Abstract
Bak and Bax are critical apoptotic mediators that naturally localize to both mitochondria and the endoplasmic reticulum (ER). Although it is generally accepted that mitochondrial expression of Bak or Bax suffices for apoptosis initiated by BH3-only homologues, it is currently unclear whether their reticular counterparts may have a similar potential. In this study, we show that cells exclusively expressing Bak in endoplasmic membranes undergo cytochrome c mobilization and mitochondrial apoptosis in response to BimEL and Puma, even when these BH3-only molecules are also targeted to the ER. Surprisingly, calcium was necessary but not sufficient to drive the pathway, despite normal ER calcium levels. We provide evidence that calcium functions coordinately with the ER-stress surveillance machinery IRE1α/TRAF2 to transmit apoptotic signals from the reticulum to mitochondria. These results indicate that BH3-only mediators can rely on reticular Bak to activate an ER-to-mitochondria signalling route able to induce cytochrome c release and apoptosis independently of the canonical Bak,Bax-dependent mitochondrial gateway, thus revealing a new layer of complexity in apoptotic regulation.
Keywords: Bak, endoplasmic reticulum, ER-to-mitochondria apoptotic communication, mitochondrial apoptosis
Introduction
Bcl-2 family members have important functions in the integration of multiple apoptotic pathways (Danial and Korsmeyer, 2004). These proteins are structurally related by the presence of at least one of the four characteristic family domains (Bcl-2-homology domains 1–4 (BH1–4)) and can be further categorized according to functional and structural criteria (Adams and Cory, 2007). Thus, the BH3-only subgroup includes initiators of apoptosis only sharing the BH3 domain (Puthalakath and Strasser, 2002). In addition, Bak and Bax are multidomain agonists containing BH1–3, and the apoptotic activity of both subfamilies is counteracted by protective homologues presenting a BH4 domain (Adams and Cory, 2007).
The current view regarding how apoptotic signalling pathways work is that specific BH3-only molecules are induced by a wide variety of stimuli, and convey the death signal to a common downstream machinery constituted by Bak and Bax (Danial and Korsmeyer, 2004; Adams and Cory, 2007). These are critical mediators, as cells lacking both proteins are resistant to apoptosis induced by BH3-only effectors (Wei et al, 2001; Zong et al, 2001). Bak and Bax are known to have in this context a prominent mitochondrial function by promoting the release of cytochrome c. In fact, their exclusive expression in this organelle is sufficient to trigger BH3-only-induced death (Scorrano et al, 2003), thus supporting the notion that Bak and Bax constitute a mitochondrial gateway for apoptotic routes channelled through BH3-only molecules (Wei et al, 2001; Zong et al, 2001).
However, Bak and Bax also localize to the endoplasmic reticulum (ER) (Scorrano et al, 2003; Zong et al, 2003), and accumulated evidence shows that their presence in this organelle is linked to the regulation of calcium metabolism (Breckenridge et al, 2003; Rong and Distelhorst, 2008). Thus, enforced expression of Bak and Bax provokes ER calcium release (Nutt et al, 2002; Zong et al, 2003). In addition, double knockout mouse embryonic fibroblasts (MEFs) lacking Bak and Bax show decreased calcium levels in the reticular lumen that result in reduced apoptotic responses to stimuli that use ER calcium to activate the death program, like C2-ceramide or oxidative stress (Scorrano et al, 2003). Recovery of endoplasmic calcium by alternative means other than reexpressing Bak or Bax restores death induced by these agents, thus underscoring that the main apoptotic function of both proteins in the reticulum is the regulation of calcium levels (Scorrano et al, 2003). Therefore, ER-localized Bak or Bax seem to act as an apoptotic gateway for stimuli that rely on reticular calcium to activate programmed cell death.
Certain BH3-only mediators are known to function at the ER to induce apoptosis and, although calcium fluxes have been involved in these pathways, other mechanisms have also been proposed. For example, Puma is a key participant in p53-induced cell death (Villunger et al, 2003), and activates apoptosis by inducing ER calcium liberation (Shibue et al, 2006). Spike is a less-studied homolog that localizes to the ER, where it regulates the cleavage of Bap31 to trigger mitochondrial apoptosis (Mund et al, 2003). Bim mediates death induced by ER stress (Puthalakath et al, 2007), a process that involves Bim translocation to reticular membranes (Morishima et al, 2004). In addition, Bik is known to induce mitochondrial apoptosis by promoting both the oligomerization of reticular Bak and ER calcium release (Mathai et al, 2005). However, to what extent these death signals initiated at the ER by BH3-only molecules are autonomous inducers of apoptosis or simple facilitators of the conventional Bak,Bax-dependent mitochondrial gateway is not entirely clear.
Here, we address this issue by generating cells expressing reticular Bak in a cellular background devoid of both Bak and Bax. We found that the BH3-only molecules Bim and Puma are able to fully induce cytochrome c release and apoptosis in the sole presence of reticular Bak. We also show that this activity involves an ER-to-mitochondria communication pathway mediated by calcium and a novel configuration of the IRE1α/TRAF2 stress signalling machinery.
Results and discussion
Generation of a cellular system exclusively expressing Bak at the ER
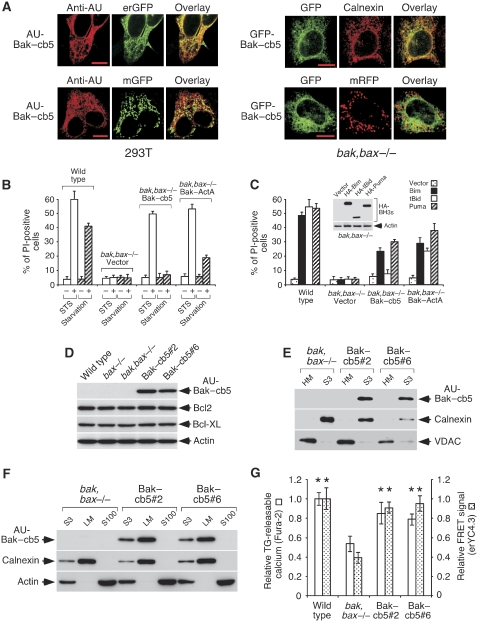
Earlier studies show that a fusion protein between Bak and the reticular targeting signal of cytochrome b5 (Bak–cb5) localizes to endoplasmic membranes (Zong et al, 2003). Consistently, transfected Bak–cb5 showed in our hands tight reticular localization in various cell types, including MEFs deficient for both Bak and Bax (bak,bax−/−) (Figure 1A). These cells were then retrovirally engineered to stably express an AU-tagged form of Bak–cb5 (AU-Bak–cb5; Supplementary Figure 1A). The resulting polyclonal cultures were tested for cell death in response to staurosporine (STS) or serum starvation, two conventional apoptotic inducers. Interestingly, STS treatment, but not starvation, killed the Bak–cb5-expressing cells (Figure 1B), whereas control bak,bax−/− MEFs were insensitive (Figure 1B). Double-deficient MEFs expressing a version of Bak targeted to mitochondria (Bak–ActA; Zong et al, 2003) were susceptible to both stimuli (Figure 1B; Supplementary Figure 1A and B), thus confirming previous indications that mitochondrial Bak or Bax suffice to transmit a wide range of apoptotic signals (Scorrano et al, 2003).
