EMBO J 28, 1757–1768 (2009); published online 17 June 2009
Proteins of the BCL-2 family are key mitochondrial actors in the intrinsic pathway of apoptosis. However, they also exert housekeeping functions at the level of the endoplasmic reticulum (ER), controlling Ca2+ homeostasis and the unfolded protein response (UPR). Klee et al (2009) in an article published in this issue, identified that proapoptotic proteins of the BCL-2 family can activate the mitochondrial pathway of death also when restricted only at the ER, by engaging an atypical arm of the UPR requiring Ca2+. This paper elucidates how unconventional cascades overcome the spatial restriction of cellular signalling components and add a further layer of complexity to the regulation of cell death.
BCL-2 family proteins are key regulators of apoptosis. They can be subdivided in proapoptotic multidomain proteins (as BAX and BAK), antiapoptotic multidomain proteins (e.g. BCL-2), and BH3-only proteins (as BID or BIM), all of which performing a deeply studied—and yet not clear—choreography ending up in the regulated permeabilization of outer mitochondrial membrane, release of cytochrome c, and therefore activation of effector caspases. A few years ago, evidence was presented that this family of proteins could play other roles when in different subcellular locations. Members of the three subgroups are indeed also retrieved in the ER.
ER and mitochondria are closely connected both physically and functionally, impacting on mitochondrial metabolism and on apoptosis (de Brito and Scorrano, 2008). The two main functions of the ER are calcium storage and protein production and folding. Both appear crucially linked to regulation of the apoptotic cascade. BCL-2, as well as BAX and BAK have been shown to regulate Ca2+ handling in this organelle (Pinton et al, 2000; Scorrano et al, 2003). Overexpression of BCL-2, similarly to ablation of both BAX and BAK decreases steady-state calcium levels in the ER and reduces apoptotic response to Ca2+-dependent stimuli such as C2-ceramide and oxidative stress. This can be rescued by restoring Ca2+ levels in the ER by overexpressing SERCA (sarcoplasmic-endoplasmic reticulum Ca2+ adenosine triphosphatase), thus proving that the apoptotic defect is mainly due to Ca2+ dysregulation (Scorrano et al, 2003). Some BH3-only proteins, such as PUMA and BIK, have also been implicated in apoptosis-related ER-Ca2+ release (Mathai et al, 2005; Shibue et al, 2006). The accumulation of unfolded or misfolded proteins in the ER is detrimental for the cell and triggers a compensatory cascade christened ‘unfolded protein response'. At least three pathways, mediated by the sensors IRE 1α (inositol requiring kinase 1α), PERK (protein kinase-like ER kinase), and ATF6 (activating transcription factor 6) are activated. In a first phase, IRE1α and ATF6 upregulate ER chaperone genes, to deal with the misfolded proteins. IRE1α activation and interaction with TRAF2 (tumour necrosis factor receptor-associated factor 2) also induces JNK (Jun N-terminal kinase) phosphorylation. PERK dimerization leads to phosphorylation of eIF2α (eukaryotic translation initiation factor 2α), which inhibits translation, to avoid further accumulation of proteins in the ER. If these mechanisms are not enough to overcome the overload of proteins in the ER, the pro-survival pathway turns into an apoptotic response. The pathway that leads to cell death after UPR is a matter of debate, being unclear whether it is dependent or not on mitochondria. The controversial mitochondria independent pathway relies on the activity of caspase 12, which might activate caspase 9 without cytochrome c release (Morishima et al, 2004). However, ER stress is unable to cause death in cells lacking Bax and Bak, thereby strongly suggesting that the mitochondrial pathway is required. In addition, the PERK pathway inhibits the synthesis of BCL-2 (McCullough et al, 2001), and JNK activation can phosphorylate and inactivate BCL-2. BCL-2 members, besides localizing in the ER and regulating calcium levels, have also been related to UPR. BAX seems to directly interact with IRE1α, controlling its autophosphorylation and oligomerization (Hetz et al, 2006). BIM translocates to the ER, in which it promotes caspase activation after UPR stimuli (Morishima et al, 2004). Signalling between ER and mitochondria during UPR has been proposed to be achieved through BH3-only proteins. Bad is activated by ER stress inducers (Wang et al, 1999), and NOXA and PUMA are transcriptionally induced during ER stress. Thus, it seems that BCL-2 family proteins play a role in apoptosis at the ER level and in ER-calcium and UPR regulation under non-apoptotic conditions. However, the link between calcium, UPR, and BCL-2 family members is not completely understood. Several questions remain open: do proapoptotic BCL-2 family members regulate apoptosis at the ER level solely by regulating Ca2+ levels? Is there a role for IRE1α in this process? What is the role of spatial subcellular restriction in the different functions of BAX and BAK?
