Abstract
Literature highlights that serotonergic descending pathways [14] are implicated in somatosensory functions in the spinal cord and that serotonin (5-HT) in the dorsal horn might play a role in motor function through proprioceptive feedback [3, 8, 9, 13]. We hypothesized that 5-HT release in dorsal horn might represent an important factor in the completion of locomotion by facilitation of the spinocerebellar tract [8] and/or by modulation of spinal reflex pathways [9]. The present study demonstrates that during locomotor activity, 5-HT is released in layers II, III, IV, V of Rexed laminae. Microdialysis in combination with HPLC was used to measure concentrations of neurotransmitters in the lumbar dorsal horn before, during, and after a treadmill running exercise. Our results show a significant 41% increase of 5-HT release within the dorsal horn during a running exercise. 5-HT release is temporally related to exercise. The present study demonstrates that dorsal horn 5-HT release might modulate locomotion.
Keywords: Microdialysis, Spinal cord, Exercise, Motor function, 5-HT
Introduction
This study addresses the question of whether 5-HT control of afferent feedback is relevant to limb movement during locomotion or not. This control is assessed by 5-HT release variations in dorsal horn during rest and motor activity.
Several recent publications stress the role of 5-HT in modulation of motor activity, such as its role in control of pelvic musculature [3], its activation in the commissural region, its facilitatory effect on the activation of spinocerebellar tract neurons (in feline) [8] and, its increase in the effectiveness of pre-setting motor responses [9]. We show here that 5-HT release within the lumbar dorsal horn is increased during locomotor activity. Therefore our data corroborate with previous data suggesting [2, 3, 8, 9] that 5-HT innervation controls motor afferent feedback in hindlimbs.
This study addresses the hypothesis that locomotor activity affects the kinesis of 5-HT release by descending systems ending in the lumbar enlargement of the rat dorsal horn. Microdialysis probes implanted and maintained in place [6] into layers II, III, IV and V of Rexed [12] were used to sample 5-HT release.
Materials and Methods
Experimental animals
Seven Sprague Dawley male rats (100±10 g at their arrival in our vivarium; Iffa-Credo) received standard food and drinking water ad libitum. Conditioning exercise took place before any surgery and began 12 days after arrival of animals in our vivarium. The exercise consisted of a progressive 5-day-a-week treadmill program, in order to obtain spontaneous and continuous running for 60 min.
Stereotactic microdialysis probe placement into the dorsal horn of the spinal cord
The probe was placed at the maximum convex point of the spinal kyphosis (T13-L1) where minimal cephalo-caudal translation of the cord within the vertebral canal is observed thus preventing from potential movement of the spinal cord relative to the probe. For surgery, animal (235±10 g) anaesthesia was induced by 50 mg/kg of sodium pentobarbital i.p.; additional doses were given i.p. (3mg/kg) as needed. Body temperature was monitored. Animals were placed in a spinal stereotactic aparatus (David Kopf Instruments, USA). A microdialysis probe (membrane diameter of 240μm, cut off of 6000 Daltons, CMA11/1 mm, CMA/Microdialysis, Sweden) was stereotactically inserted into the L4 dorsal horn segment of the spinal cord (vertebral body T13) [6] in layers II, III, IV, V of Rexed [10, 11]. As proprioceptive areas are located within laminae III, IV, V the specific placement of the membrane of the probe allowed for sampling from proprioceptive areas within the dorsal horn. Stereotactic coordinates were set with reference to T13 spinous process apex: AP= -4.0 mm; L= 0.64±0.14 mm; H= +0.72±0.06 mm (from dura), with an angle of 16°to the horizontal fixation of the probes to the vertebra. Muscles and skin were sutured plan by plan [6].
Post-operative care
Post-operative care and treadmill walking was initiated immediately (usually 2 hours) after recovery from anesthesia [6]. Pain management has been assessed (no prostrated behavior, weight gain, proper grooming …) to make sure that animals were not suffering from surgery.
