Abstract
Fish are elusive prey with a short-latency escape behavior—the C-start—initiated to either the left or right by a “race” between 2 giant Mauthner neurons in the fish brainstem. Water disturbances usually excite the ipsilateral neuron, which massively excites contralateral motor neurons, resulting in a rapid turn away from striking predators. Here, it is reported that tentacled snakes (Erpeton tentaculatus) exploit this normally adaptive circuitry by feinting with their body, triggering the Mauthner cell that is furthest from their head milliseconds before a ballistic strike is initiated. As a result, fish that were oriented parallel to the long axis of the snake's head most often turned toward the approaching jaws, sometimes swimming directly into the snake's mouth. When strikes were instead directed at fish oriented at a right angle to the snake's head, snakes anticipated future fish behavior by striking to where fish would later be if they escaped from the snake's body feint, which fish usually did. The results provide an example of a rare predator taking advantage of a prey's normally adaptive escape circuitry and suggest that the snake's sensory-motor system is adapted to predict future behavior.
Keywords: escape, Mauthner, predator, fish, evolution
Tentacled snakes (Erpeton tentaculatum) are fishing specialists that assume an unusual “J”-shaped posture while waiting to strike (1, 2). Once positioned, they typically remain motionless, waiting for fish to move into the concave space between their head and body before striking. The strike is explosive [supporting information (SI) Movie S1], reaching the position of a nearby fish in only 15–20 ms. However, fish are specialized to elude sudden attacks through a ubiquitous and well-studied escape response—the C-start (3–11). The response consists of a sudden bend of the body into a C-shape (followed by propulsion away from the water disturbance) initiated in as little as 5–6 ms (4) (see Methods for fish in this study). The timing of tentacled snake strikes and fish escape responses pits these 2 high-speed behaviors against each other, with the outcome critical to both predator and prey. For fish, turning in the correct direction for escape—away from an approaching predator—is an important component of the escape response, and this decision occurs within a few milliseconds of stimulus onset (12, 13). Once the escape direction has been determined (to either the left or right), the fish is committed and the neural circuitry massively excites muscles on one side of the trunk while inhibiting muscles on the other. In this study, slow-motion video analysis and hydrophone recordings were used to investigate the dynamics of this predator-prey interaction. Here, it is reported that the tentacled snake's unusual hunting position and strike dynamics provide a mechanism to elicit C-starts in the wrong direction—toward the attacking snake.
Results
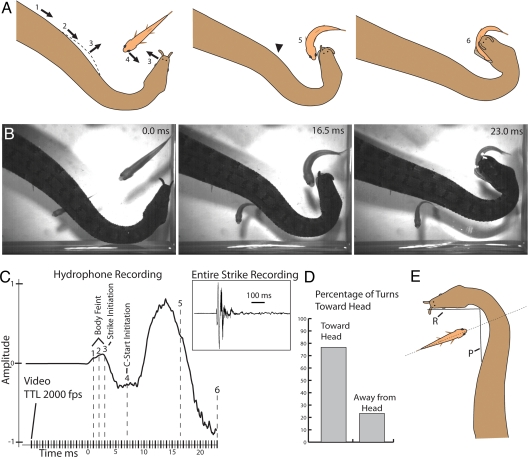
To examine the details of tentacled snake predation, strikes of 4 snakes were filmed at 500–2,000 frames per second (fps) at short shutter speeds using a Redlake MotionPro HS-3 camera as they fed on fathead minnows (Pimephales promelas) in an aquarium filled to a depth of ≈4–5 cm with water. This arrangement confined the interactions between snakes and fish primarily to 2 dimensions and facilitated observations by increasing the encounter rate between fish and snakes. Snakes preferred to strike at fish that had entered the region between their head and body (1) (Fig. 1). Just 1–3 ms before initiating a strike, snakes successively moved portions of their body in a sequence beginning on the neck and moving toward the head. This began as a horizontal translation of the skin surface, accompanied by a slight downward rotation of the body, followed by a distinctive outward bend of the neck region just as the head began to move forward to strike [Fig. 1A (Arrows 1–3) and SI]. During initial observations of striking snakes, it was surprising to see that fish seemed to respond to this movement of the snake's body rather than the striking head, as indicated by turns (C-starts) away from the body and toward the approaching jaws [Movie S2 (Clips 1–10)]. To examine this possibility, turn direction in response to strikes was quantified in 30 trials for each of the 4 snakes for trials during which fish were oriented roughly parallel to the long axis of the snake's head as determined by plotting the point of intersection of the long axis of the fish on the snake's neck (Fig. 1E), not including 9 strikes during which no C-starts were elicited. The fish turned toward the snake's head during the strike in 78% of the trials (25 of 30, 23 of 30, 22 of 30, and 24 of 30 strikes), and for each snake, this tendency was significant (P < 0.05, binomial test). Although snakes captured fish 65% of the time when the fish turned toward the snake and 50% of the time when fish turned away, the handful of trials (between 2 and 4) for each snake during which fish turned away from the head and were captured did not allow for statistical analysis. Nevertheless, for fish, it seems that turning toward the strike is not adaptive.
