Abstract
Phenotypic modulation of airway smooth muscle (ASM) is an important feature of airway remodeling in asthma that is characterized by enhanced proliferation and secretion of pro-inflammatory chemokines. These activities are regulated by the concentration of free Ca2+ in the cytosol ([Ca2+]i). A rise in [Ca2+]i is normalized by rapid reuptake of Ca2+ into sarcoplasmic reticulum (SR) stores by the sarco/endoplasmic reticulum Ca2+ (SERCA) pump. We examined whether increased proliferative and secretory responses of ASM from asthmatics result from reduced SERCA expression. ASM cells were cultured from subjects with and without asthma. SERCA expression was evaluated by western blot, immunohistochemistry and real-time PCR. Changes in [Ca2+]i, cell spreading, cellular proliferation, and eotaxin-1 release were measured. Compared with control cells from healthy subjects, SERCA2 mRNA and protein expression was reduced in ASM cells from subjects with moderately severe asthma. SERCA2 expression was similarly reduced in ASM in vivo in subjects with moderate/severe asthma. Rises in [Ca2+]i following cell surface receptor-induced SR activation, or inhibition of SERCA-mediated Ca2+ re-uptake, were attenuated in ASM cells from asthmatics. Likewise, the return to baseline of [Ca]i after stimulation by bradykinin was delayed by approximately 50% in ASM cells from asthmatics. siRNA-mediated knockdown of SERCA2 in ASM from healthy subjects increased cell spreading, eotaxin-1 release and proliferation. Our findings implicate a deficiency in SERCA2 in ASM in asthma that contributes to its secretory and hyperproliferative phenotype in asthma, and which may play a key role in mechanisms of airway remodeling.
Asthma is a chronic inflammatory disease which is accompanied by extensive changes in normal airway tissue architecture, termed remodeling (1, 2). Airway remodeling in asthma comprises epithelial dysfunction, hypertrophy of the mucus glands, subepithelial vascularization, and changes in extracellular matrix composition (2). In addition, airway smooth muscle (ASM) from people suffering with asthma exhibits enhanced proliferative (3) and migratory responses (4, 5), as well as increased secretion of a myriad of pro-inflammatory cytokines/chemokines and growth factors (6). The mechanisms that underly the exaggerated function of ASM in asthma are unknown.
Smooth muscle responses to diverse stimuli are controlled by changes in the concentration of free cytosolic Ca2+ ([Ca2+]i). Elevation of [Ca2+]i results from increased Ca2+ influx across the plasma membrane following activation of Ca2+-permeable ion channels and the Na+-Ca2+-exchanger (NCX, 3Na+:1Ca2+), and by release of stored Ca2+ from the sarcoplasmic reticulum (SR), in turn triggered by inositol 1,4,5-triphosphate (IP3) or ryanodine receptor (RyR) channels (7). Termination of the cytosolic Ca2+ signal occurs by extracellular removal of cytosolic Ca2+ by the NCX and by its rapid sequestration into SR stores by the sarco/endoplasmic reticulum Ca2+ (SERCA) pump (7). Impaired replenishment of SR stores arising from reduced activity of the SERCA pump could impact on a wide range of Ca2+-dependent smooth muscle functions (8) and abnormal Ca2+ handling by ASM has previously been proposed to be an important determinant of the airway hyperresponsiveness that is characteristically present in asthma (9, 10).
There are 3 tissue-specific members of the mammalian SERCA family, SERCA1, SERCA2 and SERCA3, each encoded by a separate gene (ATP2A1, ATP2A2, and ATP2A3) (11), with SERCA2 being the most highly expressed in smooth muscle (12, 13). The function of the different isoforms of SERCA2 is similar (14). We have investigated if the secretory and hyperproliferative phenotype of ASM in asthma is associated with impaired SERCA isoform expression.
Results
SERCA2 Expression.