Animals were subjected to a treadmill test session exercise
Running test sessions took place 8 days after the probe insertion in the dorsal horn. Animals were placed on a treadmill, and microdialysis probes were connected to a micro-perfusion pump (CMA100, CMA-Microdialysis, Sweden) and to the HPLC system.
Microdialysis sampling
Microdialysis probes were perfused with sterile Ringer’s solution (NaCl = 144 mM, CaCl2 = 2.0 mM, KCl = 4 mM, pH = 6.25) [15] at a flow rate of 2.0μl/min for 2.5 hour of washout period. Perfusion of the probes continued for a 4-hour period divided in 90min of rest, 60min of running exercise on a 5% slope and at a speed of 16m/min and, 90min of post-exercise rest. Sampling occurred every 15 minutes. For the 4-hour session, on-line HPLC analyses were performed.
Monoamine assay
Microdialysate samples were on-line injected (10μl) onto and separated by an analytical “microbondapack phenyl” C18 column (Waters, USA), maintained at 20°C. Mobile phase consisted of 0.1M sodium acetate and 1mM ethylenediaminetetraacetic acid (EDTA) at a pH of 4.0. Buffer was delivered at a flow rate of 1.0ml/min using a Waters’ 501 pump. Analytes were detected electrochemically (M464 detector, Waters) with Ag/AgCl reference electrode set at 574mV. Retention times, peak areas, and concentrations of 5-HT and 5-HIAA in microdialysate were computed by comparison with known standards injected before each set of six samples (Baseline 810, version 3.1, Waters). Detection limits for 5-HT, 5-HIAA were 13pg/10μl injected [6, 7].
Histology
Animals were sacrificed at the end of the entire experimental procedure by intracardiac perfusion of 5% glutaraldehyde. Spinal cords were excised and post-fixed. Spinal cords of all seven animals implanted with microdialysis probes were serially sectioned (30μm) with a vibratome to verify the location of the probe and perform immunocytochemical (ICC) analyses such as glial fibrillary acidic protein (GFAP) and 5-HT, as previously described [7]. In addition, sections were stained with cresyl violet for evaluation of the inflammatory reaction of the host tissue.
Materials
5-HT HCl, 5-HIAA, EDTA, DAB, glutaraldehyde were purchased from Sigma. Rabbit anti-GFA, was purchased from Dakopatts; primary antiserum goat anti-5-HT was a gift from M. Geffard.
Data analysis
Results were expressed as mean±s.e.m. of variation over rest concentrations. For each animal, control values were calculated as a mean of six sequential steady state samples during rest period (90min) before exercise. Control values (rest) were then compared with four exercise and six post-exercise samples. For statistical significance between groups (control, exercise, post-exercise) for microdialysate concentrations, we used repeated-factor two-way ANOVA for repeated measures (Super Anova software). We used Student’s paired two-tail t-test; p≤0.05 was considered to be significant.
Results
Clinical postoperative examination showed that wound margins were healed by day 8. Animals were completely mobile immediately after surgery. Based on our previous extensive with this preparation, which was originally introduced by us, no specific side effect has been seen as the result of the microdialysis probe [4, 5, 6, 7]. No evidence of back pain (48h after surgery) was observed neither prostrated behavior after probe implantation. Animals did not develop post-operative sensory loss or motor deficit as a consequence of surgery. Animals gained weight quickly after surgery [6].