Figure 1.
Reticular expression of Bak–cb5 in bak,bax−/− MEFs. (A) Transfected Bak–cb5 localizes to the ER. 293T cells (left panels) were co-transfected with AU-Bak–cb5 and erGFP or mGFP, and stained for AU 24 h later. Bak,bax−/− MEFs (right panels) were transfected with GFP-Bak–cb5 in the absence or presence of mRFP, and stained for calnexin (top) or directly mounted (bottom), 24 h later. Pictures show colocalization of Bak–cb5 with reticular (erGFP, left; calnexin, right), but not mitochondrial (mGFP, left; mRFP, right) markers. (B) Polyclonal Bak–cb5-expressing MEFs die in response to STS treatment, but not serum starvation. The indicated cells were treated with STS (1 μM) or serum starvation for 24 or 36 h, respectively, and death was evaluated by propidium iodide (PI) staining. (C) Polyclonal AU-Bak–cb5-expressing cells undergo Bim- and Puma-induced apoptosis. Cells were transduced with the indicated BH3-only molecules and analysed for cell death by PI staining 24 h later. The inset shows expression of HA-Bim, HA-tBid and HA-Puma in bak,bax−/− MEFs, 24 h after transduction (anti-HA western blot). Bak,bax-deficient cells were used to avoid an influence of the induced death in expression levels. (D) Expression of AU-Bak–cb5, Bcl2 and Bcl-XL in clones #2 and #6. Total lysates were subjected to western blotting. (E) AU-Bak–cb5 is excluded from mitochondria in clones #2 and #6. Equal protein amounts were subjected to western blotting for AU, calnexin and VDAC. HM, heavy membranes; S3, post-mitochondria supernatant. (F) AU-Bak–cb5 co-purifies with calnexin in the microsomal fraction. Equal amounts of protein from the post-mitochondria supernatant (S3), light membranes (LM) or the post-microsome supernatant (S100) were subjected to western blotting for AU, calnexin or actin, as shown. (G) Recovered levels of reticular calcium in Bak–cb5-expressing clones. The amount of TG-releasable calcium (left bars) and the FRET signals provided by transduced erYC4.3 (right bars) are represented as a fraction of the values obtained for wild-type MEFs. Shown are averages and standard deviations (error bars) of the values obtained from three independent experiments. Asterisks indicate significant differences (Student's t-test) with respect to bak,bax−/− MEFs (P<0.05).
These results suggest that certain stimuli can fully activate the death program in the sole presence of reticular Bak, revealing the existence of signalling cascades capable of transmitting these signals. To explore these routes, we excited molecularly-defined apoptotic pathways by overexpressing the BH3-only molecules BimEL (Bim hereafter), tBid and Puma, three family members known to be particularly potent activators of apoptosis (Adams and Cory, 2007). Expression of Bim and Puma, but not tBid, provoked cell death in MEFs expressing Bak–cb5 (Figure 1C), whereas Bak–ActA-expressing MEFs were susceptible to all three inducers (Figure 1C). These data point to the notion that reticular Bak might suffice to relay death signals in response to certain BH3-only activators.
To prevent drifting of the polyclonal population, we cloned the Bak–cb5-expressing cells and chose two clones for further studies (#2, #6). Both strains showed expression of the transgene (Figure 1D) and presented unaltered levels of Bcl-2 and Bcl-XL (Figure 1D), suggesting that the cloning process did not involve a parallel selection for these protective molecules. Such bias was conceivable, given that excessive expression of Bak–cb5 induces cell death (Zong et al, 2003). Biochemical fractionation studies indicated that Bak–cb5 was excluded from the mitochondrial (Figure 1E) and cytoplasmic fractions (Figure 1F), and localized to light membranes (Figure 1F). Expression levels of the subcellular markers VDAC (mitochondria) and calnexin (ER) were comparable in all strains (Supplementary Figure 2). Bak and Bax are necessary to maintain high calcium concentrations in reticular cisternae (Scorrano et al, 2003). Consistently, the clones showed increased calcium mobilization induced by thapsigargin (TG, an ER calcium-releasing agent; Breckenridge et al, 2003) as well as elevated FRET signals provided by the calcium reporter erYC4.3 (Palmer et al, 2004), when compared with bak,bax−/− MEFs (Figure 1G), indicating recovered calcium levels in the ER lumen. ErYC4.3 was in fact able to measure reticular calcium, as it showed responsiveness to forced ER calcium depletion (Supplementary Figure 3). Both clones remained susceptible to cell death induced by STS, but not serum starvation (Supplementary Figure 4).
Bim and Puma induce cytochrome c release and apoptosis in Bak–cb5-expressing cells
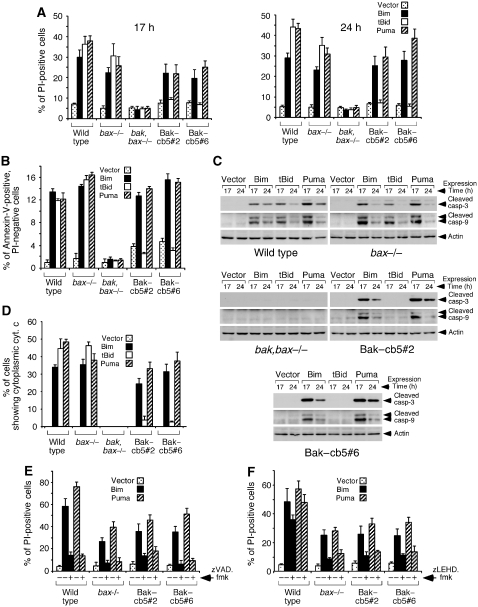
We used the clones expressing Bak–cb5 as a model system to dissect the apoptotic role of reticular Bak in response to BH3-only mediators. Expression of Bim and Puma in these cells induced significant levels of cell death, similar to those observed in bax−/− MEFs (Figure 2A). However, although tBid efficiently killed wild-type and bax−/− cells, both clones were strikingly resistant (Figure 2A). This result further suggests that no functional Bak is expressed at the mitochondria in these cells, given that mitochondrial expression of multidomain pro-apoptotic effectors is sufficient for tBid-induced apoptosis (see Figure 1C; Scorrano et al, 2003). The death process activated by Bim and Puma in Bak–cb5-expressing MEFs had apoptotic features, because the dying cells presented Annexin-V reactivity (Figure 2B), cleavage of caspases 9 and 3 (Figure 2C) and cytochrome c release (Figure 2D). However, no signs of caspase-12 cleavage were detected (Supplementary Figure 5), thus arguing against a role of this protease in the pathway. An involvement of the general caspase machinery was revealed by the capacity of the pan-caspase inhibitor zVAD.fmk to block death (Figure 2E). Treatment with the caspase-9 inhibitor zLEHD.fmk also diminished cell death (Figure 2F), a result that, together with the data showing concomitant cytochrome c mobilization (see Figure 2D), points to mitochondria as the main executioners of the apoptotic program. Taken together, these results indicate that certain BH3-only activators, like Bim and Puma, can induce cytochrome c mobilization and mitochondrial apoptosis in the sole presence of reticular Bak.