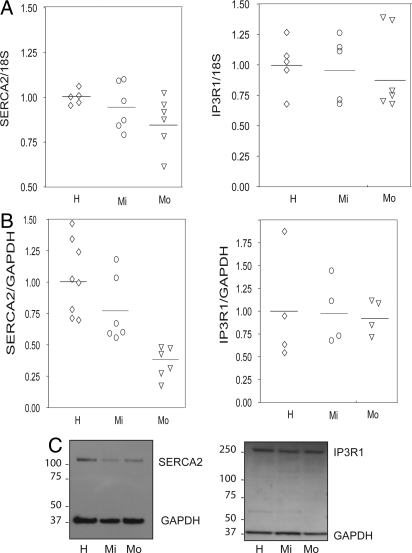
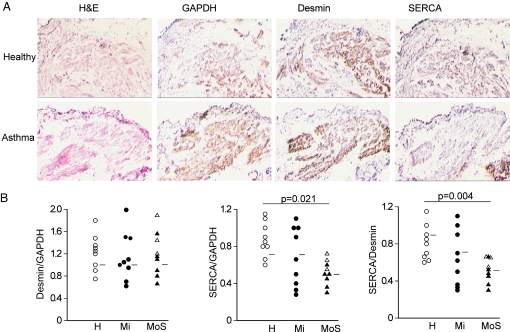
SERCA2 mRNA expression was reduced in ASM cells cultured from patients with moderate, but not mild asthma compared with cells derived from healthy subjects (P = 0.04, Fig. 1A). Western immunoblot showed a single band for SERCA2 at the expected size (≈110 kDa) in ASM lysates (Fig. 1). SERCA2 protein expression was correspondingly reduced in ASM cells from patients with moderate asthma (P = 0.015, Fig. 1B). In contrast, IP3R1 mRNA and protein expression did not differ between asthmatics and controls (Fig. 1 A and B), suggesting the change in SERCA2 was not the result of a reduction in total SR. Transcripts for SERCA1, and SERCA3 were not detected in ASM. Further experiments using SERCA2A, SERCA2B, and SERCA2C specific primers demonstrated that predominant isoform in ASM is SERCA2B with the other isoforms expressed at very low levels around the limit of detection. The pattern of expression of SERCA2B was similar to that shown in Fig. 1A for total SERCA2. Reduced SERCA2 expression in asthma was also detected in ASM in vivo in tissue sections of endobronchial biopsies, when compared with desmin (31 ± 6%) or GAPDH expression (33 ± 11%) in 9 moderate to severe asthmatics (Fig. 2). There was no statistical correlation between SERCA expression and FEV1 and MCh PC20. The correlation coefficients were 0.44 for FEV1 (P = 0.09) and 0.29 for PC20 (P = 0.29).
Fig. 1.
Reduced expression of SERCA2, but not IP3 receptor-1 in airway smooth muscle cells from subjects with asthma. (A) Expression of SERCA2 and IP3 receptor-1 (IP3R1) mRNA evaluated using real-time PCR in 5 healthy (H, diamonds), 6 mild asthmatic (Mi, circles), and 6 moderate asthmatic (Mo, triangles) subjects. (B) SERCA2 and IP3R1 protein expression evaluated using western immunoblot followed by densitometry in 8 healthy (H, diamonds), 6 mild asthmatic (Mi, circles), and 6 moderate asthmatic (Mo, triangles) subjects. (C) Western immunoblots for SERCA2 and IP3R1 and the internal control, GAPDH, showing a band around 110 kDa (SERCA2), a band around 37 kDa (GAPDH), and a band around 240 kDa (IP3R1).
Fig. 2.
SERCA2 expression in airway smooth muscle tissue sections from subjects with and without asthma. Histology and immunohistochemistry in serial frozen sections of deep endobronchial biopsies from healthy subjects (H, open circle, n = 9) or those with mild (Mi, closed circle, n = 9) or moderate/severe asthma (MoS, open triangle/closed triangle, n = 9). (A) Airway smooth muscle is shown with hematoxylin-eosin (H&E) staining and by immunoreactivity for desmin (smooth muscle marker) as well as GAPDH or SERCA2. (B) Quantitation of SERCA2 immunoreactivity. (Mann-Whitney U test for statistical comparison).
Calcium Homeostasis.
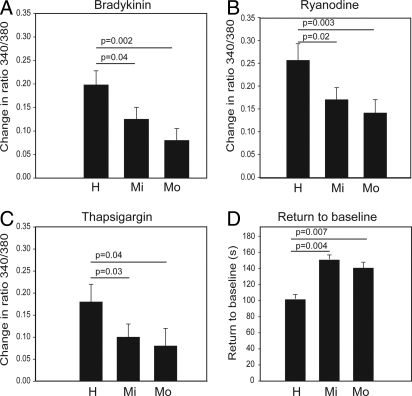
Transient peak Ca2+ responses to bradykinin (BK) were reduced by 36 ± 12% (P = 0.04) and by 59 ± 13% (P = 0.002) in 11 mild and 13 moderate asthmatic subjects, respectively, compared with 9 controls (Fig. 3A). Because BK induces Ca2+ release from the SR through an IP3-dependent pathway in ASM (15), the reduced response suggests that SR stores are partially depleted in ASM cells from asthmatics. This was confirmed by the finding that SR emptying by ryanodine (Fig. 3B) and by thapsigargin (TSG) (Fig. 3C) also elicited less intracellular Ca2+ release in asthmatic ASM cells. The ryanodine peak response was reduced by 33 ± 9% in 6 mild and 45 ± 11% in 6 moderate asthmatics when compared with 5 healthy subjects (P = 0.02 and P = 0.003, respectively). TSG-induced Ca2+ release was reduced by 44 ± 12% and 56 ± 19% in 11 mild asthmatics (P = 0.03) and 13 moderate asthmatics (P = 0.04), respectively, compared with 9 healthy subjects. Further support implicating diminished SERCA2 in the altered Ca2+ regulation in ASM from asthmatics was evident from the increased time interval taken for BK-elevated [Ca2+]i to return to baseline in asthmatic ASM (148 ± 5 s, P = 0.004; 145 ± 6 s, P = 0.007 in 6 mild and 6 moderate asthmatic subjects, respectively) compared with cells from 5 healthy subjects (101 ± 8 s) (Fig. 3D).
