Abstract
Background: Preclinical evidence suggests synergy between docetaxel and irinotecan, two drugs active in esophagogastric cancer. We previously demonstrated the safety of docetaxel 35 mg/m2 and irinotecan 50 mg/m2 given on days 1 and 8 of a 21-day schedule.
Materials and methods: Patients who had unresectable/metastatic squamous cell carcinoma or adenocarcinoma of the esophagus, measurable disease, Eastern Cooperative Oncology Group performance status of zero to two, and normal bilirubin were eligible. Tumor assessment was carried out every three cycles.
Results: We enrolled 29 chemotherapy-naive (CN) and 15 chemotherapy-exposed (CE) eligible patients. Principal toxic effects were diarrhea, neutropenia, and hyperglycemia. There were no toxic deaths. There was one early death, from myocardial infarction. Among 26 CN and assessable patients, there were seven (26.9%) with a partial response (PR) and one (3.8%) with a complete response (CR). There were two PRs and one CR among the patients with CE disease. Median time to progression for CN patients was 4.0 months and for CE patients 3.5 months. Median survival for CN eligible patients was 9.0 months and for CE patients 11.4 months.
Conclusions: Docetaxel–irinotecan combination given on a weekly × 2 of 3 schedule is promising in the treatment of advanced esophageal cancer.
Keywords: chemotherapy, docetaxel, esophageal cancer, irinotecan
introduction
Esophageal cancer incidence is increasing in the United States, with 16 470 cases and 14 280 deaths expected in 2008 [1]. Esophageal cancer accounted for 3% of cancer deaths in European males in the 1990s [2]. Esophageal cancer is not curable when metastatic. The predominant histology has shifted from squamous cell to adenocarcinoma; however, without clear evidence of a difference in chemosensitivity between the histologic types, regimens based on 5-fluorouracil and cisplatin continue as the mainstay of palliative treatment of both histologies. A randomized phase II study of cisplatin and 5-fluorouracil combination compared with cisplatin monotherapy in patients with squamous cell carcinoma demonstrated median survivals of 33 and 28 weeks for combination and monotherapy, respectively [3]. Toxicity with the combination included treatment-related death. Chemoradiation or trimodality therapy in patients who present with locally confined disease is also reliant on 5-fluorouracil and cisplatin [4]; the standard palliative regimens which include cisplatin may have less utility and more toxicity when used a second time in patients who relapse after chemoradiation.
Among the cytotoxic agents with activity in esophageal cancer are docetaxel and irinotecan. Docetaxel is a semisynthetic taxane, which promotes assembly of tubulin and inhibits microtubule depolymerization [5]. A randomized phase II trial of docetaxel–cisplatin or docetaxel–cisplatin–5-fluorouracil (DCF) in patients with gastric or gastroesophageal cancer demonstrated objective responses in 43% and 59% of patients in the two-drug and three-drug arms, respectively [6]. Phase III comparison demonstrated a significant survival advantage for the three-drug regimen, although at the cost of increased toxicity [7]. Median overall survival (OS) was significantly longer for DCF versus CF (hazard ratio 1.29, P = 0.02). Two-year survival was 18% for DCF and 9% for CF. Quality of life was preserved for a significantly longer period of time [8]. Irinotecan, a semisynthetic camptothecin derivative, and its principal metabolite SN-38 are inhibitors of topoisomerase I, acting to stabilize the cleavable complex, with resulting DNA strand breaks [9, 10]. Irinotecan is also a component of active, cisplatin-based regimens for esophagogastric cancer, with a response rate of 57% reported from a phase II trial [11]. A phase III study of the substitution of irinotecan for cisplatin in advanced esophagogastric cancer did not demonstrate superiority, but the irinotecan-containing regimen was active with a response rate of 32% [12].