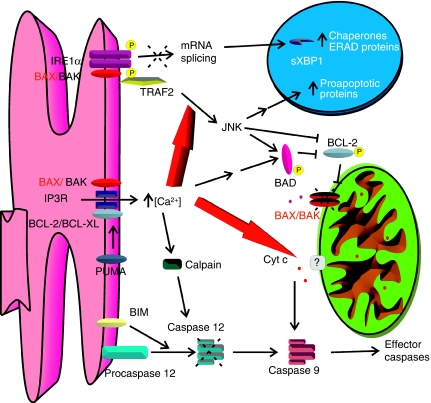
In this issue of the EMBO Journal, Pimentel-Muiños and colleagues tackled this problem using a new approach. By restricting BAK expression in the ER of BAX, BAK double knockout cells, they show that the extramitochondrial BAK can fully complement death of these cells to certain BH3-only molecules such as PUMA and BIM, but not BID. This indicates that the mitochondrial localization is dispensable when death is triggered by stimuli that can directly engage ER localized multidomain proapoptotics. These, in turn, activate a specific ‘ER stress' signalling pathway dependent on a peculiar IREα-TRAF2 activation. Surprisingly, this activation does not cause XBP1 splicing, suggesting that either BH3-only proteins are not able of activating other components of ER stress machinery (such as ATF6, whose activation is required for upregulation of XBP1), or that the signal is differentially transmitted by IRE1α to the downstream pathways—an exciting possibility. However, what is the role of Ca2+ in this cell death paradigm? Klee and co-workers clearly show that calcium plays two roles in this model: it directly enables mitochondrial susceptibility to cell death, because its pharmacological chelation reduces cytochrome c release, and it influences the IRE1α-TRAF2 ‘apoptotic' signalling pathway, favouring JNK activation. Importantly, Klee and co-workers show that certain BH3-only molecules can bypass the need to be localized on mitochondria, by engaging the subset of the multidomains that are retrieved on the ER. Furthermore, they identify a novel pathway of ER–mitochondria crosstalk dependent on persistent JNK activation (Figure 1). Whether this can occur also in normal cells, in which both mitochondrial and ER BAX/BAK are present, remains to be ascertained, but it could open promising avenues to bypass the resistance to mitochondrial apoptosis of cancer cells. Finally, the surprising discovery that ER and mitochondria can communicate during apoptosis through UPR-mediated selective activation of JNK adds a layer of complexity to our understanding of the apoptotic cascade, which should be kept in mind when dissecting the molecular details of death signalling.
Figure 1.
Schematic representation of the ER–mitochondria apoptotic signalling pathway operating in cells devoid of mitochondrial BAK and BAX, and of ER-located BAX. The high concentration of Ca2+ favours the IRE1α–TRAF2–JNK pathway, and enables mitochondrial susceptibility to cell death in these cells. See text for details.
Acknowledgments
VL is an EMBO long-term postdoctoral fellow. LS is a senior scientist ofthe Dulbecco-Telethon Institute and EMBO YIP. This work was supported by SNF 3100A0-118171 Switzerland, Telethon Switzerland, AFM.
Footnotes
The authors declare that they have no conflict of interest.
References
- de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 [DOI] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312: 572–576 [DOI] [PubMed] [Google Scholar]
- Klee M, Pallauf K, Alcala S, Fleischer A, Pimentel-Muinos F (2009) Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J 28: 1757–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai JP, Germain M, Shore GC (2005) BH3-only BIK regulates BAX, BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem 280: 23829–23836 [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Tsuchiya K, Shibata T, Seiwa E (2004) Translocation of Bim to the endoplasmic reticulum (ER) mediates ER stress signaling for activation of caspase-12 during ER stress-induced apoptosis. J Biol Chem 279: 50375–50381 [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R (2000) Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol 148: 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300: 135–139 [DOI] [PubMed] [Google Scholar]
- Shibue T, Suzuki S, Okamoto H, Yoshida H, Ohba Y, Takaoka A, Taniguchi T (2006) Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways. EMBO J 25: 4952–4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC (1999) Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science 284: 339–343 [DOI] [PubMed] [Google Scholar]