Abstract
Agonist-induced ubiquitination of the β2 adrenergic receptor (β2AR) functions as an important post-translational modification to sort internalized receptors to the lysosomes for degradation. We now show that this ubiquitination is reversed by two deubiquitinating enzymes, ubiquitin-specific proteases (USPs) 20 and 33, thus, inhibiting lysosomal trafficking when concomitantly promoting receptor recycling from the late-endosomal compartments as well as resensitization of recycled receptors at the cell surface. Dissociation of constitutively bound endogenously expressed USPs 20 and 33 from the β2AR immediately after agonist stimulation and reassociation on prolonged agonist treatment allows receptors to first become ubiquitinated and then deubiquitinated, thus, providing a ‘trip switch' between degradative and recycling pathways at the late-endosomal compartments. Thus, USPs 20 and 33 serve as novel regulators that dictate both post-endocytic sorting as well as the intensity and extent of β2AR signalling from the cell surface.
Keywords: arrestin, cAMP, GPCR, lysosomes, recycling
Introduction
The receptor intracellular trafficking is an essential process that allows plasma-membrane localized seven-transmembrane receptors (7TMRs, also known as G-protein-coupled receptors, GPCRs) to generate adaptive responses to external stimuli (Tan et al, 2004; Drake et al, 2006; Moore et al, 2007). Adaptor proteins called β-arrestins 1 and 2, which bind the receptor on receptor phosphorylation by the G-protein-coupled receptor kinases (GRKs), mediate agonist-stimulated 7TMR endocytosis (Ferguson, 2001; DeWire et al, 2007). Owing to its involvement in severe pathologies, including heart failure and asthma, β2 adrenergic receptor (β2AR) trafficking has been extensively studied. The β2AR and β-arrestin interaction leads to receptor desensitization as well as internalization into clathrin-coated pits. After internalization into early endosomes, the β2ARs are rapidly recycled back to the plasma membrane promoting functional resensitization of receptor signalling (Pippig et al, 1995). However, during prolonged exposure to agonists, β2ARs also sort from endosomes to lysosomes and are degraded (Moore et al, 2007; Shenoy et al, 2008). This downregulation process represents a critical means of regulating the number of receptors at the cell surface (Drake et al, 2006), thereby influencing the intensity of physiological responses to β-agonists, many of which are used as therapeutic agents.
For the β2AR and several other 7TMRs, receptor ubiquitination serves as a necessary modification for receptor degradation in the lysosomal compartments (Shenoy, 2007). On the other hand, ubiquitination of β-arrestin2 regulates the initial step of receptor endocytosis and signalling of 7TMRs (Shenoy et al, 2001; DeWire et al, 2007). Additionally, β-arrestin2 recruits the HECT-domain containing E3 ligase Nedd4 and mediates ubiquitination and subsequent lysosomal degradation of the β2ARs (Shenoy et al, 2008). Internalized ubiquitinated receptors are also recognized by a conserved set of endosome-associated proteins known as the ESCRT complexes, which are suggested to promote receptor translocation to the intralumenal vesicles of multi-vesicular bodies and facilitate lysosomal degradation (Saksena et al, 2007).
Ubiquitination is a reversible process and deubiquitinating enzymes (DUBs) remove the ubiquitin moieties from ubiquitinated substrates. About 100 putative genes in the human genome encode DUBs, which are divided into five distinct subclasses of which the ubiquitin-specific protease (USP) subclass represents the bulk of the human DUBs (Nijman et al, 2005). It is thought that ubiquitination and deubiquitination processes are choreographed by adaptors that bind both these enzymes. As β-arrestin2 serves as an adaptor to bring the E3 ubiquitin ligase Nedd4 to the β2AR, we wondered if it could have a dual role in escorting a DUB to modulate receptor ubiquitination. Recently, we discovered that β-arrestins bind the DUB , USP33 (Shenoy et al, 2009), and herein we report our findings on how USP33 and its homologue USP20 modulate post-endocytic trafficking of the β2AR.
Results
USP33 inhibits the agonist-stimulated ubiquitination and lysosomal trafficking of the β2AR
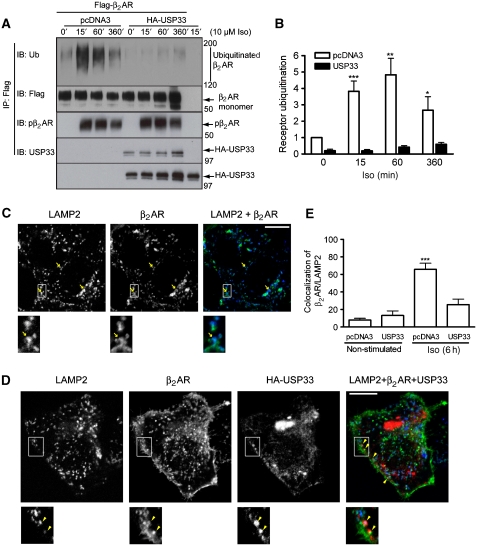
To evaluate if USP33 affects receptor ubiquitination, we overexpressed HA-USP33 in HEK-293 cells stably transfected with Flag-β2ARs and analysed receptor ubiquitination in response to isoproterenol (Iso) stimulation by immunoprecipitation and western blotting (Figure 1A and B). Iso stimulation resulted in receptor ubiquitination in cells with endogenous USP33 expression; however, this response was lost on overexpression of HA-USP33 in both HEK-293 (Figure 1A and B) and COS-7 cells (Supplementary Figure S1). This diminution of ubiquitin signals in USP33-containing samples is not due to a defect in receptor activation because agonist-stimulated receptor phosphorylation at the earlier documented GRK sites was normal (Figure 1A; Supplementary Figure S1, pβ2AR blot). The low level of receptor ubiquitination detected under basal conditions was eliminated on USP33 overexpression suggestive of a constitutive β2AR–USP33 interaction. HA-USP33 specifically interacted with the β2AR under basal as well as in agonist-stimulated conditions as assessed by immunoblotting (Figure 1A).
Figure 1.
USP33 inhibits agonist-stimulated ubiquitination and lysosomal trafficking of the β2AR. (A, B) Ubiquitination of the β2AR was measured in HEK-293 cells stably transfected with Flag-β2AR-mYFP. Cells were transfected transiently with pcDNA3 or HA-USP33 and stimulated with Iso (10 μM) for indicated times. The receptor was immunoprecipitated with M2 anti-Flag affinity gel and ubiquitinated receptor detected with an anti-ubiquitin antibody, FK2H (A, upper panel). The amounts of Flag-tagged β2AR, phosphorylated β2AR and USP33 in the IP are shown in the three panels below the Ub panel. Expression of USP33 is shown in the bottom panel (A). The electrophoretic mobility of molecular weight markers (in kD) is indicated at the right side of each panel. (B) The graph represents fold increases in receptor ubiquitination obtained after Iso stimulation and is the mean±s.e.m. of four independent experiments. ***P<0.001, **P<0.01 between pcDNA3 and USP33 conditions, two-way ANOVA Boniferroni comparison. (C, D) The 293 cells with stable Flag-β2AR-mYFP expression were transfected either with (C) pcDNA3 or (D) USP33. After Iso (10 μM) stimulation for 6 h, cells were fixed and immunostained for USP33 and LAMP2 as described in ‘Materials and methods'. Confocal images are displayed in black and white for each individual channel and merged images are shown with the Flag-β2AR-mYFP in green, USP33 in red (Alexa 633) and LAMP2 in blue (Alexa 594). A portion of each image is enlarged and displayed below. Arrows in (C) indicate colocalization of the β2AR and LAMP2 and arrowheads in panel (D) indicate colocalization of USP33 and β2AR. (E) Bar graph represents quantification of colocalization in merged images for the two channels LAMP2 and β2AR in each sample (see Materials and methods). ***P<0.001 pcDNA3-6 h versus all others, one-way ANOVA, Boniferroni comparison.