Figure 2.
Expression of Bim and Puma in MEFs expressing Bak–cb5 induces mitochondrial apoptosis. (A) Retroviral transduction of Bim and Puma leads to a progressive accumulation of PI-permeable cells in clones #2 and #6. Cells were transduced with the shown BH3-only molecules, and cell death was evaluated by PI-staining at the indicated times. (B) Bim and Puma induce Annexin-V reactivity before membrane disruption. PI-negative cells were analysed for Annexin-V binding 24 h after transduction. (C) Processing of caspases-3 and -9. Transduced cells were lysed at the shown times for western blotting. (D) Induction of cytochrome c release. Cells were transduced in the presence of zVAD.fmk (100 μM), and the percentage of cells showing diffuse cytochrome c staining was evaluated 17 h after transduction. (E) Cell death provoked by Bim and Puma is blocked by the pan-caspase inhibitor zVAD.fmk. Cells were transduced in the presence of zVAD.fmk (80 μM) and analysed for death by PI-staining 24 h later. (F) Inhibition of cell death by the caspase-9 inhibitor zLEHD.fmk. Cells were transduced in the presence of zLEHD.fmk (80 μM). Death was evaluated 17 h after transduction.
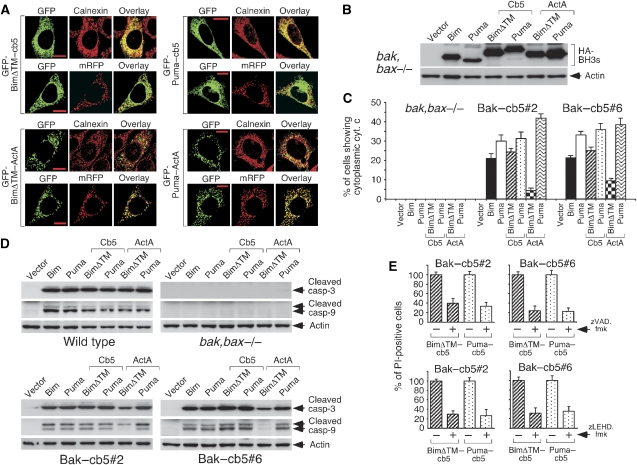
Bim and Puma targeted to the ER retain the capacity to activate mitochondrial apoptosis in Bak–cb5-expressing cells
Bim and Puma are thought to act at the level of mitochondria as well as the ER (Yamaguchi and Wang, 2002; Morishima et al, 2004; Shibue et al, 2006). To explore the relative contribution of both subcellular localizations to their apoptotic effect in the Bak–cb5 clones, we generated versions of these BH3-only effectors directed to the ER (cb5 fusions) or the mitochondrial outer membrane (ActA fusions). Bim chimeras lacking the native C-terminal hydrophobic region (BimΔTM–cb5 or BimΔTM–ActA) showed superior targeting specificity than constructs based on full-length Bim (data not shown) and, therefore, were used in these studies. All chimeric proteins presented proper subcellular localization (Figure 3A). We found that mitochondria-localized BimΔTM–ActA had a diminished capacity to activate cytochrome c exit and cleavage of caspases 9 and 3 compared with wild-type Bim (Figure 3C and D), despite similar expression levels (Figure 3B). On the other hand, BimΔTM–cb5 retained both activities (Figure 3C and D) and induced a death process blocked by caspase-9 and pan-caspase inhibitors (Figure 3E). These results argue that the apoptotic effect of unaltered Bim in Bak–cb5-expressing cells (see Figure 2) is likely to be mainly reticular.
Figure 3.
Reticular Bim and Puma stimulate mitochondrial apoptosis in MEFs expressing Bak–cb5. (A) The cb5 and ActA derivatives of BimΔTM and Puma are targeted to the ER and mitochondria, respectively. GFP-tagged versions of Bim and Puma were transfected into clone #2, sometimes in combination with a plasmid expressing mRFP (as shown), in the presence of zVAD.fmk (100 μM). After 48 h, cells were stained for calnexin or directly mounted (mRFP samples). Pictures show co-localization of the cb5 and ActA chimeras with reticular (Calnexin) and mitochondrial (mRFP) markers, respectively. Shown are representative examples. (B) Expression of the different constructs in bak,bax−/− MEFs. Cells were lysed for anti-HA western blotting 24 h after transduction. Bak,bax-deficient cells were used to prevent cell death. (C) Cytochrome c release induced by BimΔTM and Puma–cb5 and ActA fusions. Cells were transduced in the presence of zVAD.fmk (100 μM) and stained for cytochrome c 17 h later. (D) Caspase cleavage provoked by the chimeras. Cells were transduced and lysed 17 h later for western blotting. (E) BimΔTM–cb5 and Puma–cb5-induced death is inhibited by zVAD.fmk and the capase-9 inhibitor zLEHD.fmk. Cells were transduced and treated with zVAD.fmk (80 μM) or zLEHD.fmk (80 μM). Cell death was evaluated 24 h (zVAD.fmk) or 17 h (zLEHD.fmk) later by PI staining and is represented as a fraction of the value obtained in the absence of inhibitor.
Unexpectedly, however, Puma–ActA retained the potential of the wild-type molecule to excite the pathway in both Bak–cb5-expressing clones (Figure 3C and D). Given that bak,bax−/− MEFs remained unresponsive (Figure 3C and D), this result suggests that reticular Bak can somehow restore susceptibility of double-deficient mitochondria to certain apoptotic insults. The effect of Puma–ActA was inhibited by 2-aminoethyl-diphenyl-borinate (2-APB; Supplementary Figure 6A), an agent that reduces the overall calcium content of the cell (Peppiatt et al, 2003), thus pointing to a role of calcium in this phenomenon. How a global calcium decrease can inhibit apoptosis caused by direct mitochondrial engagement is unclear but, as expected, 2-APB reduced the basal calcium levels present in mitochondria (Supplementary Figure 6B), suggesting that mitochondrial calcium could play a relevant role. Importantly, 2-APB did not have a significant impact on ATP levels (Supplementary Figure 7). These data suggest that Bak–cb5 might be able to influence mitochondrial susceptibility to apoptosis by regulating their calcium metabolism, perhaps through its effect on reticular stores. This is a plausible notion, given that both organelles have a privileged interaction in terms of calcium metabolism (Walter and Hajnoczky, 2005). But more interesting in the context of this work, Puma–cb5 provoked cytochrome c release (Figure 3C) and caspase cleavage (Figure 3D) as efficiently as wild-type Puma, and induced a death process mediated by caspases 9 and 3 (Figure 3E). Therefore, unlike Bim, the apoptotic effect of native Puma in the Bak–cb5 clones (see Figure 2) is probably a mixture of events initiated at both reticular and mitochondrial membranes.