Fig. 3.
Ratiometric measurements of calcium store release in airway smooth muscle cells from subjects with and without asthma. Cytosolic calcium was measured by fura PE-3 in healthy controls (H), mild (Mi), and moderate (Mo) asthmatic subjects. (A and B) mean (SEM) peak responses after activation by 1 μM bradykinin and 1 μM ryanodine, respectively in 5 healthy, 6 mild, and 6 moderate asthmatic subjects; (C) mean (SEM) peak responses after emptying of sarcoplasmic reticulum (SR) stores by SERCA blockade with 0.1 μM thapsigargin in the absence of extracellular calcium in 9 healthy, 11 mild and 13 moderate asthmatic subjects; and (D) mean ± SEM time interval taken for the peak response to return to baseline following SR store emptying with 1 μM bradykinin (Mann-Whitney U test for statistical comparison).
Functional Consequences of SERCA2 Knockdown.
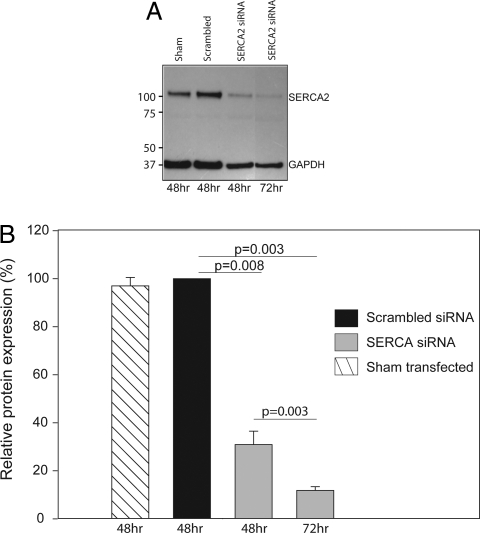
Western immunoblot confirmed knockdown of SERCA2 protein (>90%) after transfection for 72 h with a SERCA2 targeting siRNA (P = 0.003, Fig. 4 A and B). This was associated with a >50% reduction in TSG (10 μM)-induced Ca2+ store emptying, showing that Ca2+ signaling responses continued to function at low levels of SERCA. SERCA2 levels and responses to TSG were unaffected in ASM cells transfected with the scrambled siRNA (Fig. 4) and those that had been sham-transfected. In addition, there was a delayed recovery of BK-elevated [Ca2+]i to baseline (scrambled siRNA: 133 ± 21 s; SERCA2 siRNA: 221 ± 40 s; n = 4, P = 0.004) in the healthy ASM cells, consistent with the impaired SERCA function and similar to the delayed recovery seen in asthmatic ASM (see above and Fig. 3D).
Fig. 4.
siRNA-mediated knock-down of SERCA2 in ASM from subjects with and without asthma. ASM cells were either sham-tranfected, transfected with scrambled non-targeting siRNA or SERCA siRNA. Protein was isolated after nucleofactor mediated transfection. (A) Expression of SERCA2 and GAPDH protein in total cell lysates using western immunoblot in 1 representative experiment. (B) SERCA2 protein levels are quantified by denstometry. Bars, mean ± SEM SERCA expression in ASM from 4 different subjects.
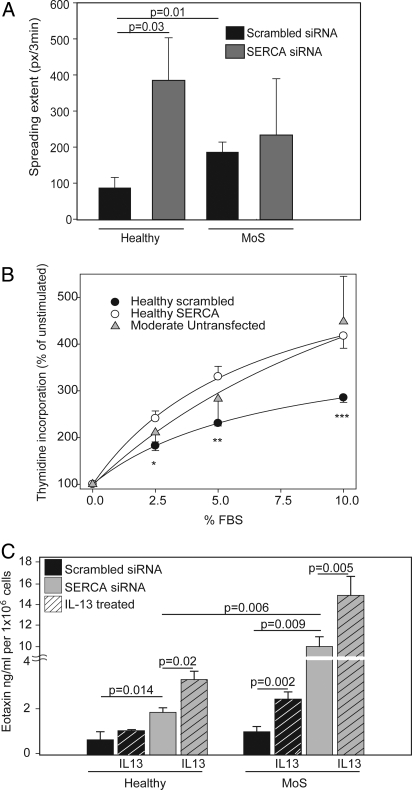
In a cell spreading assay, ASM cells from asthmatics treated with the scrambled siRNA oligosequence spread faster ex vivo than similarly transfected ASM from healthy subjects (P = 0.01, Fig. 5A). SERCA2 knockdown by siRNA in healthy ASM cells markedly enhanced spreading (P = 0.03). Increased spreading was also observed with SERCA2 knockdown in asthmatic ASM cells, although this was not significantly elevated (P > 0.05).