Agonist-stimulated receptor ubiquitination has been shown earlier to serve as a signal for lysosomal degradation (Shenoy et al, 2008). As HA-USP33 overexpression inhibits receptor ubiquitination, we anticipated that it would cause a lag in lysosomal trafficking of the β2AR. To assess this, we stimulated HEK-293 cells stably expressing Flag-β2AR-mYFP with or without co-expressed HA-USP33 with Iso and determined receptor colocalization with the late-endosomal/lysosomal marker protein LAMP2. In cells with endogenous USP33 expression, we observed ∼60% colocalization of the β2AR and LAMP2 (Figure 1C and E). In contrast, in cells overexpressing HA-USP33, we detected only ∼20% colocalization of LAMP2 and the β2AR (Figure 1D and E). In HEK-293 cells, on immunostaining with anti-USP33 (Figure 1D; Supplementary Figure S2) or anti-HA (Supplementary Figure S2A) antibodies, overexpressed USP33 is visualized in the perinuclear regions and in small vesicles distributed throughout the cytoplasm and near the plasma membrane. Unfortunately, we were unable to detect endogenous USP33 by immunostaining with anti-USP33 antibodies. In quiescent cells, we detected colocalization of HA-USP33 and the β2AR at the plasma membrane (Supplementary Figure S2B), and in agonist-stimulated cells, we observed the β2AR-positive vesicles being partially or wholly enveloped by USP33-positive vesicles (Figure 1D; Supplementary Figure S2B). We did not observe any differences in receptor distribution between cells with endogenous or overexpressed USP33 under unstimulated conditions or on Iso stimulation for <6 h (Supplementary Figure S2B and data not shown). However, after 6 h of Iso stimulation, in cells overexpressing HA-USP33, the β2ARs re-localized at the plasma membrane or were found in small vesicles close to the plasma membrane, suggesting that receptors have recycled (compare the β2AR panels in Figure 1C and D). Overall, USP33 overexpression leads to an inhibition of receptor ubiquitination accompanied by a significant decrease in lysosomal trafficking as well as an increase in the plasma-membrane reappearance of the β2AR after 6 h Iso stimulation.
Catalytically inactive USP33 mutants do not inhibit the agonist-stimulated ubiquitination and lysosomal trafficking of the β2AR
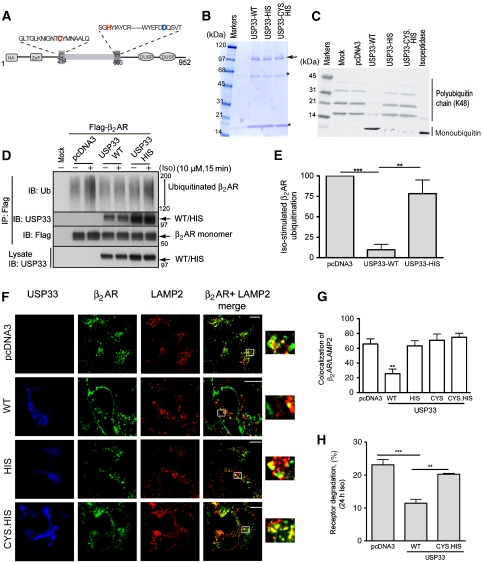
USP33 is a cysteine protease and its enzymatic activity relies on the thiol group of a cysteine in the active site. Deprotonation of this cysteine (C214 in HA-USP33) is assisted by a histidine (H683 in HA-USP33), which is polarized by an aspartate residue. These three residues make up the catalytic triad (Figure 2A). We generated inactive USP33 mutants by altering the cysteine 214 to a serine (USP33-CYS) and/or the histidine 683 to glutamine (USP33-HIS) and tested the deubiquitinating activity of HA-tagged (wild type) WT and mutant enzymes purified from COS-7 cells in an in vitro assay using polyubiquitin chains as substrates (Figure 2B and C). Monoubiquitin accumulated due to the disassembly of either lysine48- or lysine63-linked polyubiquitin chains in the presence of USP33-WT and a generic DUB (isopeptidase T), but not USP33 mutants (Figure 2C and data not shown).
Figure 2.
USP33 active site mutants do not inhibit receptor ubiquitination and lysosomal trafficking. (A) Schematic indicates the positions of cysteine (red) and histidine (red), which form the catalytic triad along with an aspartate (blue). ZnF, zinc finger; DUSP, domain in USPs. (B) Coomassie gel displays purified preparations of HA-USP33: WT, HIS mutant and CYS.HIS mutant. The arrow indicates mobility of HA-USP33. Asterisks indicate the heavy and light chain IgG bands eluting from the HA-affinity beads. (C) Enzymatic activity measured by in vitro DUB assay. After purification, USP33 WT and mutants (as indicated) were incubated at 37°C with the polyubiquitin chain (K48). The isopeptidase T-enzyme is known to cleave polyubiquitin chains to yield monoubiquitin and represents a positive control. (D) COS cells co-expressing the Flag-β2AR with either HA-USP33 or HA-USP33-HIS were stimulated or not with 10 μM Iso for 15 min. The β2AR was then immunoprecipitated with M2 anti-Flag affinity gel and ubiquitinated receptors detected with an anti-ubiquitin antibody P4D1 (upper panel). The amount of USP33 (WT or mutant) and Flag-tagged β2AR in the IP are seen in the second and third panel, respectively. Expression of USP33 WT and USP33-HIS is detected with an anti-USP33 antibody (lowest panel). Mobility of molecular weight markers are indicated at the right. (E) The graph represents the ratio between the β2AR ubiquitination signals obtained after Iso stimulation and the ubiquitination signals obtained in the nonstimulated condition. The result is the mean±s.e.m. of four independent experiments. ***P<0.001, **P<0.01 between indicated samples, one-way ANOVA. (F) The 293 cells expressing Flag-β2AR-mYFP stably are transfected with pcDNA3, USP33-WT or indicated USP33-mutants. The distributions of USP33 (blue), β2AR (green) and LAMP2 (red) after 6 h Iso (10 μM) stimulation are shown for each sample as detected on immunostaining USP33 and LAMP2. Merged images for the two channels, β2AR in green and LAMP2 in red (Alexa 594), are shown. (G) Quantification of β2AR and LAMP2 colocalization at 6 h Iso is shown; ***P<0.001 WT versus all others, one-way ANOVA. (H) The bar graph represents β2AR degradation in HEK-293 cells on coexpression of vector, USP33-WT or USP33-CYS.HIS mutant after 24 h Iso stimulation measured by 125I-CYP binding. **P<0.01, ***P<0.001, one-way ANOVA (n=4); in all above experiments, ANOVA was followed by Bonferroni comparison.
We tested the effects of USP33-HIS, USP33-CYS and the double mutant USP33-CYS.HIS, which had comparable receptor interactions (Supplementary Figure S3; Figure 2D) on both ubiquitination and trafficking of the β2AR (Figure 2D–G and data not shown). In cells coexpressing Flag-β2AR and either USP33-HIS, USP33-CYS or USP33-CYS.HIS, receptor was ubiquitinated to identical levels as in cells transfected with vector and Flag-β2AR (Figure 2D and E and data not shown). In contrast, as in Figure 1, no agonist-induced ubiquitination was seen on WT USP33 overexpression along with Flag-β2AR (Figure 2D and E). After 6 h of Iso stimulation, unlike USP33-WT that led to a decrease in lysosomal trafficking (Figure 2F, right panel second row), USP33-HIS, USP33-CYS.HIS or USP33-CYS did not affect β2AR-LAMP2 colocalization (Figure 2F and G and data not shown). At 6 h of Iso stimulation, receptor degradation as measured by [125I]-(−)iodocyanopindolol (125I-CYP) binding was <10% in cells with endogenous USP33 expression, whereas there was no degradation in cells overexpressing HA-USP33 (not shown). Receptor degradation after 24 h Iso treatment was also dramatically reduced by the coexpression of USP33 (24±2%: pCDNA3 versus 11±1.2%: USP33-WT, Figure 2H), but not catalytically inactive USP33 (21±0.5%: USP33-CYS.HIS, Figure 2H). These results show that the enzymatic activity of USP33 regulates β2AR ubiquitination and modulates receptor degradation in the late-endosomal/lysosomal compartments.