Taken together, these results show that Bim and Puma targeted to the ER retain the ability to provoke cytochrome c release and mitochondrial apoptosis in Bak–cb5-expressing MEFs. This scenario implies the activation of ER-to-mitochondria apoptotic communication mechanisms able to induce cytochrome c mobilization in the absence of Bak and Bax expressed in mitochondrial membranes, although a minor fraction of Bak–cb5 mislocalized to mitochondria might also contribute to cytochrome c release. We sought to study the signalling events that participate in this pathway.
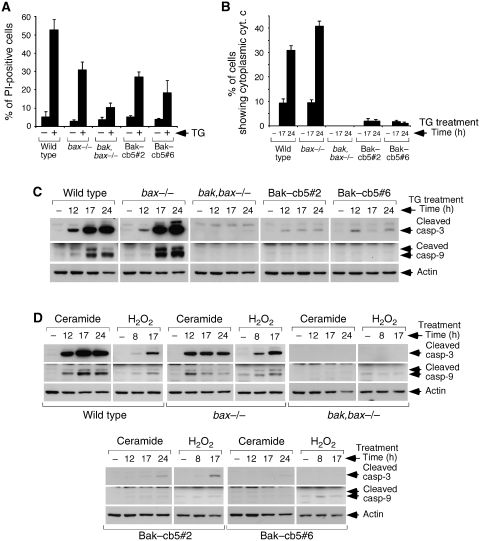
ER calcium mobilization is insufficient to activate mitochondrial apoptosis in cells expressing Bak–cb5
Calcium transits have been involved in the apoptotic communication between the reticulum and mitochondria (Walter and Hajnoczky, 2005). To evaluate whether a discharge of reticular calcium is sufficient to activate mitochondrial apoptosis in our system, we tested the death response of both Bak–cb5-expressing clones to stimuli that induce ER calcium liberation. TG treatment induced a higher degree of death in these cells when compared with bak,bax−/− MEFs (Figure 4A), a result consistent with earlier observations in bak,bax−/− cells engineered to have recovered ER calcium levels by overexpression of the SERCA pump (Scorrano et al, 2003). However, TG-induced death was not accompanied by cytochrome c release (Figure 4B) or cleavage of caspases 9 and 3 (Figure 4C), thus excluding an involvement of the mitochondrial apoptotic machinery. Similarly, other stimuli that rely on ER calcium to trigger apoptosis (C2-ceramide, H2O2; Scorrano et al, 2003) provoked a higher death rate in Bak–cb5-expressing MEFs compared with bak,bax−/− cells (Supplementary Figure 8), and were also unable to induce caspase processing (Figure 4D). These data indicate that calcium released from the reticulum, although capable of promoting death, is inefficient in inducing cytochrome c exit and apoptosis in cells lacking mitochondrial Bak and Bax. Consequently, it seems unlikely that ER calcium mobilization, if involved at all, is the only mediator of apoptosis induced by reticular Bim and Puma in our Bak–cb5-expressing cells. How calcium-dependent agents can activate death without mitochondrial involvement is unclear, but calcium mobilization can also trigger alternative death pathways like necrosis (Leung and Halestrap, 2008) or calpain-dependent apoptosis (Mathiasen et al, 2002).
Figure 4.
ER calcium agonists are unable to stimulate mitochondrial apoptosis in MEFs expressing Bak–cb5. (A) TG induces increased death in clones #2 and #6. Cells were treated with TG (5 μM) and cell death was measured by PI-staining 24 h later. (B) TG does not induce cytochrome c release. Cells were treated as in (A) in the presence of 100 μM zVAD.fmk, and stained for cytochrome c at the shown times. (C) Absence of caspase-3 and -9 processing induced by TG. Cells were treated with TG (5 μM) for the indicated times and lysed for western blotting. (D) C2-ceramide and H2O2 fail to activate caspases in Bak–cb5-expressing cells. Cells were treated for the indicated times with C2-ceramide (100 μM) or H2O2 (1 mM).
ER-to-mitochondria apoptotic communication mediated by a cooperative action of calcium and the IRE1/TRAF2 stress signalling system
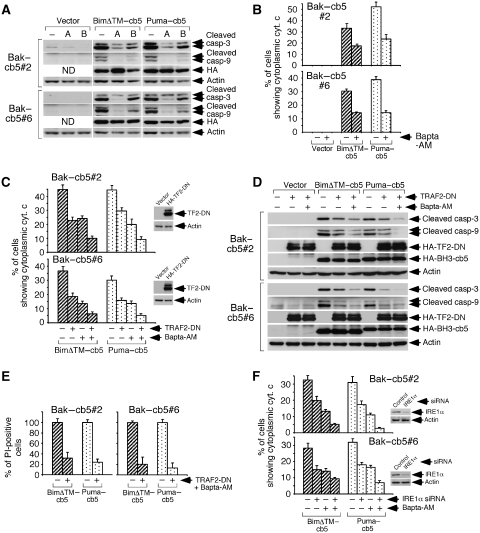
To explore a potential necessary role of calcium in this system, we used calcium antagonists like 2-APB or the calcium chelator Bapta-AM. Treatment with these agents inhibited cleavage of caspases 9 and 3 caused by BimΔTM–cb5 and Puma–cb5 in both clones (Figure 5A). Bapta-AM also inhibited cytochrome c release (Figure 5B) and, similar to 2-APB, did not cause reduction in ATP levels (see Supplementary Figure 7). Therefore, calcium mobilization is involved in this signalling system. However, since calcium cannot autonomously activate the pathway (see Figure 4), these data suggest that additional mechanisms mediate the apoptotic crosstalk between the ER and mitochondria in the Bak–cb5-expressing cells.
Figure 5.
ER-to-mitochondria apoptotic communication in MEFs-Bak–cb5 is mediated by calcium and IRE1/TRAF2. (A) 2-APB and Bapta-AM reduce cleavage of caspases 9 and 3 induced by BimΔTM–cb5 and Puma–cb5. Cells were transduced, treated with 2-APB (A; 75 μM) or Bapta-AM (B; 15 μM) 8 h later, and lysed 17 h after transduction for western blotting. (B) Treatment with Bapta-AM inhibits cytochrome c release. Cells were transduced in the presence of zVAD.fmk (100 μM), treated with Bapta-AM and stained for cytochrome c 17 h after transduction. (C) Effect of TRAF2-DN and Bapta-AM in cytochrome c release. Cells were pretransduced twice with retroviruses expressing HA-TRAF2-DN (HA-TF2-DN) and, 36 h later, treated as in (B). The insets show TRAF2-DN expression (anti-HA western blot). (D) Effect of TRAF2-DN and Bapta-AM in caspase processing. Cells were treated as in (C) without zVAD.fmk, and lysed 17 h later for western blotting. (E) Effect of TRAF2-DN plus Bapta-AM in cell death. Death was evaluated by PI staining, 24 h after transduction. Data are represented as percentages of the values obtained in the absence of TRAF2-DN and Bapta-AM. (F) Effect of IRE1α depletion and Bapta-AM in cytochrome c release. Cells were transfected with the siRNAs, transduced 48 h later in the presence of 100 μM zVAD.fmk, treated with Bapta-AM as in (C) and stained for cytochrome c 17 h after transduction. The insets show IRE1α depletion (anti-IRE1α western blot).