Histological analysis showed the accuracy of microdialysis probe placements, the reaction of host tissue, and 5-HT fibers surrounding the probe
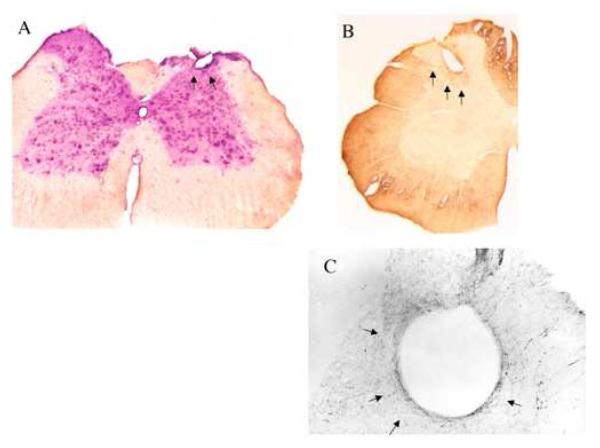
Transverse sections of spinal cord at the level of the dialysing part of microdialysis probes (1mm) confirmed that probes were successfully inserted in the L4 segment of the dorsal horn (layers II, III, IV, V of Rexed [10, 11]), (Fig.1; Fig. 2-A). Histology (Fig. 2-A, -B, -C) did not show any sign of additional lesion other than the round-shaped hole due to the physical implantation of the probe. There was no evidence of oedema, supporting that there was no injury due to the probe being chronically implanted. ICC analysis shows that a one-cell thick ring of astrocytes loosely surrounded the probe site, thus indicating a very moderate reaction [4, 5, 6] in the dorsal horn. Adjacent sections IR to 5-HT and GFAP show that the astrocytic ring was penetrated by a 5-HT axon network (Fig. 2-B and 2-C).
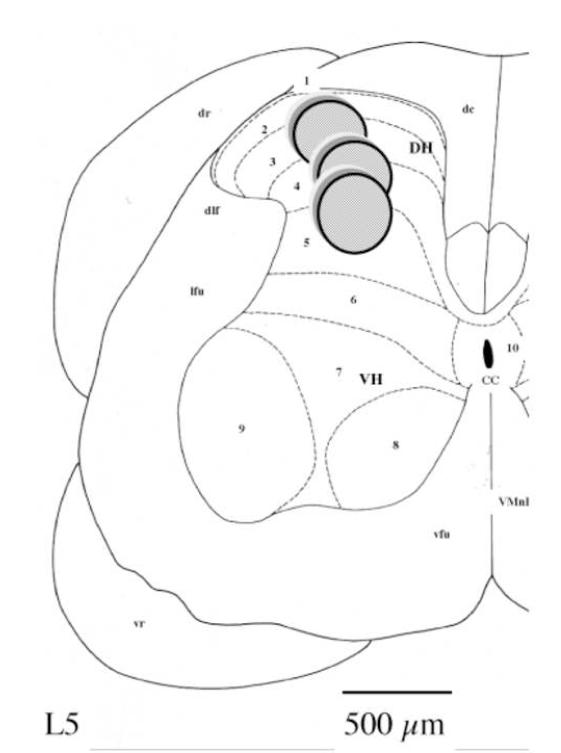
Figure 1 represents a diagram of a hemi-spinal cord section adapted from Paxinos and Watson (1982). This diagram shows the location of the dialysing part of the microdialysis probe in the lumbar enlargement within layers 2,3,4,5 of Rexed. Solid gray circles represent the microdialysis membrane (Gerin et al. 1994). L5: lumbar segment number 5; CC: central canal; dc: dorsal columns; dr: dorsal root of spinal nerve; dlf: dorsolateral funiculus of the spinal cord; lfu: lateral funiculus; vr: ventral root of spinal nerve; vfu: ventral funiculus; VMnF: ventral median fissure of the spinal cord; DH: dorsal horn of the spinal cord; VH: ventral horn. (Scale bar: 500μm)
Figure 2-A shows the tissue reaction to the probe in the dorsal horn sections (Rexed’s layers II, III, IV, V) (30μm thickness, using vibratome) as revealed by cresyl violet staining. Haemorrhage, oedema or massive tissue reaction in the vicinity of the microdialysis probe is absent.