Role of deubiquitination in β2AR recycling
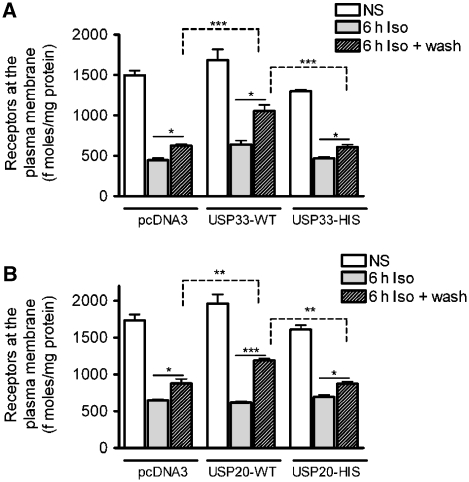
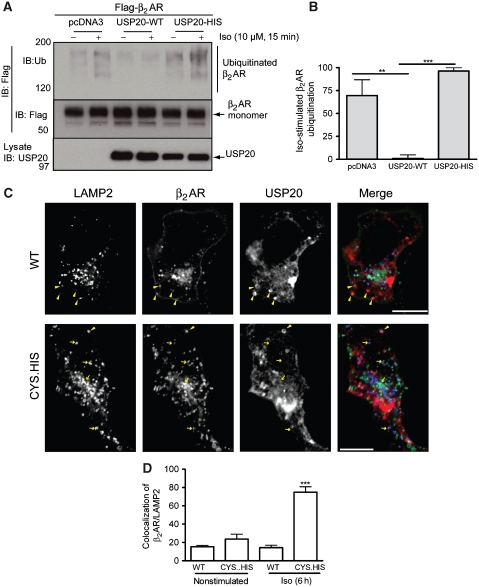
As USP33 activity appears to not only inhibit lysosomal trafficking and degradation, but actually promote the plasma-membrane localization of receptors (Figures 1D and 2F), we sought to quantify the resulting change in the absolute amount of cell-surface receptors using radioligand binding. We stimulated HEK-293 cells with stable β2AR expression for 6 h with Iso, performed agonist washout to enhance recycling and measured cell-surface receptors by 3H-CGP12177 binding before and after agonist washout. In cells transfected with pcDNA3, a 30–40% increase in surface receptors was detected at the end of the 2-h wash period (Figure 3A). This increase was not affected by the addition of 5 μg/ml cycloheximide (protein synthesis inhibitor) before Iso stimulation and, therefore, is not due to newly synthesized receptors (data not shown). Overexpression of USP33 resulted in a 65–70% increase in cell-surface receptors—as that of USP33-HIS, which led to ∼35–45% increase (Figure 3A). If the catalytically inactive mutant functioned as a ‘dominant negative', we would have observed some inhibition of receptor recycling. We theorized that the lack of a ‘dominant-negative' effect could be attributed to the presence of a related enzymatic activity that functions in receptor deubiquitination and trafficking. In fact, mammalian cells express a homologue of USP33, namely USP20 (Supplementary Figure S4), and its overexpression produced equivalent increasing effects on receptor recycling as that by USP33 (Figure 3B). Overexpression of USP20-HIS (H643Q) mutant had similar effects on recycling as that induced by USP33-HIS mutant (Figure 3B). Moreover, USP20 overexpression inhibited receptor ubiquitination, whereas catalytically inactive mutants, USP20-HIS, USP20-CYS (C154S) and USP20-CYS.HIS (C154S.H643Q), did not (Figure 4A and B and data not shown). USP20 inhibited lysosomal trafficking of the β2AR, whereas USP20-CYS.HIS mutant led to ∼74% colocalization of β2AR-LAMP2 after 6 h of Iso stimulation (Figure 4C and D). As a reciprocal effect, we found more membrane receptors in USP20-containing cells than USP20-CYS.HIS after 6 h of Iso stimulation. Similar to USP33, USP20 was also detected in vesicles by immunostaining. These vesicles mostly enveloped the β2AR-containing vesicles; some colocalization with LAMP2 was also seen, which was more pronounced for the USP20-CYS.HIS mutant than the WT (Figure 4C). These results suggest that both USPs 33 and 20 function to recognize and deubiquitinate the internalized β2AR cargo and play key roles in promoting receptor recycling.
Figure 3.
USP33 and its homologue USP20 function redundantly to regulate recycling of the β2AR. The graphs show cell-surface receptor expression as determined by [3H]CGP12177 radioligand binding in cells with stable β2AR expression, which were transiently transfected with vector, USP33-WT or USP33-HIS (A) and vector, USP20-WT or USP20-HIS (B). The values corresponding to NS, 6 h Iso and 6 h Iso+wash within each sample were analysed by one-way ANOVA. Two-way ANOVA (n=4 for A and n=7 for B) was used to compare the three samples pcDNA3, WT and HIS. *P<0.05, **P<0.01, ***P<0.001 between indicated samples, Bonferroni comparison. Each 6 h Iso sample was significantly different (***P<0.001, one-way ANOVA, not indicated) from the respective NS samples and there was no difference in recycling between pcDNA3 and USP mutant samples (two-way ANOVA).
Figure 4.
USP20 reverses β2AR ubiquitination and modifies its trafficking. (A) Cells stably transfected with Flag-β2AR-mYFP were transiently transfected with pcDNA3, USP20 or USP20-HIS and stimulated with Iso (10 μM) for 15 min and immunoprecipitates probed with an anti-ubiquitin antibody (upper panel). The amount of receptor in the IP was detected by the anti-Flag M2 antibody in the middle panel. Expression of USP20 in the lysates is displayed in the lower panel. Mobility of molecular markers is indicated at the left. (B) Agonist-stimulated ubiquitination quantified as the difference between 15 min and unstimulated samples is plotted here. The maximum signal in each experiment is designated as 100%. **P<0.01 and ***P<0.001, one-way ANOVA (n=3), Bonferroni comparison. (C) The confocal panels show the distributions of USP20 (WT or CYS.HIS), Flag-β2AR-mYFP and LAMP2 in HEK-293 cells after 6 h Iso stimulation. Merged images show USP20 (red), β2AR (green) and LAMP2 (blue). Regions of colocalization are indicated by yellow arrowheads (containing mostly USP20 and β2AR with lesser/no LAMP2) or yellow arrows (mostly LAMP2 and β2AR with lesser/no USP20). (D) β2AR-LAMP2 colocalization was quantified as in Figure 1E, ***P<0.001, CYS.HIS 6 h versus all others, one-way ANOVA, Bonferroni comparison.
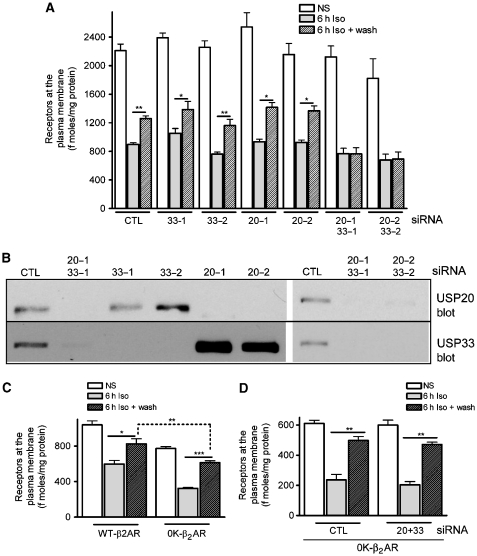
To address the combined and individual roles of the two DUBs, we resorted to siRNA-mediated knockdown of endogenously expressed USPs 20 and/or 33. We tested the effects of either single or double knockdowns of USPs 20 and 33 by two separate siRNA oligonucleotides (20-1, 20-2, 33-1 and 33-2 targeting different regions) on receptor recycling (Figure 5A). When either USPs 33 or 20 was depleted, we did not observe a significant decrease in receptor recycling. In contrast, when we depleted both USPs 33 and 20 by siRNA transfections, we observed a complete inhibition of receptor recycling (Figure 5A). We could achieve >95% decrease in each protein by the simultaneous knockdown of USPs 20 and 33 (Figure 5B), whereas individual knockdowns had an effect of increasing (20-1, 20-2 and 33-2) or decreasing (33-1) protein levels of the second DUB. As a result, we detected either a slight increase or decrease in receptor recycling in the single knockdown cells compared with control cells. These data suggest that USPs 20 and 33 have redundant functions and one of the enzymes is able to compensate for the absence of the other and mediate β2AR trafficking. Receptor recycling is impaired only when both activities are eliminated.
Figure 5.