Reticular Bak is known to participate in the cellular response to ER stress by binding and activating the stress sensor IRE1α (Hetz et al, 2006). Stimulated IRE1α directly generates a spliced, active form of the transcription factor XBP1 (sXBP1) that in turn promotes transcription of stress-relieving molecules. A second signalling branch emanates from active IRE1α, and sequentially engages the adaptor TRAF2 and the kinases ASK1 and JNK (Ron and Walter, 2007). Although a recent study argues that the main function of IRE1α is to promote survival (Lin et al, 2007), other reports show that the IRE1α/TRAF2/ASK1 route contributes to apoptosis if the reticular damage is improperly managed (Nishitoh et al, 2002; Szegezdi et al, 2006).
To evaluate whether the IRE1α signalling machinery might be involved in promoting apoptosis in the Bak–cb5-expressing clones, we tested a possible dominant-negative effect of a deleted version of TRAF2 (TRAF2-DN) known to interfere with this ER stress route (Ron and Walter, 2007). Expression of TRAF2-DN reduced cytochrome c release induced by BimΔTM–cb5 or Puma–cb5 in both clones expressing reticular Bak (Figure 5C). Interestingly, such diminution was turned into a nearly complete blockade by the additional treatment with Bapta-AM (Figure 5C). This cooperative reduction in cytochrome c release had a parallel impact both in caspase processing (Figure 5D) and cell death (Figure 5E). In addition, knock-down of IRE1α inhibited cytochrome c liberation induced by reticular Bim and Puma (Figure 5F) and, again, Bapta-AM further reduced the activity (Figure 5F). These results reveal that IRE1α and TRAF2 mediate the apoptotic consequence of Bak activation by Bim and Puma at the ER, working in coordination with calcium to transmit death signals to Bak,Bax-devoid mitochondria. Since the simultaneous impairment of both pathways routinely reduced cytochrome c mobilization, caspase processing and cell death to marginal levels (see Figure 5), the IRE1α/TRAF2 and calcium routes are likely to be the main players involved.
Unconventional IRE1 signalling in response to reticular Bim and Puma
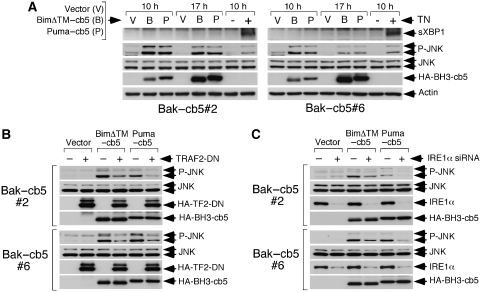
To further explore whether reticular Bim and Puma can activate IRE1α, we studied the induction of downstream signalling events. Treatment of Bak–cb5-expressing cells with tunicamycin (an inducer of ER stress) provoked both upregulation of sXBP1 and JNK phosphorylation (Figure 6A), a result consistent with earlier observations showing that reticular Bak suffices to support proper functioning of IRE1α (Hetz et al, 2006). Surprisingly, however, ER-targeted Bim and Puma were unable to cause processing of XBP1, despite a clear capacity to induce JNK phosphorylation (Figure 6A). Activation of JNK in this context was in fact mediated by TRAF2 and IRE1α, as inhibition of these molecules reduced JNK phosphorylation (Figure 6B and C). These data confirm that reticular Bim and Puma can activate IRE1α-dependent signalling. However, unlike ER stress, the BH3-only effectors seem to selectively engage the TRAF2/JNK route without inducing the XBP1 pathway, thought to have a more pro-survival role (Szegezdi et al, 2006).
Figure 6.
Downstream signalling induced by reticular Bim or Puma and ER stress in the Bak–cb5-expressing clones. (A) BimΔTM– and Puma–cb5 do not activate XBP1. MEFs were either transduced or treated with tunicamycin (TN, 10 μg/ml) and lysed at the shown times for western blotting. (B) TRAF2-DN inhibits JNK phosphorylation promoted by reticular Bim and Puma. Cells were pretransduced twice with retroviruses expressing HA-TRAF2-DN (HA-TF2-DN), transduced 24 h later with the BH3-only molecules and lysed 17 h later for western blotting. (C) Depletion of IRE1α reduces JNK phosphorylation induced by BimΔTM– and Puma–cb5. Cells were transfected with the indicated siRNAs, transduced 36 h later with retroviruses expressing BimΔTM– and Puma–cb5, and lysed for western blotting 13 h later.
Understanding how ER stress and BH3-only molecules differentially regulate both pathways requires further investigation, but one possibility might be that Bim and Puma cannot target other components of the ER-stress surveillance machinery (like ATF6) whose activation is needed for upregulation of the XBP1 mRNA (Ron and Walter, 2007). In this scenario, IRE1α would be unable to splice XBP1 simply because ATF6 did not previously cause accumulation of its specific mRNA. Alternatively, IRE1α might be able to regulate both signalling arms independently. But irrespective of the underlying mechanism, these results reveal an unexpected flexibility of the IRE1α signalling system that could explain why this molecule, although believed to deliver survival signals in response to ER stress (Lin et al, 2007), can also promote apoptosis in the face of a persistent stressful situation (Nishitoh et al, 2002) or, as we show here, after BH3-only stimulation. Thus, IRE1α emerges as a central coordinator of multiple survival/death pathways at the ER.
Calcium promotes sustained JNK activation that drives mitochondrial apoptosis in cells expressing Bak–cb5
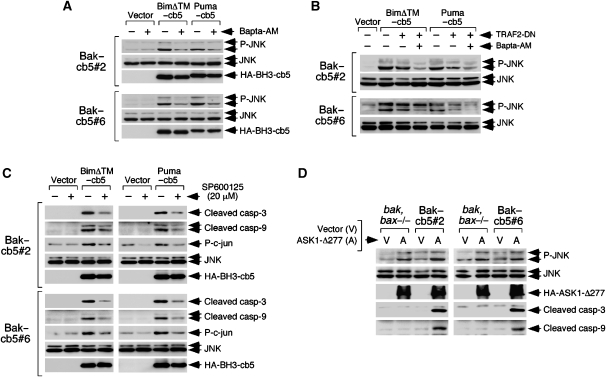
Relevant issues regarding the pathway described here are the mechanisms that mediate cytochrome c release, and how calcium and the ER-stress signalling machinery might interact to induce such effect. Mitochondrial calcium overloads can activate apoptosis through the induction of mitochondrial permeability transition (MPT), a sudden increase in inner membrane permeability that provokes depolarization, swelling, rupture of the outer membrane and cytochrome c release (Brenner and Grimm, 2006; Hajnoczky et al, 2006). Interestingly, most Bak–cb5-expressing cells that showed cytochrome c release in response to BimΔTM–cb5 and Puma–cb5 also exhibited mitochondrial depolarization (Supplementary Figure 9), suggesting a possible implication of MPT. However, direct measurements of mitochondrial integrity using the calcein-based method (Poncet et al, 2003) showed that reticular Bim and Puma do not induce inner membrane permeabilization in Bak–cb5-expressing MEFs (Supplementary Figure 10A). In contrast, STS activated the calcein reporter in both clones, indicating that their mitochondria are not intrinsically impaired to undergo MPT (Supplementary Figure 10A). In addition, cyclosporine A (CsA), an MPT inhibitor, was unable to reduce cytochrome c release induced by reticular Bim and Puma (Supplementary Figure 10B and C). Together, these data rule out a role for calcium-induced MPT in this system. How mitochondria might become depolarized by reticular Bim and Puma is not known, but earlier reports show that depolarization can occur in the absence of permeability transition during apoptosis (Poncet et al, 2003). Taken as a whole, these results suggest that other mechanisms of cytochrome c release alternative to MPT or a Bak,Bax-dependent pore are likely to operate in this apoptotic model, although some contribution of Bak–cb5 mislocalized to mitochondria cannot be excluded.