Figure 2-B shows the general innervation pattern at the site of implantation of the microdialysis probe, visualized by immuno-detection of 5-HT.
Figure 2-C shows that at a higher magnification, the IR for 5-HT is slightly denser at the contact of the membrane of the probe suggesting local sprouting. Moreover, the proximity of serotonergic axons and terminals to the probe suggests that 5-HT in the microdialysate originates from neighboring fibers and terminals.
Release of 5-HT in dorsal horn showed its direct implication in motor function
Release of 5-HT was measured and, in order to assess the catabolic pathway of serotonin we also measured 5-HIAA. Both 5-HT and 5-HIAA were measured before, during and after treadmill exercise.
Release of 5-HT during rest period
During rest, mean of 5-HT concentration was 28.10 ± 4.52pg/10μl of microdialysate injected onto the HPLC column (n=7) (Table 1).
Table 1 shows extracellular concentration (pg/10μl) of 5-HT and 5-HIAA from the dorsal horn of the rat spinal cord expressed as mean ±s.e.m. of time point concentrations during rest (6 time points), treadmill exercise (4 time points), and post-exercise rest periods (6 time points)
| MEAN ± s.e.m. (pg/10μl) | RATIO | ||
|---|---|---|---|
| 5-HT | 5-HIAA | 5-HT/5-HIAA | |
| REST | 28.10 ± 4.52 | 14.98 ± 1.52 | 1.88 |
| EXERCISE | 39.73 ± 2.76 | 15.63 ± 0.5 | 2.54 |
| POST-EXERCISE | 36.98 ± 4.12 | 16.10 ± 0.87 | 2.3 |
Concentration of 5-HIAA during rest
Concentration of 5-HIAA gave an indication of serotonergic turnover in dorsal horn. 5-HIAA concentrations were 14.98 ± 1.52pg/10μl of microdialysate injected onto HPLC column (n=7) (Table 1). The ratio 5-HT to 5-HIAA is of 1.88 in dorsal horn.
Release of 5-HT during exercise period
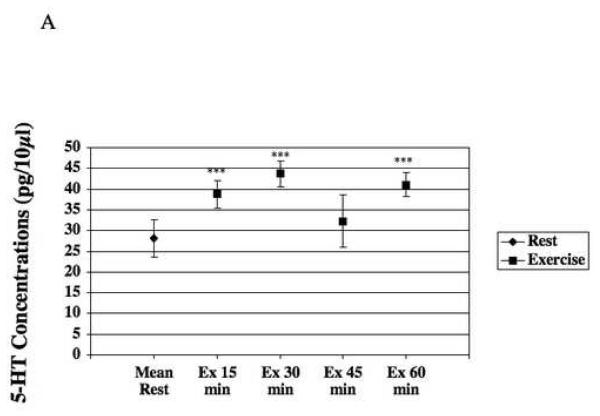
Exercise induced an increase in 5-HT release in dorsal horn (Fig. 3-A). During exercise, mean of 5-HT release was 39.73 ± 2.76pg/10μl (n=7), representing a significant (p≤0.005) increase of 41% when compared to mean at rest (Table 1). Figure 3-A illustrates the time course of changes in concentrations of 5-HT in the dialysate. There was a significant (p≤0.001) increase in 5-HT release of 38% within the first 15 minutes after the start of exercise when compared to rest, and this release was maintained at that level for 30 minutes. 5-HT release was significantly increased until the end of the exercise showing a continuous effect of exercise on 5-HT increased release.
Figure 3-A represents microdialysate concentrations of 5-HT (mean±s.e.m.) during rest, and exercise. There is a significant increase of 5-HT release (n=7; p≤0.001) 30, and 60 minute time points. At 45 minutes there was a higher variability and the increase does not show any significance. The release of 5-HT in the dorsal horn stays elevated during the entire motor activity.