Effect of single or combined knockdown of USPs 20 and 33 on β2AR recycling. (A) The bar graph shows cell-surface receptor expression at basal, 6 h Iso and 6 h Iso+wash (recycling) conditions as determined by [3H]CGP12177 binding. HEK-293 cells with stable β2AR expression were transiently transfected with either control (CTL), or two separate siRNA targeting each type of USP singly or combined. *P<0.05, **P<0.01, one-way ANOVA between 6 h Iso and 6 h Iso+wash in each group as indicated. Each 6 h sample is significantly different (***P<0.001) from the NS sample (not indicated). Additionally, the double siRNA transfections are significantly different (***P<0.001) from the CTL at 6 h Iso+wash, by two-way ANOVA, (n=5–10). (B) The 30 μg of lysate samples from each siRNA transfection were analysed for USP20 (top) and USP33 (bottom) levels by immunoblotting. (C) Receptor recycling was determined in HEK-293 cells transiently transfected with the β2AR WT or the 0K-β2AR and cell-surface receptor expression analysed as in (A). Each receptor type showed significant amount of recycling as indicated (*P<0.05 and ***P<0.001) and internalization at 6 h Iso (not indicated, ***P<0.001). The 0K-β2AR recycling was significantly higher than WT recycling (**P<0.01), two-way ANOVA, n=3. (D) The 0K-β2AR recycling was analysed as above in cells with CTL or USPs 20+33 double knockdown. **P<0.01, between 6 h Iso and 6 h Iso+wash in each case, one-way ANOVA, Bonferroni comparison. CTL and USP siRNA groups did not differ, two-way ANOVA (n=4).
If receptor deubiquitination subserves sorting of receptors to a recycling route, then the absence of ubiquitin modification on the β2AR should have a similar effect. To evaluate this, we compared recycling of WT-β2AR with that of a lysine-less β2AR (0K-β2AR) that is not ubiquitinated (Shenoy et al, 2001). The 0K-β2AR recycled to a significantly higher extent than the WT-β2AR, confirming that absence of ubiquitin moieties on the receptor efficiently directs them to recycle back to the plasma membrane (Figure 5C). Moreover, the simultaneous knockdown of USPs 20 and 33 that inhibits WT recycling (Figure 5A) did not affect 0K-β2AR recycling (Figure 5D), suggesting that USP activity targets only ubiquitinated receptors during post-endocytic sorting.
USPs 33 and 20 are required for β2AR resensitization on recycling from late-endosomal/lysosomal compartments
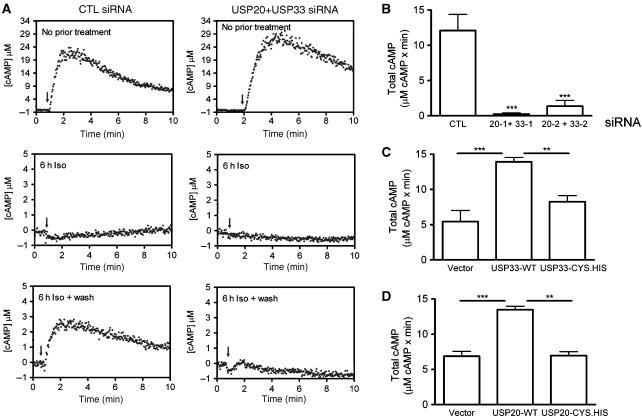
On recycling to the plasma membrane, β2ARs are known to functionally recover or ‘resensitize' such that their responsiveness to agonist is restored. To ascertain the effects of USPs 33 and/or 20 knockdowns on receptor resensitization, we used the cAMP biosensor (ICUE2) (Violin et al, 2008) and tested the cAMP responses by FRET microscopy. In cells with stable transfections of ICUE2 and the β2AR, the Iso-stimulated cAMP responses were identical irrespective of control or USPs 20 and 33 siRNA transfections (Figure 6A, top row panels). In cells that have been pre-exposed to agonist for 6 h, we did not observe any cAMP response in control as well as USP-depleted cells (Figure 6A, middle panels). However, in cells that have been treated with agonist for 6 h followed by a 2-h washout, we observed cAMP accumulation only in the control cells, but not in the USP-depleted cells (Figure 6A, lowest panels and 6B). Single knockdown of either USP leads to a similar response as in control cells (not shown). As receptor recycling is close to zero in cells lacking both USPs 20 and 33 (Figure 5A), this defect in resensitization could be attributed to a lack of repopulation of receptors at the cell surface. If the USP enzymes regulate just recycling, then we should observe an increase only in recycling, but not in receptor resensitization, if we overexpress these enzymes. On the other hand, if USPs 33 and 20 regulate receptor recycling as well as resensitization, then overexpression of USPs 33 or 20 would result in an augmentation of both recycling and resensitization events. Indeed, when we overexpressed USPs 33 or 20 in the ICUE2 stable cells, we observed a significant increase in cAMP production measured after agonist wash (Figure 6C and D). Additionally, this effect was lost on coexpression of the catalytically inactive mutants (Figure 6C and D), suggesting that USPs 20 or 33 enzymatic activity is required for β2AR resensitization. These data suggest that receptor deubiquitination, recycling and resensitization are intimately linked events and that USPs 20 and 33 are important regulators that connect and balance these pathways.
Figure 6.
USPs 33 and 20 regulate resensitization of the β2AR. (A) HEK-293 cells with stable expression of Flag-β2AR and cAMP biosensor ICUE2 were transfected with CTL or USPs 33 and 20 siRNA. After each type of treatment, [cAMP] (μM) was determined in live cells after an acute Iso stimulation (10 μM) as indicated by the arrow. The cAMP responses shown in the top panels were obtained from cells not exposed earlier to agonist. In the middle panels, cells have been stimulated for 6 h and in the bottom panels, cells were stimulated for 6 h and agonist washed for 2 h with new media. (B) The [cAMP] (μM) responses (Figure 6A, lowest panels) were converted into total cAMP by integrating cAMP concentration over time (total cAMP (area under the curve)=[cAMP] (μM) × min). The mean±s.e.m. of total cAMP from multiple measurements are plotted as bar graphs. ***P<0.001, one-way ANOVA, Bonferroni comparison. (C, D) HEK-293 cells with stable expression of ICUE2 were transiently transfected with pcDNA3 (vector), WT or CYS.HIS mutants of (C) USP33 or (D) USP20 and total cAMP was determined as in (B). **P<0.01, ***P<0.001, one-way ANOVA, Bonferroni comparison.
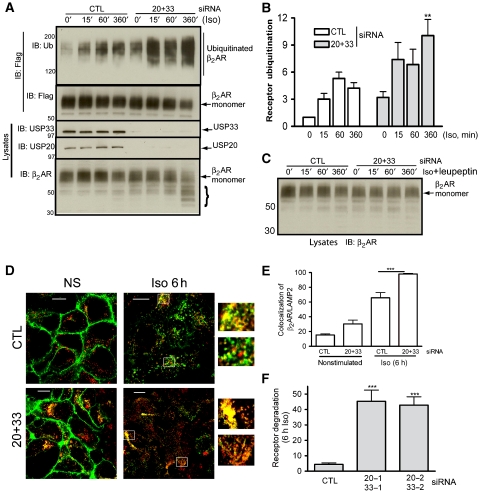
Knockdown of USPs 20 and 33 results in increased agonist-stimulated ubiquitination, lysosomal trafficking and degradation of the β2ARs
As shown in Figure 7A and B, simultaneous depletion of USPs 20 and 33 resulted in a dramatic increase in receptor ubiquitination in response to Iso stimulation. Furthermore, on analysing the cell lysates, we found a dramatic reduction in the monomeric form of the receptor at 6 h of Iso stimulation along with the appearance of numerous low-molecular weight bands as detected by a β2AR-specific antibody (Figure 7A, lowest blot panel). When we treated cells with a lysosomal inhibitor (leupeptin) along with Iso, the monomeric β2AR was stabilized and the low-molecular weight bands were minimized (Figure 7C). Correlating with the increase in receptor ubiquitination, we also detected a more robust colocalization of β2AR and LAMP2 on depleting USPs 20 and 33 than in control cells (Figure 7D and E; Supplementary Figure S5). This effect was most pronounced at 6 h stimulation, although we did observe a small increase in receptor-LAMP2 colocalization in quiescent cells and on 1 h Iso treatment (Figure 7D; Supplementary Figure S5). In cells that are depleted of both USPs 20 and 33, immunostaining of the β2AR is much weaker than in control cells at 6 h Iso stimulation, suggesting that internalized receptors are taken through a rapid path of destruction. Additionally, as determined by 125I-CYP binding, receptor degradation increased by about 10–12 folds at 6 h of Iso stimulation in the absence of USPs 20 and 33 expression (Figure 7F). The above effects on receptor ubiquitination, trafficking and degradation were not observed in cells with individual depletion of these DUBs (data not shown). These data suggest that USPs 20 and 33 activities are critical for preventing lysosomal degradation of the β2AR and prolonging the lifetime of β2ARs during chronic agonist treatments.