Fig. 5.
Functional consequences of SERCA2 knockdown. (A) ASM cells from 4 healthy controls and 4 moderate/severe (MoS) asthmatics were imaged by phase-contrast microscopy during spreading (0 to 240 min) after transfection with either SERCA2 targeting siRNA or non-targeting (scrambled) siRNA. (B)Proliferation of subconfluent cultures of ASM cells from 3 healthy volunteers after transfection with SERCA2-targeting siRNA (open circles) compared with the same cells transfected with non-targeting scrambled siRNA (filled circles) in response to increasing concentrations of FBS (FBS); *, P = 0.017; **, P = 0.002; ***, P = 0.001 (RM ANOVA). Proliferation of ASM cells from 3 subjects with moderate asthma is included for comparison (gray triangles). (C) Eotaxin-1 (CCL-11) release quantified by ELISA by ASM cells from 6 healthy subjects (left hand panels) or 6 moderate/severe asthmatics (MoS; right hand panels). Cells were transfected with either non-targeting (scrambled) siRNA (black bars) or SERCA2-targeting siRNA (gray bars). All data are depicted as the mean±SEM. Statistical analysis by Mann-Whitney U test.
FBS-dependent proliferation of cultured healthy ASM cells was enhanced following siRNA-mediated SERCA2 knockdown, compared with cells transfected with a scrambled control RNA sequence (Fig. 5B). The increased proliferation of healthy ASM after SERCA2 knockdown was similar to that observed in ASM grown directly ex vivo from subjects with moderate asthma (Fig. 5B). Cells from healthy subjects following SERCA2 knockdown showed earlier formation of laminopodia (fan-like projections) accompanied by several outgrowths (filapodia) than cells receiving non-targeted siRNA. Similarly, cells from subjects with moderate asthma developed earlier laminopodia compared with healthy subjects.
Interleukin (IL)-13 (10 ng/mL) induced the release of eotaxin-1 (CCL-11) from ASM cells cultured from either healthy or asthmatic subjects. IL-13-stimulated eotaxin-1 release was greater in ASM cells cultured from asthmatics, compared with similar cells from healthy subjects. siRNA-mediated SERCA2 knockdown potentiated both the constitutive and IL-13-stimulated components of eotaxin-1 release in ASM cultured from healthy subjects. A further increase in eotaxin-1 release was observed after siRNA-mediated SERCA2 knockdown in ASM from asthmatics in which SERCA2 protein expression was already constitutively reduced (Fig. 5C).
Discussion
ASM cells from asthmatic patients showed diminished expression of SERCA2 to a degree which correlated with the severity of disease. siRNA-targeted SERCA2 knockdown in healthy ASM reproduced many features of the asthmatic ASM cell phenotype observed directly ex vivo including slowed recovery from an elevation of [Ca2+]i, enhanced cellular motility, proliferation and secretion. These findings provide compelling evidence that abnormal Ca2+ homeostasis associated with constitutively depleted SERCA2 expression underlies the abnormal secretory and hyperproliferative phenotype of asthmatic ASM.
mRNA transcripts for either the skeletal (SERCA1) or non-muscle (SERCA3) isoforms were not detected in human ASM cells, consistent with previous findings (12). Alternative splicing of exon 20 of ATP2A2 gives rise to SERCA2A, SERCA2B, and SERCA2C mRNA transcripts, which differ in the final exon usage (16). SERCA2C results from inclusion of a short intronic sequence containing an in-frame stop codon between exons 20 and 21 of SERCA2A and has only recently been identified (12). The real time PCR primer we used for most of the experiments was intron spanning and amplified all 3 splice variants. In some experiments, we used SERCA2A, SERCA2B, and SERCA2C specific primers and demonstrated that the predominant isoform in ASM is SERCA2B. The other splice variants are expressed at very low levels around the limit of detection. The SERCA2B specific primers reveal a similar pattern of diminished SERCA2 expression in the moderate asthmatics as seen for the total SERCA2 probeset in Fig. 1A. The SERCA2 antibody we used did not distinguish the 110 kDa SERCA2A, SERCA2B, and SERCA2C protein isoforms, although SERCA2B is reported to be the most abundant isoform in smooth muscle (13). Thus, the major isoform contributing to the abnormal phenotype of ASM present in remodeled airways in asthma is probably SERCA2B.