Mitochondrial calcium uptake can trigger the production of reactive oxygen species (ROS; Hajnoczky et al, 2006) that are known ASK1 activators (Fujino et al, 2007), thus providing a possible link between both signalling arms. However, ROS quenchers like N-acetyl-L-cysteine (NAC) or MitoQ were unable to reduce cytochrome c mobilization by reticular Bim and Puma (Supplementary Figure 11), indicating that ROS are not relevant mediators in this system. In addition, neither MPT nor ROS were found to be involved in apoptosis induced by Puma–ActA (Supplementary Figures 10 and 11).
To further explore a possible interaction between both signalling branches, we tested whether calcium inhibition might have an impact in the IRE/TRAF2-dependent signalling arm. Treatment with Bapta-AM reduced the levels of phosphorylated JNK induced by reticular Bim and Puma (Figure 7A), suggesting that calcium released by these BH3-only effectors promotes sustained JNK activation. In contrast, inhibition of the IRE1α route had no effect on ER calcium (Supplementary Figure 12). While transient JNK activity is preferentially involved in gene regulation, persistent activation has been linked to the induction of cell death (Ventura et al, 2006). Interestingly, the levels of phospho-JNK induced by reticular Bim and Puma nicely paralleled those of processed caspases or cytochrome c release in cells expressing TRAF2-DN and/or treated with Bapta-AM (Figure 7B), pointing to the notion that long-lasting JNK activation might participate in this signalling system. Consistently, pharmacological inhibition of JNK reduced caspase processing (Figure 7C), cytochrome c mobilization and mitochondrial depolarization (Supplementary Figure 13) induced by reticular Bim and Puma, thus arguing that JNK plays a role in the pathway. It is worth mentioning that the JNK inhibitor caused in some experiments a less pronounced but detectable reduction in the expression levels of the two BH3-only mediators (data not shown), suggesting that JNK might also be involved in a feedback regulatory loop that promotes stability of reticular Bim and Puma. A role for the MAP kinase family in regulating Bim stability has been shown before in other systems (Hubner et al, 2008). Also in line with the idea that persistent JNK activation can drive the pathway in the Bak–cb5-expressing clones, expression of a constitutively active ASK1 mutant (ASK1-Δ277; Fujino et al, 2007) induced JNK activation (Figure 7D), and sufficed to cause processing of caspases 3 and 9 in these cells (Figure 7D). Interestingly, ASK1-Δ277 was inactive in control bak,bax−/− MEFs (Figure 7D), further supporting the notion that their mitochondria are not fully competent to undergo apoptosis.
Figure 7.
Persistent JNK activation induced by reticular Bim and Puma is mediated by calcium and drives mitochondrial apoptosis in Bak–cb5-expressing cells. (A) Bapta-AM inhibits JNK activation induced by BimΔTM– and Puma–cb5. Cells were transduced, treated with Bapta-AM 8 h later and lysed 17 h later for western blotting. (B) TRAF2-DN and Bapta-AM synergistically inhibit JNK activation. Cells were pretransduced with viruses encoding TRAF2-DN and treated as in (A) 36 h later. Samples are the same as in Figure 5D. (C) JNK inhibition reduces caspase processing induced by BimΔTM– and Puma–cb5. Cells were transduced, treated with the JNK inhibitor SP600125 (20 μM) 8 h later and lysed 17 h after infection. The figure shows that SP600125 inhibits JNK activity (phospho-c-jun) without reducing total JNK levels. (D) Expression of ASK1-Δ277 drives caspase processing in clones #2 and #6. Cells were transduced twice with retroviruses encoding an irrelevant insert (V) or HA-ASK1-Δ277 (A) and lysed 36 h later for western blotting.
All these results are consistent with a model where calcium has at least two roles in the pathway described here. First, the reticular calcium reservoir maintained by Bak–cb5 in bak,bax−/− MEFs appears to be involved in restoring mitochondrial susceptibility to apoptosis. Second, ER calcium mobilized by the continuous action of BH3-only molecules likely influences the IRE1/TRAF2 signalling branch by promoting persistent JNK activation, thus favouring the apoptotic potential of this kinase. Therefore, calcium seems to have complex and multifaceted roles in this system, and additional functions cannot be ruled out.
Concluding remarks
Together, our results indicate that reticular expression of Bak is sufficient to fully trigger mitochondrial apoptosis in response to Bim and Puma, and support the involvement of an ER-to-mitochondria apoptotic pathway participated by calcium and the ER-stress signalling system in the induction of this phenomenon. Such observations reveal that, by not relying on mitochondrial Bak and Bax to activate the death program, ER-localized multidomain effectors may function as autonomous integration sites for apoptotic signals transduced by BH3-only mediators, thus adding a new layer of complexity to the regulation of apoptosis. Under which physiological conditions this route might become more notable in normal cells where there is expression of Bak and Bax in mitochondrial membranes remains to be determined. However, anti-apoptotic family members like Bcl-2 and Bcl-XL have different subcellular distribution and protective potency in different compartments (Kaufmann et al, 2003; Fiebig et al, 2006). One could conceive a situation where the Bak,Bax-dependent mitochondrial gateway is inhibited so that the reticular pathway described here gains prominence.
Materials and methods
Cell lines and reagents
HEK-293T cells were obtained from the American Type Culture Collection, and the different MEFs were kindly shared with us by Dr S Korsmeyer (Wei et al, 2001). Cells were cultured at 37°C and a humidified 5% CO2 atmosphere, in DMEM with 10% heat-inactivated FBS and 100 U/ml of penicillin/streptomycin (Invitrogen). ZVAD.fmk, zLEHD.fmk and Annexin-V-FITC were purchased from Becton Dickinson. TG, 2-aminoethoxydiphenyl borate, puromycin, C2-ceramide, propidium iodide, staurosporine, N-acetyl-L-cysteine, cyclosporine A and H2O2 were from Sigma. Bapta-AM and Pluronic were from Molecular Probes, and SP600125 was purchased from Calbiochem. MitoQ was kindly provided by Dr M Murphy.