Figure 7.
Simultaneous depletion of USPs 20 and 33 leads to robust increases in agonist-stimulated β2AR ubiquitination and lysosomal degradation. (A) Flag-β2ARs from control or USPs 20- and 33-depleted HEK-293 cells were immunoprecipitated after Iso stimulation for indicated times and analysed for ubiquitination and receptor levels by western blotting. The amounts of USPs 20, 33 and β2AR in the lysates are also shown. (B) The ubiquitin signals in each sample in (A) were quantified and the mean data from four experiments are shown. **P<0.01, 6 h samples, two-way ANOVA. (C) Lysates from control or USPs 20+33 knockdown cells treated with Iso and leupeptin for the indicated times were immunoblotted for Flag-β2AR levels. (D) Flag-β2AR-mYFP stable HEK-293 cells transfected with control or USPs 20 and 33 siRNA were stimulated or not with 1 μM Iso for 6 h, fixed, permeabilized and immunostained for LAMP2 (red). Overlay images (LAMP2, red+β2AR, green) for a group of 8–18 cells are shown. Two regions from the 6 h panels are enlarged on the right to show moderate and robust colocalization (yellow) in each case. (E) Quantitation of receptor and LAMP2 colocalization in unstimulated and Iso-stimulated cells are shown in (D). ***P<0.001, CTL versus USPs 20+33 siRNA, one-way ANOVA. (F) The bar graphs represent the decrease in total receptor levels (125I-CYP binding) in cells with control and USPs 20+33 knockdowns after 6 h Iso stimulation. ***P<0.001, versus control, one-way ANOVA, n=4, Bonferroni Comparison.
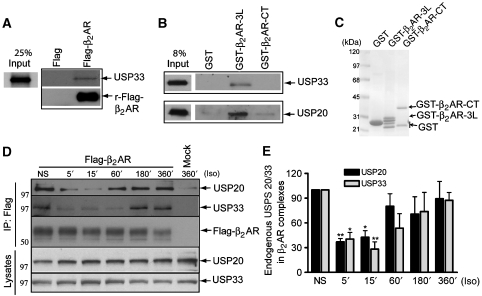
In vitro and agonist-dependent interactions of USPs 20 and 33 and the β2AR
To assess if β2ARs and USPs 20 and 33 interact directly, we tested the binding between recombinant Flag-β2AR purified from Sf9 insect cells (Shenoy et al, 2001) and in vitro translated [35S]-labelled USPs. In these assays, we detected a better interaction of the purified β2AR with [35S]-USP33 than with in vitro translated [35S]-USP20 (Figure 8A and data not shown). Earlier identified proteins such as NHERF1/EBP50, N-ethylmaleimide (NEM) sensitive fusion protein and GASPs, which regulate receptor trafficking, interact at the carboxyl-tail (CT) region of the β2AR (Marchese et al, 2008). However, we could not detect USP33 binding to GST–β2AR C tail (residues 329–413) fusion protein. In these GST-pull down assays, we detected a weak interaction of USP20 with the receptor tail (Figure 8B and C). On the other hand, both USPs 20 and 33 bound to the receptor third loop (residues 218–276) GST-fusion protein (Figure 8B and C). The above in vitro data suggest that receptor USP interaction is direct and USPs 20 and 33 are likely recruited to membrane-resident receptors in quiescent cells. Indeed, isolated β2AR immunoprecipitates contained detectable amounts of endogenous USP33 as well as USP20 in the absence of any agonist stimulation, suggesting that these enzymes are complexed with the cell-surface receptors (Figure 8D and E, NS lanes). Interestingly, agonist stimulation for 5–15 min resulted in a significant decrease in the amounts of endogenous USP enzymes co-precipitating with the β2AR (Figure 8D and E), suggesting that these enzymes have dissociated from the activated receptor complexes. However, the total levels of USPs 20 and 33 did not decrease on agonist stimulation as assessed by western blotting whole cell extracts (Figure 8D), and hence, this decrease in the detection of USPs 20 and 33 was not from protein degradation. The exact reason for the dissociation of endogenous USPs 20 and 33 is not known. At endogenous levels, increased affinity of other proteins that dynamically interact with activated receptors could compete off bound endogenous USPs, whereas on USP overexpression such displacements may be ineffective (Figures 1 and 2; Supplementary Figure S3). However, beyond 3 h of Iso treatment, we observed a reassociation of β2AR–USP enzymes, with the interaction levels reaching close to basal conditions. Thus, although endogenous USPs dissociate from the receptors immediately after agonist activation, they evidently recognize and reassociate with internalized receptors. After 6 h of Iso stimulation, internalized β2ARs are mostly localized in LAMP2-positive compartments; therefore, USPs 20 and 33 recognize the ubiquitinated receptor cargo that traffic to late-endosomal/lysosomal compartments, deubiquitinate the receptors and promote their recycling.
Figure 8.
In vitro and agonist-dependent interactions of USPs 20 and 33 with the β2AR. (A) Interaction between in vitro translated USP33 and recombinant Flag-β2AR. From a 100-μl of in vitro translated HA-USP33 reaction mix (see Materials and methods), 10 and 40 μl were used for input and binding, respectively. The [35S]-labelled USP33 bands were detected by autoradiography. The Flag lane indicates use of anti-Flag affinity beads (no receptor). Lower panel shows western blotting of Flag-β2AR in the immunoprecipitated sample. (B) GST–β2AR-fusion proteins (third intracellular loop (3L) or carboxyl tail) or GST bound to glutathione sepharose beads was mixed with equal amounts of whole cell extracts from COS-7 cells transfected with USPs 33, 20 or pcDNA3 (not shown). Bound proteins were eluted and analysed by western blotting with anti-USP33 (top) or anti-USP20 (bottom) antibodies. No signal was detected with extracts from pcDNA3 expressing cells (not shown). (C) The total amount of GST, GST–β2AR-3L and GST–β2AR-CT proteins on the membrane as visualized by Ponceau S staining is shown. (D) After O/N starvation, cells with stable Flag-β2AR-mYFP expression were stimulated by Iso (10 μM) for the indicated times. Endogenous USPs 33 or 20 bound to the receptor were detected by the corresponding specific antibodies (panels 1 and 2 from the top). The lysate expression levels (5% of cell extracts used in the IP) of both USPs are shown in the lower two panels. The amount of receptor in the IP as detected by anti-Flag M2 is also shown. (E) The bar graphs have been obtained by calculating the ratio between the endogenous USP and β2AR signals for each time point. The result is the mean±s.e.m. of 4–7 independent experiments. **P<0.01, *P<0.05 versus NS, one-way ANOVA, Bonferroni comparison. NS, nonstimulated.
The tag team of β2AR, USP33 and β-arrestin2
As we observed β2AR–USP33 binding in the absence of agonist stimulation and as it is well established that β-arrestins are transiently recruited to the β2ARs at the plasma membrane only on agonist activation, we believe that β2AR–USP33 interaction can prevail before β-arrestin recruitment. However, as β-arrestins bind USP33 as well (Shenoy et al, 2009), we sought to analyse how these various interactions correlate with agonist treatment. As seen above, at early time points of Iso stimulation, endogenous USP33 dissociates from the activated β2ARs. In marked contrast, when we isolated β-arrestin2 immunoprecipitates (representing the total cellular pool of β-arrestin), we observed an increase in β-arrestin–USP33 interaction at early time points of Iso stimulation and the interaction diminished beyond 1 h of agonist treatment (Figure 9A and B). Thus, although we observe a decrease in receptor–USP33 interaction at short times of agonist activation, we detect a reciprocal pattern of increase in β-arrestin2–USP33 interaction. Collectively, our findings suggest that when β-arrestin2 facilitates β2AR ubiquitination by recruiting Nedd4 (Shenoy et al, 2008), its adaptor function might actually serve to remove the DUB from the activated β2AR to facilitate receptor ubiquitination.