In addition to diminished SERCA2 expression, our data clearly suggest diminished ability of SERCA2 to replete SR Ca2+ stores in asthmatic ASM cells, since partial emptying of these stores in these cells by bradykinin and ryanodine, or complete emptying by thapsigargin (TSG) resulted in an attenuated rise in free [Ca2+]i, which was also slower to return to the steady state. To investigate the functional consequences of SERCA2 depletion we chose a series of asthma-relevant, Ca2+-dependent functional endpoints, including cell spreading/motility (5, 17–19), NFAT-dependent production of mediators such as eotaxin-1 (6) and proliferation (3). In each case, siRNA-mediated functional knockdown of SERCA2 in ASM cells from healthy donors reproduced, and in some cases enhanced, the abnormalities found in ASM cells obtained directly from asthmatics ex vivo.
Our findings share broad similarities with an earlier study by Trian and colleagues who suggested that enhanced proliferation of ASM cells from asthmatics results from increased mitochondrial biogenesis (20). This was attributed to their finding that Ca2+ responses induced by acetylcholine in ASM from asthmatics appeared more sustained and prolonged. It was proposed that enhanced external Ca2+ entry was responsible because removal of external Ca2+ normalized the altered Ca2+ homeostasis in asthmatic ASM. However, the prolonged removal of external Ca2+ also depletes SR stores and prevents refilling, thereby interrupting normal SERCA2 function (21, 22). Both studies agree that Ca2+ homeostasis is deregulated in ASM cells from subjects with asthma, and that this contributes to the development of the asthmatic phenotype of these cells.
Overall our study demonstrates that total SERCA2 protein expression is reduced in ASM from asthmatics. Depleted SERCA-function leading to abnormal calcium homeostasis in asthmatic ASM was associated with a sustained increase in [Ca2+]i and enhanced proliferative and secretory function. The highly non-linear 4:1 stoichiometry of the calcium-calmodulin-interaction implies that even a small subthreshold increase in [Ca2+]i could greatly increase agonist induced responses (23). There are parallels here with other human diseases (12). Of particular interest is Brody's disease (24), an autosomal recessive condition most commonly caused by premature stop codons or point mutations in ATP2A1 (encoding a truncated SERCA1). Although the expression of SERCA1 in these patients is normal, its function is reduced by approximately 50%. This does not affect the amplitude of [Ca2+]i transients evoked by agonists, but it markedly slows their decay. Delay in reuptake of Ca2+ is consistent with the known pathology of muscle cramping and impairment of relaxation in this disease.
In conclusion, disruption in expression and activity of the pivotal SERCA2 Ca2+ pump could be fundamental to many features of exaggerated smooth muscle function in the airways of asthmatics, such as the increased proliferation and enhanced cytokine expression, that contribute to airway remodeling. Our data directly support the proposal that Ca2+ handling in ASM from asthmatics is abnormal (11, 12). Restoring SERCA2 function in ASM from asthmatics could provide a therapeutic approach in asthma.
Methods
Patients.
Fibreoptic bronchoscopy was performed with approval by the Research Ethics Committees of King's College Hospital (study #11–03-209) and Guy's and St. Thomas' Hospitals (study #05/Q0704/72). All volunteers provided written informed consent. Deep endobronchial biopsies were obtained using Olympus FB-35C-1 forceps, as previously described (6) from 11 healthy volunteers (6M; 5F), 12 steroid naïve patients with mild asthma (7M; 5F), and 13 patients with moderate to severe asthma as defined by GINA guidelines (25) of whom 3 were steroid naïve (8M; 5F). Healthy subjects had a mean predicted forced expiratory volume in 1 s (FEV1) of 111 ± 3.8% (mean ± SEM) and a PC20 to methacholine (MCh) of >16 mg/mL; mild asthmatics were all steroid naïve with an FEV1 of 98 ± 3.7% predicted and a PC20 MCh of <8 mg/mL (1.9 ± 0.6 mg/mL); and moderate/severe asthmatics were on a stable dose of inhaled corticosteroids (fluticasone propionate <500 μg/daily or equivalent) with a mean FEV1 of 72 ± 2.6% predicted and a PC20 to MCh <1 mg/mL (0.6 ± 0.2 mg/mL) and >15% reversibility in FEV1 to 400 μg inhaled salbutamol. All participants were either non-smokers or an ex-smoker of at least 6 months with less than a 10-pack year history.
Cell Culture.
ASM cells were grown from bronchial biopsies by explant culture, as described previously (6). Fluorescent immunocytochemistry was used routinely to confirm that near-confluent, FBS (FBS)-deprived ASM cells labeled positively (>95%) for the muscle phenotype marker proteins smooth muscle-specific α-actin, desmin, and calponin (26). Cells in passages 3–8 were used in all experiments.
Gene Expression.