Preclinical evidence of synergy between taxanes and irinotecan or SN-38 has been obtained in several laboratories [13–15]. The interaction may be schedule dependent, with administration of taxane followed by irinotecan predicted to be optimal. We and others have conducted phase I studies of docetaxel–irinotecan combination [16–18]. Dose-limiting toxic effects were predominantly neutropenia and diarrhea in each study. Recommended phase II doses are docetaxel 35 mg/m2 followed by irinotecan 60 mg/m2, for administration on a weekly schedule for 2 weeks of a 3-week schedule, albeit associated with a >20% rate of grade 3 or 4 diarrhea and dehydration [16]. Objective partial response (PR) was observed among three patients with esophageal cancer treated on our phase I study. We conducted this phase II study to determine the activity of docetaxel–irinotecan combination administered on a weekly schedule in advanced esophageal cancer. We capped the irinotecan dose at 50 mg/m2 because patients with advanced esophageal cancer may have dysphagia or be feeding tube dependent and thus not cope well with high-grade diarrhea seen at the highest irinotecan dose in the phase I study.
materials and methods
patient selection
Patients 18 years of age or older who had histologic evidence of adenocarcinoma or squamous cell carcinoma of the esophagus and who had metastases were eligible. Patients were required to have measurable disease, Eastern Cooperative Oncology Group (ECOG) performance status of zero to two, ability to provide informed consent, and no concomitant medical problems that could interfere with the ability to receive therapy. Patients were required to have an absolute neutrophil count ≥1500 cells/μl and platelet count >100 000/μl. Estimated creatinine clearance >60 ml/min was required. Patients who had normal bilirubin, aspartate aminotransferase and alanine aminotransferase <1.5× the institutional upper limit of normal, and alkaline phosphatase <2.5× the upper limit of normal were eligible. Prior systemic chemotherapy for metastatic disease was not permitted; however, prior chemotherapy as part of a neoadjuvant or adjuvant regimen was permitted, with the exception of irinotecan or docetaxel. Radiation therapy to >20% of the bone marrow was not allowed. Patients who were human immunodeficiency virus (HIV) positive, pregnant or lactating, had preexisting neuropathy >grade 1, or had brain metastases were excluded. Written informed consent was obtained from all patients. The protocol and consent form were approved by the Yale University Human Investigation Committee and the Institutional Review Boards of the Johns Hopkins Medical Institutions and Anne Arundel Medical Center.
treatment plan
Computed tomography (CT) scans of the chest and abdomen were obtained within 3 weeks of initiating treatment. Laboratory studies, carcinoembryonic antigen, electrocardiogram, chest X-ray, and HIV screening were completed within 2 weeks of initiating treatment.
Patients were premedicated with dexamethasone 8 mg p.o. 12 h before chemotherapy and 10 mg i.v., and i.v. 5-HT3 antagonist antiemetic therapy given within 1 h before chemotherapy. Docetaxel (Taxotere; Aventis Pharmaceuticals, Collegeville, PA) 35 mg/m2 was infused over 1 h on days 1 and 8 of a 21-day cycle. Immediately following docetaxel administration (days 1 and 8), irinotecan (Pharmacia & Upjohn, Kalamazoo, MI) 50 mg/m2 was infused over 30 min. Dexamethasone 8 mg p.o. was given again 12 h after chemotherapy administration. Oral antiemetic therapy was prescribed and patients were instructed in use of the intensive loperamide regimen in the event of diarrhea [19].
Treatment was held for absolute neutrophil count <1200/ml, platelet count <100 000/ml, or diarrhea ≥grade 2. Dose modifications were to be made to docetaxel dosing for hypersensitivity reactions, neutropenia, or hepatic toxicity. Dexamethasone was continued full dose for the first cycle. For patients who did not develop fluid retention but suffered excess dexamethasone toxicity, dexamethasone could be tapered in subsequent weeks to gradually eliminate the 12-h pre- and 12-h post-chemotherapy doses. The dose immediately before chemotherapy was not eliminated. Modifications to irinotecan dosing were mandated for neutropenia and diarrhea. If, for any reason, a patient received no treatment for more than three successive weeks, continuation on study was permitted only in the absence of progressive disease.
response assessment
Response assessment was carried out with CT scanning at 9 weeks and thereafter every 9 weeks. In the event of a PR or complete response (CR), the response was confirmed with CT 4–6 weeks after it was first documented. World Health Organization criteria were used to determine response status [20].
statistical design
The primary end point of the study was objective response rate. There were two strata: previously untreated and previously treated patients. The response rates of interest were 30% for previously untreated patients and 20% for previously treated patients. Response rates not of interest (null hypotheses) were 10% for previously untreated and 5% for previously treated patients. The study used a Simon’s two-stage accrual design for each stratum. For previously untreated patients, two or more responses were required among the first 10 patients enrolled. If these were seen, an additional 19 previously untreated patients would be enrolled. If they were not seen, this stratum would close to accrual after the first stage. The combination would be rejected for chemotherapy-naive (CN) patients unless there were six responders among these 29 patients. The first stage for the previously treated stratum was to accrue 10 patients. If one response was seen, an additional 19 patients would be added. The combination would be rejected unless four responses were documented among 29 eligible and previously treated patients. This design provided a power of 80% and significance of 0.05 [21]. We accounted for the two-stage design in the estimation of response rate confidence intervals (CIs) [22].
results
CN patients
patient characteristics.