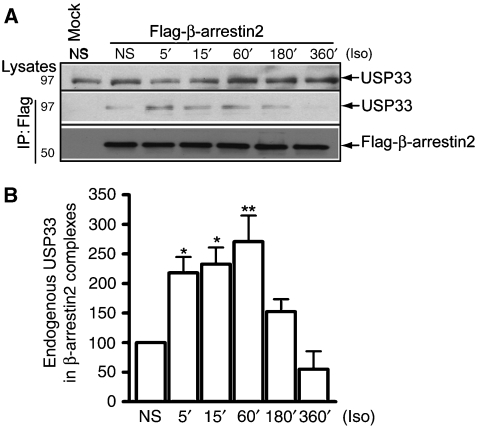
Figure 9.
β-arrestin2 and USP33 interaction. (A) HEK-293 cells transiently transfected with HA-β2AR and Flag-β-arrestin2 was stimulated with Iso (10 μM) for the indicated times. Endogenous USP33 in lysates (upper panel) and USP33 bound to the β-arrestin2 (middle panel) were detected by anti-USP33 antibody. Flag immunoblots show the amounts of β-arrestin2 in the IP (lowest panel). (B) Bar graphs show the quantification USP33 bands in β-arrestin2 immunoprecipitates. Data are mean±s.e.m. from four independent experiments. *P<0.05, **P<0.01 versus NS, one-way ANOVA, Bonferroni comparison.
Discussion
We show that two closely related DUBs, USP33 (also known as VDU1) (Li et al, 2002a) and USP20 (also known as VDU2) (Li et al, 2002b), can bind the β2AR and orchestrate receptor recycling and resensitization. Agonist stimulation of the β2AR leads to ubiquitination and lysosomal degradation of the receptor, but overexpression of USPs 33 and 20 counteracts these effects and promotes receptor recycling and resensitization. Additionally, knockdown of both USPs 33 and 20 abolishes receptor recycling and resensitization, but enhances ubiquitination as well as lysosomal degradation. Thus, USPs 20 and 33 act as novel regulators that dictate the post-endocytic fate of internalized β2ARs.
β-arrestin2 serves as an essential endocytic adaptor for the β2AR, recruits endocytic and signalling proteins and escorts the E3 ubiquitin ligase Nedd4 to the activated receptor complex (DeWire et al, 2007; Shenoy et al, 2008). As USP33 was identified as a β-arrestin interacting partner (Shenoy et al, 2009), we expected that β-arrestin2 would also bridge the interaction between the β2AR and USP33. Our data prove otherwise that USP33 is bound to the receptor before agonist-stimulation and β-arrestin translocation. Agonist-activated β2AR forms a weak and transient complex with recruited β-arrestins and the dissociation of β-arrestin2 has been correlated with its deubiquitination (Oakley et al, 2000; Shenoy and Lefkowitz, 2003). In a related work (Shenoy et al, 2009), we show that conformational changes induced in β-arrestin2 in response to β2AR activation facilitates β-arrestin2–USP33 binding. USP33 deubiquitinates β-arrestin2 and disassembles β2AR–β-arrestin2 signalling complexes. Although the β-arrestins that are dissociating from the receptor can recruit USP33 present in the cytoplasmic compartments, the timing of these events suggests that USP33 constitutively bound to the cell-surface β2ARs could be transferred to the translocated β-arrestins leading to their deubiquitination and disengagement from the β2AR complex. We believe that there is a dynamic exchange of protein partners between the β2AR and β-arrestin when both proteins are in specific-‘activated' conformations. Thus, the E3 ligase Nedd4 is released from β-arrestin2 to act on the receptor and USP33 is transferred from the receptor to bind β-arrestin2, thus, facilitating synchronized receptor ubiquitination and β-arrestin deubiquitination events.
Ubiquitinated β2ARs begin to localize in late-endosomes/lysosomes after 3 h of Iso stimulation, and considerable colocalization of LAMP2 and β2ARs is observed only after 6 h of Iso stimulation (Hanyaloglu and von Zastrow, 2007; Shenoy et al, 2008). As the timing of localization of internalized receptors in LAMP2-positive compartments matches with that of β2AR–USP reassociation, we believe that these DUBs recognize and bind the ubiquitinated β2AR cargo at the late endosomes. This leads to deubiquitination of the receptors preventing lysosomal degradation and facilitating receptor recycling. Overexpressed USPs persistently associate with the β2ARs and cause more striking effects of preventing lysosomal trafficking and recycling of the β2ARs by totally inhibiting receptor ubiquitination. The differences in the binding patterns of endogenous versus overexpressed USPs could simply be due to the abundance of protein in the latter condition. As the intracellular itinerary of activated β2ARs can involve an ensemble of dynamic interactions, the dissociation of endogenous USPs 20 and 33 could be due to a competition from other endogenously expressed β2AR interactors, which cannot effectively compete with the USP-receptor interaction observed under overexpression conditions.
As such, β2ARs are known to be stable proteins with a protein half-life of ⩾24 h in the presence of Iso (Gagnon et al, 1998; Shenoy et al, 2001; Pan et al, 2003; Liang and Fishman, 2004). The slow rate of β2AR degradation could be attributed to the deubiquitinating activities of USPs 20 and 33. With the elimination of these two DUBs, both agonist-stimulated ubiquitination and receptor degradation are dramatically increased, whereas receptor recycling and resensitization are totally inhibited. We did not find any significant differences in receptor expression levels or internalization with either depletion or repletion of both USP enzymes, suggesting that their activity affects a specific step during post-endocytic sorting. However, receptor expression levels in cells transfected with WT USPs 20 or 33 were significantly elevated when compared with cells transfected with the corresponding inactive mutants. On the other hand, overexpression of USP4 that promotes cell-surface targeting of newly synthesized adenosine A2 receptors (Milojevic et al, 2006) did not affect β2AR ubiquitination or β2AR-LAMP2 colocalization (data not shown), thus, indicating that β2AR regulation involves specific USP isoforms. Interestingly, ubiquitination and lysosomal degradation of the growth factor receptor, EGFR, is regulated by two DUBs, USP8 (also known as UBPY) and AMSH (McCullough et al, 2004; Mizuno et al, 2005, 2006; Bowers et al, 2006; Clague and Urbe, 2006). AMSH is associated with endosomes and inhibits EGFR lysosomal trafficking. UBPY plays an opposite role as its deubiquitinating activity promotes EGFR downregulation. These studies indicate that DUB activity is an important check point for the lysosomal entry of internalized growth factor receptors and bear analogy with our findings on the roles of USPs 20 and 33 in β2AR trafficking. On the other hand, our studies show a novel connection between GPCR deubiquitination, recycling and receptor resensitization.
On acute agonist treatment, activated β2ARs internalize into early endosomes, subsequently become dephosphorylated and recycle to the plasma membrane. We did not find an inhibition of receptor recycling in USPs 20- and 33-depleted cells after acute agonist stimulation. As such, nonubiquitinated 0K-β2ARs, which internalize to the same extent as the ubiquitinated WT-β2ARs, recycle more efficiently at early (data not shown) as well as late time points than the WT-β2AR. The 0K-β2ARs do not colocalize with LAMP2 (data not shown), suggesting that they do not traffic to these compartments. Hence, we believe that the fast recycling route from early endosomal compartments mainly involves dephosphorylation of nonubiquitinated receptors, whereas the slow recycling of ubiquitinated receptors from deeper subcellular compartments is regulated perhaps by both DUB and phosphatase activities (Pitcher et al, 1995). Future studies should reveal if the DUB-dependent recycling pathway could parallel or intersect other pathways showed for β2AR recycling (Odley et al, 2004; Hanyaloglu and von Zastrow, 2007; Millman et al, 2008). It would be of interest also to determine if USPs 33 and 20 could functionally link some of the scaffolds involved in β2AR recycling and resensitization, although these earlier studies mostly characterized recycling events induced by acute agonist treatments (Hall and Lefkowitz, 2002; Hanyaloglu and von Zastrow, 2007). Future challenges will also involve unravelling the mechanisms by which ubiquitination of the β2AR is preserved until the receptors sort to lysosomes as well as the identification of cofactors that facilitate or prevent the reassociation of receptors and DUBs at the late-endosomal compartments. The mechanisms that suppress DUB activity at further time points of Iso stimulation (24 h or more) to promote receptor degradation remain to be discovered.