RNA was isolated from whole cell lysates of approximately 1 × 106 cells using RNeasy mini kits (Qiagen, Inc.). RNA quality and quantity were assessed using a NanoDrop Bioanalyzer and 1 μg total RNA from each ASM culture was reverse transcribed (You prime first beads). Reaction mixtures containing cDNA (2 ng/reaction) were incubated for 2 min at 50 °C followed by 5 min at 95 °C and run for 38 cycles. Assays-on-Demand primers (Applied Biosystems) were used in the PCR as follows: 4319413E (18S rRNA), Hs00181881_m1 (IP3R1), Hs00544877_m1 (ATP2A1/SERCA1), Hs00544877_m1 (total ATP2A2/SERCA2), Hs00155939_m1 (ATP2A2A/SERCA2A), and Hs00193090_m1 (ATP2A3/SERCA3). Analysis of relative gene expression was by the 2−ΔΔCT method (27). The gene accession numbers for SERCA2A, SERCA2B, and SERCA2C are NM_001681.3, NM_170665.3, and NM_001135765.1. Isoform specific real time probes for SERCA2B and C were designed using the Universal Probe Library (UPL) (Roche). SERCA2B flanking primers were: TTAAAGCAACTGTCTATTTCTGCTG and AGTCAGAAAAAGCAAAACAAAATCTA with UPL9. SERCA2C flanking primers were: CTCTGCTTGAGGGGAAGAAG and GGTTTAGGAAGCGGTTACTCC with UPL15.
Protein Expression.
Total protein lysates from ASM cells were prepared and 10 μg/lane total protein separated by SDS-polyacrylamide gel electrophoresis as previously described (6). Total SERCA2 was detected using a mouse monoclonal antibody (ab2817, Abcam) at 1:2,500, GAPDH was detected with a mouse monoclonal antibody (ab8245, Abcam) at 1:3,000, and IP3R was detected using a rabbit polyclonal antibody (07–514, Upstate) at 1:2,500. Primary antibodies were detected using goat-anti-mouse IgG (for SERCA/GAPDH) and goat-anti-rabbit IgG (for IP3R) horseradish peroxidise-conjugated secondary antibody. Analysis was by densitometry as previously described (6).
Immunohistochemistry.
Processing of biopsies was performed as previously described (28). Monoclonal antibodies against the smooth muscle marker protein desmin (clone no. DE-U-10, 1:20) and against SERCA2 ATPase (clone no. IID8, 1:200) were purchased from Sigma and Abcam, respectively. GAPDH was detected using a mouse monoclonal antibody (clone no. 6C5, 1:250, ab8245, Abcam) as above. Primary antibodies were detected with donkey anti-mouse IgG (1/50) and prostatic acid phosphatase (PAP) (1/50) (Jackson Immunoresearch, 19390). Signals were developed with Fast DAB (3,'3-diaminobenzidine) and histological sections counterstained with Mayer's hematoxylin. Positive cells stained brown after development with Fast DAB. Histological sections were analyzed in a blinded fashion and desmin, GAPDH and SERCA2 immunoreactivity scored as previously described (28). The results were expressed as ratio of SERCA2/desmin and SERCA2/GAPDH.
Calcium Measurements.
ASM cells on glass coverslips (≈1 × 103/coverslip) were incubated for 90 min at room temperature in Hepes-buffered physiological salt solution (PSS) containing 0.5 μM of the ratiometric Ca2+ indicator Fura PE-3/AM. Cells on coverslips were mounted on an inverted microscope (60× fluor objective) combined with a double-excitation microfluorimeter. The light emitted by the cells at >510 nm during excitation at wavelengths of 340 and 380 nm was recorded. The ratio of the intensities of emission (R340/380) was taken as a measure of [Ca2+]i. Cells were continuously superfused with PSS containing (in mM) 140 NaCl, 5.9 KCl, 1.2 NaH2PO4, 5 NaHCO3, 1.4 MgCl2, 1.8 CaCl2, 11.5 glucose, and 10 Hepes, pH 7.4. Cells were studied for 40–60 min during continuous superfusion with PSS with test reagents added into the superfusate as described previously (29).
SERCA2 Knockdown.
ASM cells were transfected using the Basic Nucleofector Kit for Primary Smooth Muscle Cells according to the manufacturer's protocol. Briefly, 1 × 106 cells were suspended in 100 μL Basic Nucleofector Solution with 0.7 μg either SERC2A targeted siRNA (ON-TARGETplus SMARTpool, L-004082–00-0005) or a scrambled RNA oligosequence (siCONTROL Non-targeting siRNA Pool #1, D-001206–13-05) and electroporated using the Amaxa Nucleofector Device. Cells were re-suspended in 0.5 ml DMEM containing 10% FBS before seeding in 100-mm diameter dishes containing DMEM with 10% FBS. After 72 h, cells were harvested and reseeded in DMEM without FBS for Ca2+ measurements, protein isolation, eotaxin-1 release, cell spreading, and cellular proliferation.
