, Goldfarb A, Borukhov S (1995) Intrinsic transcript cleavage activity of RNA polymerase. Proc Natl Acad Sci USA 92: 4596–4600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BJ, Barker MM, Ross W, Schneider DA, Webb C, Foster JW, Gourse RL (2004) DksA: a critical component of the transcription initiation machinery that potentiates the regulation of rRNA promoters by ppGpp and the initiating NTP. Cell 118: 311–322 [DOI] [PubMed] [Google Scholar]
- Paul BJ, Berkmen MB, Gourse RL (2005) DksA potentiates direct activation of amino acid promoters by ppGpp. Proc Natl Acad Sci USA 102: 7823–7828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perederina A, Svetlov V, Vassylyeva MN, Tahirov TH, Yokoyama S, Artsimovitch I, Vassylyev DG (2004) Regulation through the secondary channel-structural framework for ppGpp-DksA synergism during transcription. Cell 118: 297–309 [DOI] [PubMed] [Google Scholar]
- Potrykus K, Cashel M (2008) (p)ppGpp: still magical? Annu Rev Microbiol 62: 35–51 [DOI] [PubMed] [Google Scholar]
- Potrykus K, Vinella D, Murphy H, Szalewska-Palasz A, D'Ari R, Cashel M (2006) Antagonistic regulation of Escherichia coli ribosomal RNA rrnB P1 promoter activity by GreA and DksA. J Biol Chem 281: 15238–15248 [DOI] [PubMed] [Google Scholar]
- Rutherford ST, Lemke JJ, Vrentas CE, Gaal T, Ross W, Gourse RL (2007) Effects of DksA, GreA, and GreB on transcription initiation: insights into the mechanisms of factors that bind in the secondary channel of RNA polymerase. J Mol Biol 366: 1243–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford ST, Villers CL, Lee JH, Ross W, Gourse RL (2009) Allosteric control of promoter complexes by DksA. Genes Dev 23: 236–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press [Google Scholar]
- Shaevitz JW, Abbondanzieri EA, Landick R, Block SM (2003) Backtracking by single RNA polymerase molecules observed at near-base-pair resolution. Nature 426: 684–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Payne SM (2006) Induction of expression of hfq by DksA is essential for Shigella flexneri virulence. Mol Microbiol 62: 469–479 [DOI] [PubMed] [Google Scholar]
- Stebbins CE, Borukhov S, Orlova M, Polyakov A, Goldfarb A, Darst SA (1995) Crystal structure of the GreA transcript cleavage factor from Escherichia coli. Nature 373: 636–640 [DOI] [PubMed] [Google Scholar]
- Szalewska-Palasz A, Johansson LU, Bernardo LM, Skarfstad E, Stec E, Brannstrom K, Shingler V (2007) Properties of RNA polymerase bypass mutants: implications for the role of ppGpp and its co-factor DksA in controlling transcription dependent on sigma54. J Biol Chem 282: 18046–18056 [DOI] [PubMed] [Google Scholar]
- Trautinger BW, Lloyd RG (2002) Modulation of DNA repair by mutations flanking the DNA channel through RNA polymerase. EMBO J 21: 6944–6953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxler MF, Summers SM, Nguyen HT, Zacharia VM, Hightower GA, Smith JT, Conway T (2008) The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol Microbiol 68: 1128–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrentas CE, Gaal T, Berkmen MB, Rutherford ST, Haugen SP, Ross W, Gourse RL (2008) Still looking for the magic spot: the crystallographically defined binding site for ppGpp on RNA polymerase is unlikely to be responsible for rRNA transcription regulation. J Mol Biol 377: 551–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M (1991) Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J Biol Chem 266: 5980–5990 [PubMed] [Google Scholar]
- Zaychikov E, Martin E, Denissova L, Kozlov M, Markovtsov V, Kashlev M, Heumann H, Nikiforov V, Goldfarb A, Mustaev A (1996) Mapping of catalytic residues in the RNA polymerase active center. Science 273: 107–109 [DOI] [PubMed] [Google Scholar]
- Zhou YN, Jin DJ (1998) The rpoB mutants destabilizing initiation complexes at stringently controlled promoters behave like ‘stringent' RNA polymerases in Escherichia coli. Proc Natl Acad Sci USA 95: 2908–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure Legends
Supplementary Table S1
Supplementary Table S2
Supplementary Material
Review Process File