As seen in our siRNA experiments, single knockdown of either USPs 20 or 33 does not affect receptor trafficking, suggesting that expression of one of these enzymes alone suffices to regulate the β2AR recycling. USPs 20 and 33 show 59% identity in their amino-acid sequence, which might explain their close functional relationship. Earlier studies have indicated their roles in proteasomal degradation of the transcription factor HIF-1 and the ER-associated type 2 iodothyronine deiodinase (Curcio-Morelli et al, 2003; Li et al, 2005). Interestingly, ubiquitin chains that are lysine48 linked are generally degraded by the 26S proteasomes, whereas the lysine63 linkage is involved in tagging proteins for vesicular trafficking (Varadan et al, 2004). In fact, in our in vitro deubiquitination assays, USP33 is capable of depolymerizing lysine48 as well as lysine63 polyubiquitin chains, indicative of its roles in lysosomal and proteasomal pathways.
This study establishes the importance of the dynamic process of ubiquitination/deubiquitination in the regulation of receptor trafficking and signalling. That GPCR recycling and resensitization could be coupled to deubiquitination occurring at late-endosomal/lysosomal compartments is a new concept. We show that USPs 20 and 33 are critical for receptor resensitization after prolonged agonist stimulation, thus, underscoring their role in rejuvenating the physiological hormonal responsiveness. Considering the pathophysiological importance of the β2AR (Rockman et al, 2002; Galandrin et al, 2007), it certainly is a process that needs to be weighed in the design of new treatments, especially for diseases that involve chronic agonist stimulation or require long-acting agonist treatment such as in asthma. A process that would rescue the recycling of the receptor and consequently its resensitization would be very helpful to overcome side effects of such diseases and treatments.
Materials and methods
Reagents and plasmids
Anti-Flag M2 affinity gel, (−)-Iso, NEM, Fibronectin (Bovine plasma) were from Sigma. Collagen (rat tail) was from Roche. Polyubiquitin chains (K48-linked; K63-linked) and Isopeptidase T were from Boston Biochem. Antibodies and their suppliers were: anti-Flag M2 (Sigma), anti-ubiquitin P4D1 (Santa Cruz), anti-ubiquitin FK2H (Biomol), anti-β2AR H20 (Santa Cruz), anti-USP33 and anti-USP20 (Bethyl laboratories) and anti-LAMP2 H4B4 (Santa Cruz). Alexa 594, 488 and 633, conjugated secondary antibodies were from Invitrogen. Horseradish peroxidase-conjugated secondary antibodies and (−)-[3H]CGP-12177 were from GE/Amersham.
The cAMP biosensor ICUE2 (indicator of cAMP using Epac) was kindly provided by Dr Jin Zhang. The human USP20/pCMV4 plasmid (NM_006676.4) is a purchase from OriGene Technologies. Mutations converting cysteine to serine residue and histidine to glutamine residue were individually introduced into USPs 33 or 20 proteins by site-directed mutagenesis. All resulting plasmids were verified by DNA sequencing.
Immunofluorescence staining and confocal imaging
HEK-293 cells stably expressing Flag-β2AR-mYFP were transiently transfected with HA-USP33 WT or mutants. The 24 h post-transfection cells were plated on collagen-coated 35-mm glass bottom plates and 24 h later, cells were starved for 1 h in serum-free medium, then stimulated, fixed with 5% formaldehyde diluted in PBS containing calcium and magnesium, permeabilized with 0.08% Triton X-100 (cat# T-9284, Sigma) in PBS containing 2% bovine serum albumin for 30 min and incubated with appropriate primary antibody O/N at 4°C, followed by the respective secondary antibody. Longer permeabilization times prevented detecting USPs associated with vesicles. Confocal images were obtained on a Zeiss LSM510 laser-scanning microscope using multitrack sequential excitation (488, 568 and 633 nm) and emission (515–540 nm, GFP; 585–615 nm, Texas Red; 650 nm, Alexa 633) filter sets. The scale bars included in all confocal panels represent 10 μm. For the quantification of β2AR/LAMP2 colocalization, β2AR was pseudo-coloured green, LAMP2 was pseudo-coloured red, the USP channel was turned off in USP-positive cells and the merged images of 10–15 randomly chosen cells for a specified condition were analysed for the percentage of yellow (colocalization of LAMP2 and β2AR) vesicles against the total β2AR (green + yellow) vesicles.
Analysis of receptor recycling
Recycling of the β2AR was quantified by (−)-[3H]CGP-12177 radioligand binding. HEK-293 cells with stable β2AR expression were transiently transfected with USP33 (WT or mutant), USP20 (WT or mutant) or USP siRNA. After 24 h of DNA or 48 h of siRNA transfections, cells were split in 24-well dishes. Cells from the same transfection were divided into three sets. The first set did not receive any treatment and represents the total receptors at the plasma membrane in unstimulated condition. The second and third sets of cells were stimulated for 6 h with Iso. The second set was washed with cold PBS to stop the agonist stimulation and to allow the measurement of the β2AR internalization, whereas the third set was washed with warm media for 2 h to allow β2AR recycling. At the end, all cells were washed with cold PBS and 3H-CGP12177 added (10 nM). Nonspecific binding was measured for each set by adding 1 μM propranolol. The same protocol was used for HEK-293 transiently transfected with the 0K- or WT-β2ARs.
cAMP measurement by FRET microscopy
Cells stably expressing ICUE2 alone or with the β2AR were transfected transiently with USP 33, USP20 DNA or USP siRNA. After 24 h of DNA or 48 h of siRNA transfections, cells were plated on fibronectin-coated 35-mm glass bottom plates. Cells from the same transfection underwent different treatment conditions: no treatment, 6 h Iso (10 μM) stimulation or 6 h Iso (10 μM) stimulation and agonist wash for several hours. After treatment, cells were placed on the FRET microscope stage and re-stimulated with 10 μM Iso (to assess resensitized receptors). FRET imaging and [cAMP] (μM) determinations were carried out as reported earlier (Violin et al, 2008). The [cAMP] (μM) responses (Figure 6A) were converted into total cAMP by integrating cAMP concentration over time (total cAMP (area under the curve)=[cAMP] (μM) × min). The mean±s.e.m. of total cAMP responses from multiple measurements are plotted as bar graphs shown in Figure 6B–D.
Receptor degradation
Degradation assays were done with 125I-CYP radioligand binding on monolayers of cells on poly-D lysine-coated 12-well dishes (Biocoat) in MEM buffered with 10 mM HEPES (pH 7.5) and 5 mM MgCl2. Experimental cells were divided into two identical sets, one that was treated with agonist and the other with vehicle. Receptor levels in both sets were determined in parallel. Binding was performed in triplicate with 400 pM 125I-CYP in the presence or absence of the hydrophobic antagonist propranolol (10 μM, to define nonspecific binding). Samples were incubated at 37°C for 1 h after which the cells were placed on ice and washed several times with ice-cold PBS buffer containing calcium and magnesium. Finally, cells were solubilized in 0.1N NaOH and 0.1% SDS and counted for 125I. The receptor number (total specific 125I-CYP binding sites) was determined after 24 h of Iso treatment and expressed as percent of receptor number assessed in nonstimulated cells.
Immunoprecipitation and immunodetection
Flag-β2AR or β-arrestin2-Flag was used to immunoprecipitate HA-USP33, ubiquitin or endogenous USPs 33 and 20 as indicated. Cells were serum starved for 4 h or O/N and then stimulated with Iso for the indicated times. COS-7 cells were solubilized in a lysis buffer containing 50 mM HEPES (pH 7.5), 0.5% Nonidet P-40, 250 mM NaCl, 2 mM EDTA and 10% (v/v) glycerol. HEK-293 cells were solubilized in RIPA buffer containing 150 mM NaCl, 50 mM Tris pH 8, 0.5 mM EDTA, 1% Nonidet P-40 and 0.5% deoxycholate. All buffers were supplemented with protease inhibitors. The 10 mM NEM was added to the lysis buffer for time course experiments to assess the binding between β2AR or β-arrestin2 and endogenous USP enzymes. After centrifugation, soluble extracts were mixed with anti-Flag M2 agarose beads and rotated overnight at 4°C. Nonspecific binding was eliminated by repeated washes with lysis or RIPA buffer, and bound protein was eluted with sample buffer containing SDS. The proteins were separated on a gradient gel (4–20%, Invitrogen) and transferred to nitrocellulose membrane for western blotting. Chemiluminescence detection was performed using SuperSignal West Pico reagent (Pierce). Signals were quantified by densitometry using GeneTools software.