Cell Spreading.
ASM cells in suspension were seeded into viewing chambers on the heated (37 °C) stage of an IX71 Olympus inverted microscope and left for 30 min to attach. TIFF images were collected every 30 s for 4 h during cell spreading and image stacks split into individual frames and viewed in Adobe Photoshop, Version 8.0 (Adobe Systems). Individual cells in each image were then outlined using the pencil tool to leave a thick white line around the cell periphery. Frame-by-frame binary images of filled white cells on a black background were produced and imported into Mathematica 6.01 (Wolfram Research) to determine cell areas. Ten frames were selected 3 minutes apart and the difference in area determined between 2 consecutive frames. Mean differences were determined to give a value for cell extension rate in pixels/3 min (30).
Cellular Proliferation.
ASM cells (≈9 × 103/well) in 96-well plates were allowed to attach (24 h) before FBS deprivation for 72 h and stimulation for 4 h with DMEM containing 2.5%, 5%, or 10% FBS. Cells were pulsed with 0.5μCi/well [3H]thymidine (Amersham) and incubated for a further 48 h before plates were frozen (−20 °C) before analysis. After thawing, cells were harvested (Perkin-Elmer cell harvester) onto fiberglass Easy tab-C self aligning filters (Packard Bioscience). Scintillation fluid was added onto the filters in 96-well holder plates and sealed before reading using a β counter.
Eotaxin-1 Release.
ASM (≈60 × 103 cells/well) were seeded into plastic 24-well plates and left overnight at 37 °C. After attachment, cells were FBS-deprived for 72 h and then incubated for 24 h in FBS-free RPMI-1640 (0.5 mL) with or without 10 ng/mL IL-13 (R&D Systems). Cell-free supernatants were collected and stored at −80 °C for later analysis by ELISA. Eotaxin-1 levels were determined in duplicate by specific sandwich ELISA as described previously (6).
Statistical Analysis.
Results are expressed as mean ± SEM; data were compared using 1-way non-parametric ANOVA, 2-way repeated measures ANOVA, or the Mann Whitney U Test as appropriate (SigmaStat, Systat Software Inc.). A difference was deemed significant if P < 0.05.
Acknowledgments.
We thank Amanda Chaytor, Wei-Yee James, Kheem Jones, Marianne Morgan, Cherylin Reinholtz, Karen Williams, Philip Aaronson, and Gianna Chadwick for their invaluable contributions. This work was supported by the Medical Research Council; Asthma U.K. Grants 05/022, 05/027, and 07/034; the Dr. Hadwen Trust for Humane Research; Wellcome Trust Grant 078075;the Guy's and St. Thomas's National Health Service Foundation Trust and King's College London National Institute for Health Research Biomedical Research Centre.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Hirst SJ, Walker TR, Chilvers ER. Phenotypic diversity and molecular mechanisms of airway smooth muscle proliferation in asthma. Eur Respir J. 2000;16:159–177. doi: 10.1034/j.1399-3003.2000.16a28.x. [DOI] [PubMed] [Google Scholar]
- 2.Solway J, Fredberg JJ. Perhaps airway smooth muscle dysfunction contributes to asthmatic bronchial hyperresponsiveness after all. Am J Respir Cell Mol Biol. 1997;17:144–146. doi: 10.1165/ajrcmb.17.2.f137. [DOI] [PubMed] [Google Scholar]
- 3.Johnson PR, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- 4.Kaur D, et al. Airway smooth muscle and mast cell-derived CCL19 mediate airway smooth muscle migration in asthma. Am J Respir Crit Care Med. 2006;174:1179–1188. doi: 10.1164/rccm.200603-394OC. [DOI] [PubMed] [Google Scholar]
- 5.Kanabar V, Simcock D, Mahn K, O'Connor BJ, Hirst SJ. Airway smooth muscle migration is increased in asthmatics. Proc Am Thorac Soc. 2006;3:A280. [Google Scholar]
- 6.Chan V, et al. Extracellular matrix regulates enhanced eotaxin expression in asthmatic airway smooth muscle cells. Am J Respir Crit Care Med. 2006;174:379–385. doi: 10.1164/rccm.200509-1420OC. [DOI] [PubMed] [Google Scholar]
- 7.Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- 8.Sathish V, et al. Regulation of sarcoplasmic reticulum Ca2+ reuptake in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294:L787–796. doi: 10.1152/ajplung.00461.2007. [DOI] [PubMed] [Google Scholar]
- 9.Parameswaran K, Janssen LJ, O'Byrne PM. Airway hyperresponsiveness and calcium handling by smooth muscle: A “deeper look. ”. Chest. 2002;121:621–624. doi: 10.1378/chest.121.2.621. [DOI] [PubMed] [Google Scholar]