Fig. 1.
Snake's strike and fish response for fish approximately parallel to jaws. (A) Schematic of snake position and events during a strike. Numbers represent sequential events. Numbers 1–3 show body movements before strike an arrowhead marks inflection of the neck during a feint. (B) Frames from high-speed video; (C) Hydrophone recording of strike showing pressure change (1–3) associated with feint—Y units arbitrary. Numbers show events illustrated in A. Tick marks represent TTL from camera (2,000 fps) correlating movie to hydrophone. Note that latency to C-start is appropriate for body feint (7 ms) but not for strike (4 ms). (D) Percent turns toward head during strike. (E) For these trials, the long axis of the fish intersected line segment P. SI contains a movie correlated to figure plates and hydrophone recording. Each TLL represents 1 frame in Movie S1.
To investigate the details of strikes further, during a number of trials, hydrophone recordings were made at 200,000 samples/s and correlated with fish and snake movements while filming at 2000 fps. The hydrophone was located ≈10 cm from the concave portion of the snake's body, with the transducer facing the concave bend of the body between the neck and head. The results revealed a sudden local pressure change occurring 1–3 ms before head translation but correlated in time with the snake's body movements [Fig. 1C and Movie S2 (Clip 1, for movie correlated to hydrophone recording)]. Additionally, C-starts were initiated after the snake's head began to move toward the fish, often 3–4 ms later. This latency is shorter than generally reported for C-starts in a range of fish species (4). C-start latency to water movements was sampled for 10 P. promelas (1 trial each) in the course of this study (see Methods) and was found to have a range of 5.5–12 ms (mean = 7.3 ms). The minimum C-start latency (5.5 ms) was longer than the average latency from the beginning of snake head translation to C-start [mean = 3.96 ms for 10 trials for each of 3 snakes; Figs. 1C and 3C and SI movies] but fell within the range of body-feint to C-start latencies (mean = 6.48 ms). Thus, both the C-start latency and its predominant direction suggest that fish responded to movement of the snake's body rather than to translation of the snake's head during the strike.
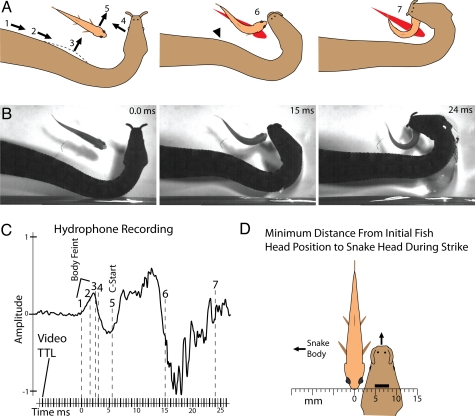
Fig. 3.
Snake strike and fish response for a fish at an approximate right angle to jaws with associated hydrophone recordings and body movements. (A-C) Details are as in Fig. 1. Note in C, latency to C-start is appropriate for response to body feint (5.5 ms) but short relative to strike (2.5 ms). (D) Schematic representation of the average closest relative position of the snake's head to the fish's initial head position during the strike (fish could be rotated 180°). This was 6.5 mm to the far side of the body. Bar marks 95% confidence interval from the means of the 4 snakes.