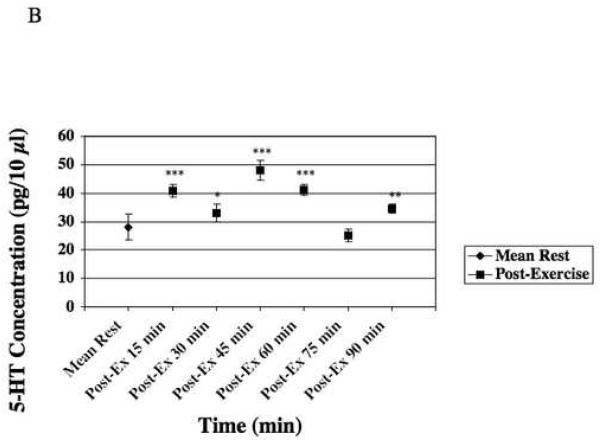
Figure 3-B represents microdialysate concentrations of 5-HT during rest, and during the 6 time points of the post-exercise rest period.
Concentrations of 5-HIAA during exercise period
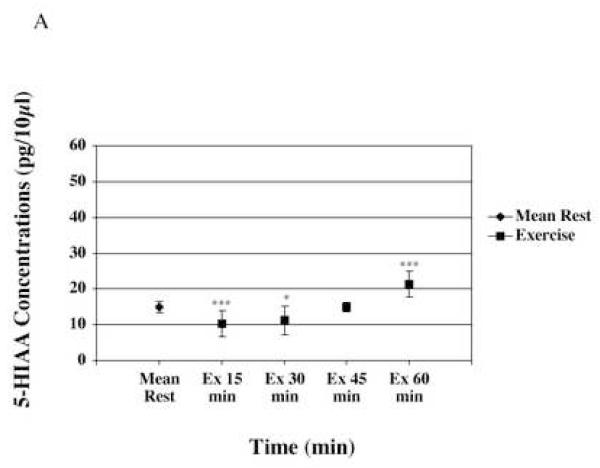
5-HIAA mean did not change during exercise when compared to rest. 5-HIAA mean value during exercise was of 15.63 ± 0.5 pg/10μl of microdialysate (n=7). However, individual time points show variations in 5-HIAA concentrations (Figure 4-A). 5-HIAA concentration was significantly (p≤0.001) decreased by 33% at the start of exercise, and this decrease persisted during the first 30 minutes of running exercise. At 60 minutes (between 45min and 60min of exercise) of exercise, 5-HIAA was increased of 33% when compared to rest.
Figure 4-A represents microdialysate concentrations of 5-HIAA during rest, and exercise. The end of exercise (60 minute time point), shows a significant increase in 5-HIAA concentration.
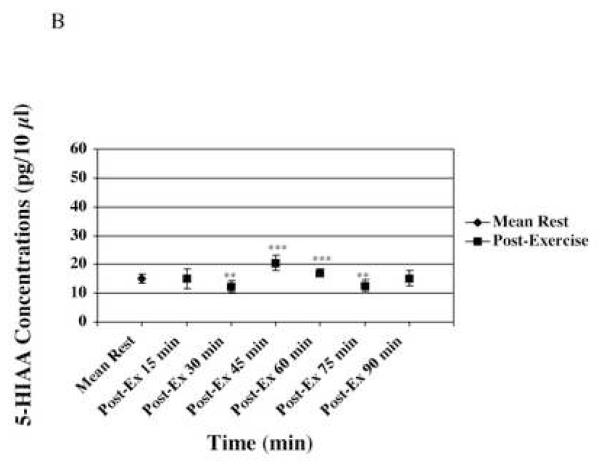
Figure 4-B represents microdialysate concentrations during rest, and the 6 time points of post-exercise rest periods. * represents comparison with the mean value during the rest period. * p≤0.05; ** p≤0.005; *** p≤0.001.