- 10.Triggle DJ. Calcium, the control of smooth muscle function and bronchial hyperreactivity. Allergy. 1983;38:1–9. doi: 10.1111/j.1398-9995.1983.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 11.Grover AK, Khan I. Calcium pump isoforms: Diversity, selectivity and plasticity. Review article. Cell Calcium. 1992;13:9–17. doi: 10.1016/0143-4160(92)90025-n. [DOI] [PubMed] [Google Scholar]
- 12.Hovnanian A. SERCA pumps and human diseases. Subcell Biochem. 2007;45:337–363. doi: 10.1007/978-1-4020-6191-2_12. [DOI] [PubMed] [Google Scholar]
- 13.Vallot O, et al. Intracellular Ca(2+) handling in vascular smooth muscle cells is affected by proliferation. Arterioscler Thromb Vasc Biol. 2000;20:1225–1235. doi: 10.1161/01.atv.20.5.1225. [DOI] [PubMed] [Google Scholar]
- 14.Prasad V, Okunade GW, Miller ML, Shull GE. Phenotypes of SERCA and PMCA knockout mice. Biochem Biophys Res Commun. 2004;322:1192–1203. doi: 10.1016/j.bbrc.2004.07.156. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Freyer AM, Hall IP. Bradykinin activates calcium-dependent potassium channels in cultured human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L898–907. doi: 10.1152/ajplung.00461.2005. [DOI] [PubMed] [Google Scholar]
- 16.Hauser K, Pavlovic N, Kissmehl R, Plattner H. Molecular characterization of a sarco(endo)plasmic reticulum Ca2+-ATPase gene from Paramecium tetraurelia and localization of its gene product to sub-plasmalemmal calcium stores. Biochem J. 1998;334(Pt 1):31–38. doi: 10.1042/bj3340031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azuma T, Witke W, Stossel TP, Hartwig JH, Kwiatkowski DJ. Gelsolin is a downstream effector of rac for fibroblast motility. EMBO J. 1998;17:1362–1370. doi: 10.1093/emboj/17.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paulhe F, Bogyo A, Chap H, Perret B, Racaud-Sultan C. Vascular smooth muscle cell spreading onto fibrinogen is regulated by calpains and phospholipase C. Biochem Biophys Res Commun. 2001;288:875–881. doi: 10.1006/bbrc.2001.5859. [DOI] [PubMed] [Google Scholar]
- 19.Wolgemuth CW. Lamellipodial contractions during crawling and spreading. Biophys J. 2005;89:1643–1649. doi: 10.1529/biophysj.105.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trian T, et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med. 2007;204:3173–3181. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble K, Matthew A, Burdyga T, Wray S. A review of recent insights into the role of the sarcoplasmic reticulum and Ca entry in uterine smooth muscle. Eur J Obstet Gynecol Reprod Biol. 2009;144(Supp)(1):S11–S19. doi: 10.1016/j.ejogrb.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Brittsan AG, et al. Chronic SR Ca2+-ATPase inhibition causes adaptive changes in cellular Ca2+ transport. Circ Res. 2003;92:769–776. doi: 10.1161/01.RES.0000066661.49920.59. [DOI] [PubMed] [Google Scholar]
- 23.Amrani Y, Panettieri RA., Jr Modulation of calcium homeostasis as a mechanism for altering smooth muscle responsiveness in asthma. Curr Opin Allergy Clin Immunol. 2002;2:39–45. doi: 10.1097/00130832-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Benders AA, et al. Ca2+ homeostasis in Brody's disease. A study in skeletal muscle and cultured muscle cells and the effects of dantrolene an verapamil. J Clin Invest. 1994;94:741–748. doi: 10.1172/JCI117393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bateman ED, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 26.Hirst SJ, Twort CH, Lee TH. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am J Respir Cell Mol Biol. 2000;23:335–344. doi: 10.1165/ajrcmb.23.3.3990. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Ying S, et al. Associations between IL-13 and IL-4 (mRNA and protein), vascular cell adhesion molecule-1 expression, and the infiltration of eosinophils, macrophages, and T cells in allergen-induced late-phase cutaneous reactions in atopic subjects. J Immunol. 1997;158:5050–5057. [PubMed] [Google Scholar]
- 29.Snetkov VA, et al. Low concentrations of sphingosylphosphorylcholine enhance pulmonary artery vasoreactivity: The role of protein kinase C delta and Ca2+ entry. Hypertension. 2008;51:239–245. doi: 10.1161/HYPERTENSIONAHA.107.104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt MR, et al. Quantifying cell–matrix adhesion dynamics in living cells using interference reflection microscopy. J Microscopy. 2008;232:73–81. doi: 10.1111/j.1365-2818.2008.02069.x. [DOI] [PubMed] [Google Scholar]