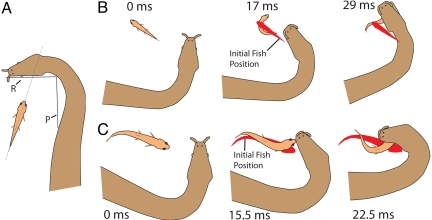
In a number of preceding trials, snakes appeared to have anticipated the future behavior of fish and struck not at the head [the area usually grasped during a successful strike (1)] but, rather, at the location where the head would be if a C-start were initiated away from the body feint [i.e., Movie S2 (Clip 10)]. To examine the possibility that snakes predicted fish behavior and accounted for their future movements when striking, trials with fish oriented at (approximate) right angles to the head were analyzed (Fig. 2A). In this orientation, the fish might turn predominantly to either the left or right of the snake's strike (in contrast to “toward or away” as previously described). To quantify this tendency, the shortest distance between the initial midline of the fish's head (before strike onset) and the midline of the snake's head during the strike was measured for 10 trials for each of the 4 snakes. Remarkably, the snakes consistently struck not toward the location of the fish's head but, rather, to the side of the fish furthest from the snake's body (Figs. 2 and 3). The midline of the snake's head was, on average, 6.5 mm from the midline of the fish's initial head position at its closest point during the strike. The 95% confidence interval for the distance between the snake's head and the initial position of the fish's head calculated from the means of the 4 snakes was 4.9–8.2 mm. Fig. 3D shows this schematically, scaled to the average-sized snake and the average-sized fish from the 40 total trials. In the course of the strike, fish usually turned toward the snake's approaching head (80%), often placing their heads directly into the oncoming jaws [Figs. 2 and 3 and Movie S3 (Clips 1–8)]. Hydrophone recordings of striking snakes in these trials also revealed a sudden local pressure change occurring in conjunction with the snake's body movement [Fig. 3C and Movie S3 (Clip 8, for video correlated to hydrophone recording)].
Fig. 2.
Snake strikes and fish response for fish at approximate right angles to jaws. (A) For these trials, the long axis of the fish intersected line segment R and was at least 5 mm from the jaws. (B and C) Two schematic examples of strikes toward the future location of fish. The red silhouette shows the initial fish position throughout the subsequent movements. Movie S3 contains these and additional video trials for this orientation.
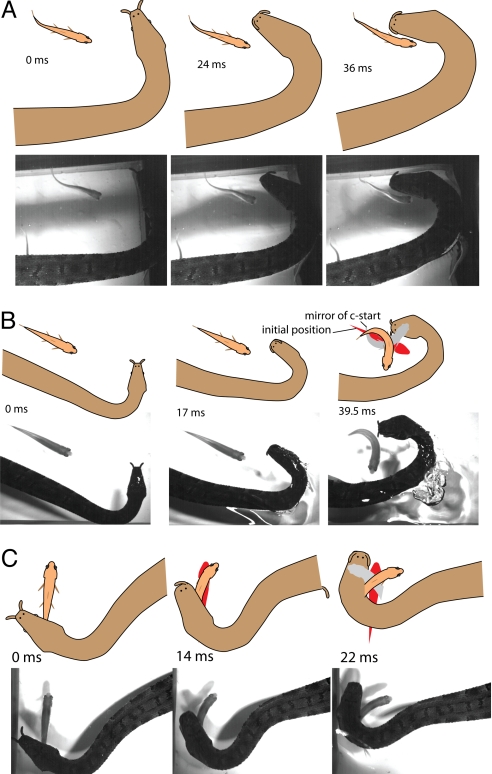
These data indicate that tentacled snakes not only startle fish with their body but predict the outcome of the feint in terms of future fish behavior. It is a prediction, because snakes began to strike before fish initiated their C-start, and the snake's strike was ballistic (Movie S1) and not compatible with midcourse correction. There are several reasons for this latter conclusion, including the speed of translation of the head during the strike, which precludes visual feedback; the short time available for using potential feedback; the momentum of the head; and because the eyes are retracted (although not covered) at the initiation of each strike (see SI movies). More telling than these considerations, however, were cases in which fish did not initiate an escape response and snakes missed by striking to the fish's side [Fig. 4A and Movie S4 (Clips 1–3)] or when fish turned toward the body feint and snakes still biased their strike to the far side of the initial fish position, missing by a wide margin (Fig. 4 B and C and Movie S4). Mirroring the fish's C-start in space [Fig. 4 B and C (gray silhouette)] suggests the intended target of the snake. Thus, snakes aimed for the most likely future location of the fish's head, irrespective of fish movements. Strike success in these trials was 48% (19 of 40) overall, but only 1 (12%) in 8 fish that remained stationary during the strike (n = 6) or turned toward the body (n = 2) was captured.