DNA constructs, transfections and retroviral transductions
The Bak–cb5, BimEL and tBid cDNAs, as well as reticular and mitochondrial GFP or RFP (erGFP, mGFP or mRFP), were described before (Klee and Pimentel-Muiños, 2005; Alcalá et al, 2008). Bak–ActA (Zong et al, 2003), the cDNA for Puma and ASK1-Δ277 were kind gifts from Dr C Thompson, Dr B Vogelstein and Dr H Ichijo, respectively. TRAF2-DN lacks the first 86 amino acids and was amplified from a human cDNA library. Constructs were tagged with GFP, AU1 or HA at the N terminus. Tagged constructs are only labelled as such in figures where tagging is essential for interpretation of the results.
To build the cb5 and ActA derivatives of BimEL, the C-terminal hydrophobic stretch that constitutes a potential transmembrane region (amino acids 181–198) (Yamaguchi and Wang, 2002) was substituted by the relevant peptides. Puma chimeras contained the full-length Puma molecule. The cb5 and ActA fragments came from constructs shared by Dr C Thompson (Zong et al, 2003). The ER-targeted version of the ratiometric calcium reporter YC4.3 (erYC4.3) was obtained from Dr R Tsien and was manipulated to substitute its N-terminal leader sequence by a cassette encoding the leader of CD5 and the HA tag. The resulting protein is N terminally tagged with HA and it is specifically expressed in the ER (data not shown).
Transfections of 293T cells were done using the calcium phosphate precipitation method. MEFs were transfected with FuGene (Roche). For retroviral transductions, all constructs were subcloned into the vector P12-MMP (Alcalá et al, 2008). Virus-containing supernatants were generated by co-transfecting 293T cells with the relevant construct together with helper plasmids expressing gag-pol (pMD.gag-pol) and env (VSV-G; pMD-G). Infections were done by diluting the viral supernatants with fresh medium (1:1), and spinning the resulting mix onto the target cells for 1 h at 2000 r.p.m., 32°C. Plasmids expressing AU-Bak–cb5 or AU-Bak–ActA plus the puromycin resistance gene under the control of an internal ribosome entry sequence were used to stably transduce bak,bax−/− MEFs. Selections were done in 1 μg/ml puromycin.
Biochemical fractionations
To evaluate whether AU-Bak–cb5-expressing MEFs (clones #2, #6) presented complete exclusion of the chimeric protein from mitochondrial membranes, we used the Mitochondria Isolation Kit for Cultured Cells (Pierce), essentially following the manufacturer's instructions with the exception that a 3000 g centrifugation was used to maximize segregation between reticular and mitochondrial markers.
Conventional fractionation techniques were used to confirm microsomal expression of AU-Bak–cb5 as well as co-segregation with calnexin. Briefly, 2 × 108 cells were resuspended in three times their pelleted volumes of hypotonic buffer (10 mM HEPES pH 7.8, 1 mM EGTA, 25 mM KCl). After 10-min incubation on ice, cells were pelleted, resuspended in two times their volume of isotonic buffer (10 mM HEPES pH 7.8, 250 mM sucrose, 1 mM EGTA, 25 mM KCl), and lysed using a dounce homogenizer (tight-fit). Post-nuclear supernatants were spun at 3000 g to remove mitochondria and other heavy membranes. The resulting supernatant was centrifuged at 100 000 g for 1 h to generate light membrane pellets and post-microsomal supernatants.
Calcium measurements
To measure reticular calcium with Fura-2-AM, cells were loaded (30 min, 37°C) in Hank's balanced salt solution (HBSS, without calcium, magnesium and phenol red) supplemented with 5% FBS and 2.5 μM of the dye premixed with an equal volume of Pluronic (20%), followed by a 10-min desterification step. Cells were resuspended in the same HBSS formulation at 2 × 106 cells/ml. Equal amounts of cells were subjected to the fluorescence assay by measuring the intensity of emission at 510 nm after excitation at 340 and 380 nm in response to TG. The assay was done at 37°C using a FluoroMax-3 fluorometer (Horiba Jobin Yvon). After obtaining a stable baseline at both 340 and 380 nm, cells were treated with TG (5 μM) and the emissions at both wavelengths were measured every 30 s up to a total of 4 min of data collection. The 340/380 ratios were represented against time and the area below the adjusted curve integrated as a measure of the amount of calcium released by TG.
ER-localized YC4.3 was transduced into cells seeded on poly-L-lysine-coated coverslips and confocal FRET measurements were conducted in vivo 36 h later as described (Klee and Pimentel-Muiños, 2005). FRET values for each pixel crossed by an arbitrary line drawn using the Zeiss LSM510 confocal software were integrated and divided by the distance covered to obtain an average value per field analysed. At least six fields and a total of 50 cells in different fields were analysed per experimental point. Experiments were repeated three times.
Immunofluorescence and quantitation of cytochrome c release
Cells were seeded onto poly-L-lysine-treated coverslips (Sigma), and either transfected or transduced the next day. For cytochrome c mobilization assays, cells were transduced in the presence of 100 μM zVAD.fmk. Coverslips were fixed in 4% paraformaldehyde and stained for 1 h with anti-AU1 (Babco), anti-calnexin (Stressgen) or anti-cytochrome c (Becton Dickinson) antibodies. Confocal microscopy was done using the 488- and 543-nm bands of the Argon and Helium-Neon lasers respectively, of a Zeiss LSM-510 confocal microscope. Scale bars represent 10 μm in all micrographs. Cytochrome c mobilization was quantitated by blindly evaluating the percentage of cells per field showing a diffuse cytochrome c staining pattern accompanied by poor mitochondrial signal. At least 600 cells were scored for each experimental point. Error bars indicate standard deviations of percentages obtained by counting at least eight different fields.
Cell death assays
MEFs were plated at a relatively low density to maximize retroviral transduction and cell death. The cumulative amount of death was measured as the percentage of cells that become permeable to PI. Both floating and adherent cells were stained for 15 min at room temperature in complete DMEM containing 1 μg/ml of PI and analysed by flow cytometry (FACScalibur, Becton Dickinson). For Annexin-V stainings, cells were resuspended in DMEM containing 2.5 mM CaCl2, and incubated for 15 min with Annexin-V-FITC (Becton Dickinson) and PI (1 μg/ml). Data are represented as averages and standard deviations (error bars) of the values obtained from at least three independent experiments. Caspase inhibitors zVAD.fmk and zLEHD.fmk were added at the time of retroviral transduction. CsA, NAC and MitoQ were added 1 h post-transduction. Control experiments showed no interference of these chemicals with the infection process (data not shown). Treatment with Bapta-AM was carried out 8 h after infection. Cells were washed with serum-free DMEM and incubated for 30 min at 37°C in serum-free DMEM containing 15 μM of Bapta-AM diluted in two volumes of Pluronic (20%). 2-APB was added 8 h after infection, as it was SP600125. Treatment with the latter was done after washing the cells to remove the retroviral supernatant.