Release of 5-HT during post-exercise rest period
During post-exercise, mean of 5-HT concentration in microdialysate was of 36.98 ± 4.12pg/10μl (n=7), (Table 1, Fig. 3-B), which represented a significant (p≤0.005) increase of 32% when compared to rest and a slight decrease of 7% when compared to exercise. However, the most important differences between rest and post-exercise concentrations occurred during the first 60 minutes after the cessation of exercise. After 60 minutes of post-exercise rest, concentrations of 5-HT were comparable to those during rest prior to exercise. During the first 60 minutes of post-exercise, 5-HT concentrations oscillated until reaching concentrations approximating control rest concentrations.
Concentrations of 5-HIAA during post-exercise rest period
5-HIAA mean concentrations did not vary during the post-exercise period when compared to rest. 5-HIAA mean value of 16.10 ± 0.87pg/10μl (n=7) represented a non-significant 8% increase when compared to rest (Fig. 4-B).
Discussion
5-HT is released from the lumbar dorsal horn specifically
Histological characteristics such as absence of haemorrhage, cell number and special organization of tissue surrounding permanently implanted probes are consistent with neuronal local origin of the 5-HT measured. Importantly, 5-HT IR fibers are found within the dorsal horn in number and location that are consistent with local release of 5-HT from axon terminals within the vicinity of the probe. Additionally, evidence of local measure of 5-HT with the microdialysis probe is provided by comparison with our previous data, showing that 5-HT release in the dorsal horn is quantitatively and qualitatively different from the ventral horn release [5, 6, 7]. Therefore, eventual diffusion might not be efficient for distances longer than 150-200μm.
Possible neural population of origin
Authors [1] have also shown that raphe magnus nucleus neurons induced an increase in serotonin concentration the dorsal horn. We suggest that in our experimental paradigm, raphe magnus nucleus neurons might provide the 5-HT that is released within the dorsal horn during exercise.
Serotonin released in dorsal horn might be specific of motor exercise
5-HT release associated with motor activity might play an indirect role in modulating motoneuron (MN) response as it has already shown by others using different preparations [8, 10, 16]. No serotonergic inhibition during basal condition (no movement) has been found and author data suggest that dorsal horn serotonin involvement in motor function may involve release of 5-HT and, its effect on multiple 5-HT receptor subtypes including 1B, 2A, 2C, 3, 4 [10]. More specifically, 5-HT1A-R activation, within the dorsal horn, increased phrenic MN frequency [16]. In our experiments, the increased release of 5-HT was maintained over the entire running exercise, inducing an intense MN activation, which further suggests that 5-HT might be specific of exercise.
Serotonin release in dorsal horn is implicated in motor function
Jankowska et al. [8] showed that serotonin facilitates the activation of some spinocerebellar tract neurons therefore facilitating motor function. Later data [9] support the idea that 5-HT release (our study) has modulatory effect during locomotion in increasing the effectiveness of pre-setting motor response. Current changes observed in 5-HT extracellular concentrations in the dorsal horn are associated with motor activity. Within the dorsal horn 5-HT asymmetric synaptic junctions might explain some changes in turn over and metabolism.
During the post-exercise period 5-HT decreases
This decrease is observed when compared to exercise however, concentrations of 5-HT stayed elevated for the first 60 minutes of post-exercise rest period when compared to rest. This suggests that exercise induces a long lasting stimulation of raphe neurons (Fig. 3-B). Individual time points provided important information on the regulatory influence of locomotor function on neurotransmitter release and metabolism.
In conclusion our biogenic 5-HT release study confirms previous data [2, 3, 8, 9, 10, 13, 16] on the importance of serotonin towards motor function in an area free of MN such as dorsal horn. We provided here neurochemical evidence of biogenic origin of the temporal link between 5-HT release within the lumbar dorsal horn and locomotion.