Fig. 4.
Examples of strikes to the side of the fish when no C-start was elicited (A) or when the start was toward the body (B and C). Strikes were still biased toward the far side of the initial fish position. The red silhouette shows the initial fish position throughout the subsequent movements. The gray silhouette shows a mirror image of the C-start suggesting the snake's target. Movie S4 contains these and additional video trials for this phenomenon.
Discussion
Tentacled snakes are fishing specialists, and results of this study suggest that they have turned the Mauthner-mediated escape response to their advantage. Typically, the Mauthner cell closest to an approaching predator rapidly and efficiently generates a C-start in the opposite direction by massively inhibiting ipsilateral musculature while exciting contralateral musculature (through intervening neurons). Tentacled snakes initiated this irreversible cascade of neuromuscular events by feinting with their body before striking, usually resulting in a C-start 5–12 ms later. In this intervening time, snakes began an explosive strike and fish were unable to change course. The benefit for the snake depends on the orientation of the fish. Fish parallel to the snake's head usually moved toward the approaching jaws. Fish at right angles to the head usually moved in a predictable direction, and snakes aimed for the future location of the head.
The results provide an explanation for the unique J-shaped hunting posture adopted by tentacled snakes and the favored location of fish when strikes are initiated (1). Both of these are necessary for generating a “feint” on the far side of the fish. Because the elicited C-start may propel fish headfirst into the mouth in some cases, this finding explains previous observations that fish may be partially swallowed (14), or disappeared completely (2), during the brief strike, contributing to a very short handling time (2). The results also suggest that tentacled snakes are acting as rare predators (15–19) that may take advantage of the neural circuitry of fish, which is normally adaptive. The most striking facet of the predator-prey interaction described here is the rapid time course, which requires tentacled snakes to plan for behavior they may never see. They strike when fish are in one position but almost always encounter fish in another position—in the midst of a C-start. In a sense, fish approaching at right angles to the snake's jaw are almost never where they appear to be. They must be viewed through the prism of a C-start. The solution, as with prism-reared owls, is to correct for the disparity by altering the position of the head relative to prey (20). This raises the question of whether individual experience (21) or evolution of the species has tailored the snake's strike to the C-start.
Materials and Methods
Snakes were housed in aquaria containing 30 cm of water, gravel, and plastic plants, with pH between 6.5 and 7. Fathead minnows (P. promelas) were used for prey because their response latency was found to be similar to wild-caught minnows (Gambusia affinis). Trials were filmed in a 25- × 50- × 15-cm aquarium containing 4–5 cm of water, allowing interactions to be filmed primarily in 2 dimensions and increasing the rate of encounters between fish and snakes. A MotionPro HS-3 camera (Redlake) with two 19-led honeycomb stroboscopic illuminators (Integrated Design Tools, Inc.) was used, and video was transferred to a MacPro laptop using MotionProX software. An Aquarian Products H2a hydrophone and rolls of MP13 mini mic preamp were used for sound pressure recordings, connected to a PowerLab 4/30 data acquisition unit using LabChart software (ADInstruments) while simultaneously recording camera TTLs for correlation of video with recordings. The hydrophone was positioned near the center of the tank ≈10 cm from the convexity of the snake's neck, with the transducer membrane facing the neck. Strikes at fish roughly parallel (P trials) to jaws were included if the fish's long axis passed through segment P in Fig. 1E. The segment was drawn for each trial by initially drawing line R (a line extending along the jaw from tentacle tip to neck intersection). Line P was the same segment extending from the endpoint of R to the neck (as illustrated). P trials did not include strikes without C-starts (9 strikes) and included fish facing both toward and away from the head. R trials included strikes with the fish's long axis passing through line segment R in Fig. 1E, included trials without C-starts, and required that the fish's head be at least 5 mm from segment R (irrespective of fish direction) and that the snake's head not rotate in the horizontal plane during the strike. Distances from the fish initial head midline to snake's head midline (Fig. 3D) were measured by exporting frames to Adobe Illustrator and measuring based on filmed scale bars. C-start latency in P. promelas was estimated with water droplets as stimuli (Movie S5) while filming at 2,000 fps without control for vision.
Supplementary Material
Footnotes
The author declares no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905183106/DCSupplemental.