Western blotting
Cells were lysed in a buffer containing 1% Igepal CA-630 detergent (Sigma), 150 mM NaCl, 50 mM Tris–HCl pH 7.5, 5 mM EDTA, protease inhibitors aprotinin (10 μg/ml), leupeptin (10 μg/ml) and PMSF (1 mM; Roche), and phosphatase inhibitors sodium orthovanadate (0.2 mM) and NaF (5 mM). To evaluate the induction of spliced XBP1, total extracts were obtained in RIPA buffer (1% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% dodecyl sulfate, 20 mM Tris–HCl pH 8, 137 mM NaCl, 1 mM MgCl2, 1 mM CaCl2).
Equal amounts of protein were resolved by SDS–PAGE, transferred to a polyvinilidene difluoride membrane (Millipore), and probed with antibodies against AU1, HA (Babco), cleaved caspase-3, cleaved caspase-9, cleaved caspase-12, IRE1α, phospho-JNK, phospho-S63-c-jun (Cell Signalling), actin (Sigma), Bak, Bcl-2, Bcl-XL (Becton Dickinson), calnexin (Stressgen), VDAC (Calbiochem), XBP1 and JNK (Santa Cruz). Blots were developed by chemiluminescence (Amersham).
siRNA studies
MEFs were transfected with predesigned pools of RNA duplexes against mouse IRE1α (On-TargetPlus, Dharmacon) using DharmaFECT1 (Dharmacon), following product instructions. The individual siRNA sequences were 5′-gaaagguggugcacaucaauu-3′, 5′-cgucauugcucgugaauuguu-3′, 5′-ugaacuacuugaggaauuauu-3′, 5′-ugacgaaacuucccuuuacuu-3′. Control siRNAs were also from Dharmacon (siControl siRNAs).
Supplementary Material
Supplementary Figures 1–13
Acknowledgments
We thank Dr JM de Pereda for help with calcium measurements, and Drs S Korsmeyer, C Thompson, B Vogelstein, R Tsien, M Murphy and XR Bustelo for kindly sharing reagents. We also appreciate Emilio Boada's experimental help. This work was funded by grants SAF2002-00193, GEN2003-20239-C06-05 and SAF2005-01208 from the Ministerio de Educación y Ciencia (MEC, Spanish Government). Additional funding comes from the FEDER program of the European Union. MK is the recipient of a predoctoral fellowship from the FPU program (MEC). KP is a graduate student funded by an FPI fellowship (MEC). SA holds a predoctoral fellowship from the Junta de Castilla y León, and AF is supported by a long-term EMBO postdoctoral fellowship. FXP was an investigator affiliated to the University of Salamanca and funded by the Ramón y Cajal program (MEC), and now holds a tenured position at the Council for Scientific Research (CSIC).
References
- Adams JM, Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26: 1324–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcalá S, Klee M, Fernandez J, Fleischer A, Pimentel-Muiños FX (2008) A high-throughput screening for mammalian cell death effectors identifies the mitochondrial phosphate carrier as a regulator of cytochrome c release. Oncogene 27: 44–54 [DOI] [PubMed] [Google Scholar]
- Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC (2003) Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 22: 8608–8618 [DOI] [PubMed] [Google Scholar]
- Brenner C, Grimm S (2006) The permeability transition pore complex in cancer cell death. Oncogene 25: 4744–4756 [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116: 205–219 [DOI] [PubMed] [Google Scholar]
- Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW (2006) Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer 6: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino G, Noguchi T, Matsuzawa A, Yamauchi S, Saitoh M, Takeda K, Ichijo H (2007) Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol 27: 8152–8163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnoczky G, Csordas G, Das S, Garcia-Perez C, Saotome M, Sinha Roy S, Yi M (2006) Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 40: 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312: 572–576 [DOI] [PubMed] [Google Scholar]
- Hubner A, Barrett T, Flavell RA, Davis RJ (2008) Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell 30: 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C (2003) Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol 160: 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee M, Pimentel-Muiños FX (2005) Bcl-X(L) specifically activates Bak to induce swelling and restructuring of the endoplasmic reticulum. J Cell Biol 168: 723–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AW, Halestrap AP (2008) Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim Biophys Acta 1777: 946–952 [DOI] [PubMed] [Google Scholar]
- Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318: 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathai JP, Germain M, Shore GC (2005) BH3-only BIK regulates BAX,BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem 280: 23829–23836 [DOI] [PubMed] [Google Scholar]
- Mathiasen IS, Sergeev IN, Bastholm L, Elling F, Norman AW, Jaattela M (2002) Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J Biol Chem 277: 30738–30745 [DOI] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Tsuchiya K, Shibata T, Seiwa E (2004) Translocation of Bim to the endoplasmic reticulum (ER) mediates ER stress signaling for activation of caspase-12 during ER stress-induced apoptosis. J Biol Chem 279: 50375–50381 [DOI] [PubMed] [Google Scholar]
- Mund T, Gewies A, Schoenfeld N, Bauer MK, Grimm S (2003) Spike, a novel BH3-only protein, regulates apoptosis at the endoplasmic reticulum. FASEB J 17: 696–698 [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, Swisher SG (2002) Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J Biol Chem 277: 9219–9225 [DOI] [PubMed] [Google Scholar]
- Palmer AE, Jin C, Reed JC, Tsien RY (2004) Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA 101: 17404–17409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL (2003) 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium 34: 97–108 [DOI] [PubMed] [Google Scholar]
- Poncet D, Boya P, Metivier D, Zamzami N, Kroemer G (2003) Cytofluorometric quantitation of apoptosis-driven inner mitochondrial membrane permeabilization. Apoptosis 8: 521–530 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129: 1337–1349 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A (2002) Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 9: 505–512 [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529 [DOI] [PubMed] [Google Scholar]
- Rong Y, Distelhorst CW (2008) Bcl-2 protein family members: versatile regulators of calcium signaling in cell survival and apoptosis. Annu Rev Physiol 70: 73–91 [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300: 135–139 [DOI] [PubMed] [Google Scholar]
- Shibue T, Suzuki S, Okamoto H, Yoshida H, Ohba Y, Takaoka A, Taniguchi T (2006) Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways. EMBO J 25: 4952–4962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7: 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura JJ, Hubner A, Zhang C, Flavell RA, Shokat KM, Davis RJ (2006) Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell 21: 701–710 [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A (2003) p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302: 1036–1038 [DOI] [PubMed] [Google Scholar]
- Walter L, Hajnoczky G (2005) Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J Bioenerg Biomembr 37: 191–206 [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Wang HG (2002) Bcl-XL protects BimEL-induced Bax conformational change and cytochrome C release independent of interacting with Bax or BimEL. J Biol Chem 277: 41604–41612 [DOI] [PubMed] [Google Scholar]
- Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB (2003) Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 162: 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB (2001) BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev 15: 1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1–13