In vitro translation
USPs 20 and 33 were in vitro translated using a TNT T7 Quick-Coupled Transcription/Translation Systems (Invitrogen Cat.# L1170) according to the manufacture's recommended procedure. Briefly, 100 μl reactions were assembled by mixing appropriate amounts of TNT Quick Master Mix, [35S] methionine (Amersham Biosciences Cat.# AG1094) and pCDNA3.HA-USP33 or pYX-Asc-USP20 plasmids in 0.5 ml microcentrifuge tubes. The reactions were incubated at 30°C for 90 min and the in vitro translated [35S]USPs were stored at −80°C before performing binding experiments. The 10 μl of the reaction was set aside to be used for the input lane. To study receptor binding, 40 μl of the in vitro translated [35S]USPs were incubated in 50 mM Tris–HCl, pH 8.0, with 5 μg of recombinant β2AR protein bound to anti-Flag affinity agarose beads on ice for 30 min. After the incubation period, samples were diluted with 0.5 ml of ice-cold buffer supplemented with protease inhibitors and rotated at 4°C. Unbound samples were separated by wash and centrifugation steps. Finally, 30 μl of SDS–PAGE buffer was added to each sample and proteins were separated by 4–20% gel. The gels were dried and the amounts of USPs bound to the β2AR were determined by autoradiography.
GST-pull down assays
Dr Lefkowitz (Duke University) kindly provided pGEX vectors with GST fusions of β2AR CT residues 329–413 (β2AR-CT) and of intracellular loop residues 218–276. These constructs were transformed into BL21(DE3)pLys-S Escherichia coli, and GST-fusion proteins were prepared according to standard procedures. Soluble extracts in lysis buffer (above) were prepared from COS-7 cells transfected with pcDNA3, USPs 20 or 33. A total of 300 μg of cell lysates were incubated on ice with either GST alone or GST-fusion proteins for 30 min, then rotated at 4°C for 2 h, unbound proteins washed by repeated centrifugation and wash steps. Bound proteins were eluted by adding SDS sample buffer and analysed by western blotting.
Supplementary Material
Supplementary Information
Acknowledgments
We thank RJ Lefkowitz, J Zhang, JD Violin and NJ Freedman for their generosity in providing reagents and suggestions, LS Barak for expert advice with confocal imaging and R Walters for critical reading. This work was supported by a grant from the National Institutes of Health (HL 080525 to SKS).
Conflict of interest The authors declare that they have no conflict of interest.
References
- Bowers K, Piper SC, Edeling MA, Gray SR, Owen DJ, Lehner PJ, Luzio JP (2006) Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J Biol Chem 281: 5094–5105 [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbe S (2006) Endocytosis: the DUB version. Trends Cell Biol 16: 551–559 [DOI] [PubMed] [Google Scholar]
- Curcio-Morelli C, Zavacki AM, Christofollete M, Gereben B, de Freitas BC, Harney JW, Li Z, Wu G, Bianco AC (2003) Deubiquitination of type 2 iodothyronine deiodinase by von Hippel–Lindau protein-interacting deubiquitinating enzymes regulates thyroid hormone activation. J Clin Invest 112: 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69: 483–510 [DOI] [PubMed] [Google Scholar]
- Drake MT, Shenoy SK, Lefkowitz RJ (2006) Trafficking of G protein-coupled receptors. Circ Res 99: 570–582 [DOI] [PubMed] [Google Scholar]
- Ferguson SS (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53: 1–24 [PubMed] [Google Scholar]
- Gagnon AW, Kallal L, Benovic JL (1998) Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the beta2-adrenergic receptor. J Biol Chem 273: 6976–6981 [DOI] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpre G, Bouvier M (2007) The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci 28: 423–430 [DOI] [PubMed] [Google Scholar]
- Hall RA, Lefkowitz RJ (2002) Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res 91: 672–680 [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M (2007) Regulation of GPCRs by membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol 48: 537–568 [DOI] [PubMed] [Google Scholar]
- Li Z, Na X, Wang D, Schoen SR, Messing EM, Wu G (2002a) Ubiquitination of a novel deubiquitinating enzyme requires direct binding to von Hippel-Lindau tumor suppressor protein. J Biol Chem 277: 4656–4662 [DOI] [PubMed] [Google Scholar]
- Li Z, Wang D, Messing EM, Wu G (2005) VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1alpha. EMBO Rep 6: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang D, Na X, Schoen SR, Messing EM, Wu G (2002b) Identification of a deubiquitinating enzyme subfamily as substrates of the von Hippel-Lindau tumor suppressor. Biochem Biophys Res Commun 294: 700–709 [DOI] [PubMed] [Google Scholar]
- Liang W, Fishman PH (2004) Resistance of the human beta1-adrenergic receptor to agonist-induced ubiquitination: a mechanism for impaired receptor degradation. J Biol Chem 279: 46882–46889 [DOI] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BR, Trejo J (2008) G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol 48: 601–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough J, Clague MJ, Urbe S (2004) AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol 166: 487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman EE, Zhang H, Zhang H, Godines V, Bean AJ, Knoll BJ, Moore RH (2008) Rapid recycling of beta-adrenergic receptors is dependent on the actin cytoskeleton and myosin Vb. Traffic 9: 1958–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, Ducza E, Ogris E, Boehm S, Freissmuth M, Nanoff C (2006) The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol 69: 1083–1094 [DOI] [PubMed] [Google Scholar]
- Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M (2005) Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell 16: 5163–5174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno E, Kobayashi K, Yamamoto A, Kitamura N, Komada M (2006) A deubiquitinating enzyme UBPY regulates the level of protein ubiquitination on endosomes. Traffic 7: 1017–1031 [DOI] [PubMed] [Google Scholar]
- Moore CA, Milano SK, Benovic JL (2007) Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol 69: 451–482 [DOI] [PubMed] [Google Scholar]
- Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123: 773–786 [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS (2000) Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 275: 17201–17210 [DOI] [PubMed] [Google Scholar]
- Odley A, Hahn HS, Lynch RA, Marreez Y, Osinska H, Robbins J, Dorn GW II (2004) Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acad Sci USA 101: 7082–7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Gurevich EV, Gurevich VV (2003) The nature of the arrestin x receptor complex determines the ultimate fate of the internalized receptor. J Biol Chem 278: 11623–11632 [DOI] [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Lohse MJ (1995) Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol 47: 666–676 [PubMed] [Google Scholar]
- Pitcher JA, Payne ES, Csortos C, DePaoli-Roach AA, Lefkowitz RJ (1995) The G-protein-coupled receptor phosphatase: a protein phosphatase type 2A with a distinct subcellular distribution and substrate specificity. Proc Natl Acad Sci USA 92: 8343–8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ (2002) Seven-transmembrane-spanning receptors and heart function. Nature 415: 206–212 [DOI] [PubMed] [Google Scholar]
- Saksena S, Sun J, Chu T, Emr SD (2007) ESCRTing proteins in the endocytic pathway. Trends Biochem Sci 32: 561–573 [DOI] [PubMed] [Google Scholar]
- Shenoy SK (2007) Seven-transmembrane receptors and ubiquitination. Circ Res 100: 1142–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ (2003) Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem 278: 14498–14506 [DOI] [PubMed] [Google Scholar]
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ (2001) Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science 294: 1307–1313 [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Modi A, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ (2009) β-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci USA 106: 6650–6655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM (2008) Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the {beta}2-adrenergic receptor. J Biol Chem 283: 22166–22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CM, Brady AE, Nickols HH, Wang Q, Limbird LE (2004) Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 44: 559–609 [DOI] [PubMed] [Google Scholar]
- Varadan R, Assfalg M, Haririnia A, Raasi S, Pickart C, Fushman D (2004) Solution conformation of Lys63-linked di-ubiquitin chain provides clues to functional diversity of polyubiquitin signaling. J Biol Chem 279: 7055–7063 [DOI] [PubMed] [Google Scholar]
- Violin JD, DiPilato LM, Yildirim N, Elston TC, Zhang J, Lefkowitz RJ (2008) Beta2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J Biol Chem 283: 2949–2961 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information