Acknowledgments
This work was supported by the INSERM, IRME, Verticale, NS023868-12, GM59218-05006, HD039879-05, RR008630-127828.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Basbaum AI, Clanton CH, Fields HL. Three bulbospinal pathways from the rostral medulla of the cat: An autoradiographic study of pain modulating systems. J. Comp. Neurol. 1978;178:209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- [2].Diaz-Rios ME, Dombeck DA, Webb WW, Harris-Warrick RM. Serotonin modulates dendritic calcium influx in commissural interneurons in the mouse spinal locomotor network. J. Neurophysiol. 2007 doi: 10.1152/jn.00430.2007. 10.1152/jn.00430. [DOI] [PubMed] [Google Scholar]
- [3].Doly S, Fischer J, Brisorgueil MJ, Verge D, Conrath M. 5-HT5A receptor localization in the rat spinal cord suggests a role in nociception and control of pelvic floor musculature. J. Comp. Neurol. 1994;476:316–329. doi: 10.1002/cne.20214. [DOI] [PubMed] [Google Scholar]
- [4].Gerin C, Privat A. Evaluation of the function of microdialysis probes permanently implanted in the rat CNS and coupled to an on-line HPLC system of analysis. J. Neurosci. Methods. 1996;66:81–92. doi: 10.1016/0165-0270(96)00003-9. [DOI] [PubMed] [Google Scholar]
- [5].Gerin C, Privat A. Direct evidence for the link in-between monoaminergic descending pathways and motor activity. II. A study with microdialysis probes implanted in the ventral horn of the spinal cord. Brain Res. 1998;794:169–173. doi: 10.1016/s0006-8993(98)00278-9. [DOI] [PubMed] [Google Scholar]
- [6].Gerin C, Legrand A, Privat A. Study of 5-HT release with a chronically implanted microdialysis probe in the ventral horn of the spinal cord of unrestrained rats during exercise on a treadmill. J. Neurosci. Methods. 1994;52:129–141. doi: 10.1016/0165-0270(94)90121-x. [DOI] [PubMed] [Google Scholar]
- [7].Gerin C, Becquet D, Privat A. Direct evidence for the link in-between monoaminergic descending pathways and motor activity. I. A study with microdialysis probes implanted in the ventral funiculus of the spinal cord. Brain Res. 1995;704:191–201. doi: 10.1016/0006-8993(95)01111-0. [DOI] [PubMed] [Google Scholar]
- [8].Jankowska E, Hammar I, Djouhri L, Heden C, Lackberg Z. Szabo, Yin XK. Modulations of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. Eur. J. Neurosci. 1997;9:1375–87. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- [9].Jankowska E, Hammar I, Chojnicka B, Heden CH. Effect of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur. J. Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- [10].Liu FY, Xing GG, Qu XX, Xu IS, Han JS, Wan Y. Roles of 5-hydroxytryptamine (5-HT) receptor subtypes in the inhibitory effects of 5-HT on C-fiber responses of spinal wide dynamic range neurons in rats. J. Pharmacol. Exp. Ther. 2007;321(3):1046–53. doi: 10.1124/jpet.106.115204. [DOI] [PubMed] [Google Scholar]
- [11].Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J. Comp. Neurol. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- [12].Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sydney: 1982. [DOI] [PubMed] [Google Scholar]
- [13].Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res. Bul. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- [14].Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- [15].Westerink BH, Tuinte MH. Chronic use of intracerebral dialysis for the in vivo measurement of 3,4-dihydroxyphenylethylamine and its metabolite 3,4-dihydroxyphenylacetic acid. J. Neurochem. 1986;46:181–185. doi: 10.1111/j.1471-4159.1986.tb12942.x. [DOI] [PubMed] [Google Scholar]
- [16].Zimmer MB, Goshgarian HG. Spinal activation of serotonin 1A receptors enhances latent respiratory activity after spinal cord injury. J. Spinal Cord Med. 2006;29:147–155. doi: 10.1080/10790268.2006.11753868. [DOI] [PMC free article] [PubMed] [Google Scholar]