References
- 1.Smith TL, Povel DE, Kardong KV. Predatory strike of the tentacled snake (Erpeton tentaculatum) J Zool Lond. 2002;256:233–242. [Google Scholar]
- 2.Murphy JC. Homalopsid Snakes, Evolution in the Mud. Melbourne, FL: Kreiger; 2007. [Google Scholar]
- 3.Zottoli SJ. Correlation of the startle reflex and Mauthner cell auditory responses in unrestrained goldfish. J Exp Biol. 1977;66:243–254. doi: 10.1242/jeb.66.1.243. [DOI] [PubMed] [Google Scholar]
- 4.Eaton RC, Hackett JT. In: Neural Mechanisms of Startle Behavior. Eaton RC, editor. New York: Plenum; 1984. pp. 213–266. [Google Scholar]
- 5.Canfield JG, Eaton RC. Swimbladder acoustic pressure transduction initiates Mauthner-mediated escape. Nature. 1990;347:760–762. [Google Scholar]
- 6.Faber DS, Fetcho JR, Korn H. Neuronal networks underlying the escape response in goldfish, general implications for motor control. Ann NY Acad Sci. 2006;563:11–33. doi: 10.1111/j.1749-6632.1989.tb42187.x. [DOI] [PubMed] [Google Scholar]
- 7.Korn H, Faber DS. The Mauthner cell half a century later: A neurobiological model for decision-making? Neuron. 2005;47:13–28. doi: 10.1016/j.neuron.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 8.Eaton RC, Bombardieri RA, Meyer DL. The Mauthner-initiated startle response in teleost fish. J Exp Biol. 1977;66:65–81. doi: 10.1242/jeb.66.1.65. [DOI] [PubMed] [Google Scholar]
- 9.Zottoli SJ, Faber DS. The Mauthner cell: What has it taught us? Neuroscientist. 2000;6:26–38. [Google Scholar]
- 10.Faber DS, Korn H, editors. Neurobiology of the Mauthner Cell. New York: Raven; 1978. [Google Scholar]
- 11.Preuss T, Osei-Bonsu PE, Weiss SA, Wang C, Faber DS. Neural representation of object approach in a decision-making motor circuit. J Neurosci. 2006;26:3454–3464. doi: 10.1523/JNEUROSCI.5259-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canfield JG, Rose GJ. Hierarchical sensory guidance of Mauthner-mediated escape responses in goldfish (Carassius auratus) and cichlids (Haplochromis burtoni) Brain Behav Evol. 1996;48:137–156. doi: 10.1159/000113193. [DOI] [PubMed] [Google Scholar]
- 13.Weiss SA, Preuss T, Faber DS. A role of electrical inhibition in sensorimotor integration. Proc Natl Acad Sci USA. 2008;105:18047–18052. doi: 10.1073/pnas.0806145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelissen T. Erpeton tentaculatum, a fishing snake. Bull Chi Herp Soc. 1989;24:26–29. [Google Scholar]
- 15.Dawkins R. The Extended Phenotype. Oxford: Oxford Univ Press; 1982. [Google Scholar]
- 16.Jablonski PG. A rare predator exploits prey escape behavior: The role of tail-fanning and plumage contrast in foraging of the painted red start (Myioborus pictus) Behav Ecol. 1999;10:7–14. [Google Scholar]
- 17.Jablonski PG. Sensory exploitation of prey: Manipulation of the initial direction of prey escapes by a conspicuous ‘rare enemy’. Proc R Soc London. 2001;268:1017–1022. doi: 10.1098/rspb.2001.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jablonski PG, Strausfeld NJ. Exploitation of an ancient escape circuit by an avian predator: Relationships between taxon-specific prey escape circuits and the sensitivity to visual cues from the predator. Brain Behav Evol. 2001;58:218–240. doi: 10.1159/000057565. [DOI] [PubMed] [Google Scholar]
- 19.Catania KC . Worm grunting, fiddling, and charming—Humans unknowingly mimic a predator to harvest bait. PLoS One. 2008;3:e3472. doi: 10.1371/journal.pone.0003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knudsen EI, Knudsen PF. Sensitive and critical periods for visual calibration of sound localization by barn owls. J Neurosci. 1990;10:222–232. doi: 10.1523/JNEUROSCI.10-01-00222.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergan JF, Ro P, Ro D, Knudsen EI. Hunting increases adaptive auditory map plasticity in adult barn owls. J Neurosci. 2005;25:9816–9820. doi: 10.1523/JNEUROSCI.2533-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.