Twenty-nine CN patients were accrued from December 2001 to October 2004. Twenty-seven (93%) were male. Median age was 60 years (range 28–74). ECOG performance status was zero in six (21%) and one in 23 (79%) patients. One patient had prior esophagectomy; the remaining 28 (97%) were newly diagnosed with metastases. Twenty-three patients (79%) had adenocarcinoma and six (21%) had squamous cell carcinoma. No patient in this stratum had received prior chemotherapy. Patient characteristics are displayed in Table 1.
Table 1.
Patient characteristics
| No prior chemotherapy | Prior chemotherapy | |
| Number | 29 | 15 |
| Age (mean, SD) | 59.9 (2.0) | 56.9 (2.1) |
| Male (%) | 93 | 93 |
| Adenocarcinoma (%) | 79 | 80 |
| Squamous cell carcinoma (%) | 21 | 20 |
| Esophagectomy* (%) | 3 | 87 |
| PS* | ||
| 0 (%) | 21 | 53 |
| 1 (%) | 79 | 47 |
P < 0.05 using Fisher's exact test.
SD, standard deviation; PS, performance status.
toxicity.
The principal toxic effects seen in this stratum were hematologic and gastrointestinal. The worst grade of neutropenia was grade 3 in four patients (14%) and grade 4 in three patients (10%), of whom one also developed neutropenic fever. Grade 3 anemia was seen in two patients (7%). No patient developed grade 3 thrombocytopenia (Table 2). Grade 3 diarrhea was seen in 10 patients (35%) and grade 4 diarrhea occurred in one (3%). Other severe gastrointestinal side-effects were grade 3 nausea (four patients, 14%) and grade 3 vomiting (two patients, 7%). Grade 3/4 hyperglycemia (six patients, 21%) and grade 3 hypophosphatemia (two patients, 7%) were likely related to the weekly administration of dexamethasone. Dose reductions were required in 13 patients (45%). Six patients required one and seven patients required two dose reductions.
Table 2.
Toxicity
| Grade 3 |
Grade 4 |
|||||||
| No prior chemotherapy |
Prior chemotherapy |
No prior chemotherapy |
Prior chemotherapy |
|||||
| n | % | n | % | n | % | n | % | |
| Any hematologic | 8 | 28 | 7 | 47 | 3 | 10 | 3 | 20 |
| WBC | 3 | 10 | 3 | 20 | 0 | 0 | 0 | 0 |
| Hemoglobin | 2 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neutropenia | 6 | 21 | 7 | 47 | 2 | 7 | 3 | 20 |
| Neutropenic fever | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 0 |
| Any non-hematologic | 18 | 62 | 9 | 60 | 5 | 17 | 1 | 7 |
| Hyperglycemia | 6 | 21 | 3 | 20 | 1 | 3 | 1 | 7 |
| Hypophosphatemia | 2 | 7 | 2 | 13 | 0 | 0 | 0 | 0 |
| Fatigue | 2 | 7 | 1 | 7 | 2 | 7 | 0 | 0 |
| Diarrhea | 10 | 34 | 4 | 27 | 1 | 3 | 0 | 0 |
| Pneumonitis | 1 | 3 | 1 | 7 | 0 | 0 | 0 | 0 |
| Syncope | 1 | 3 | 1 | 7 | 0 | 0 | 0 | 0 |
| Thrombosis/DVT/PE | 3 | 10 | 0 | 0 | 1 | 3 | 0 | 0 |
| Nausea | 4 | 14 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 2 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
DVT, deep vein thrombosis; PE, pulmonary embolus; WBC, white blood cell.
response.
The response rate for the entire enrolled cohort, including inassessable patients, was 28% (95% CI 13% to 47%). Hence, the study achieved the prespecified criteria for objective response success in this stratum. Three patients in this stratum were inassessable; two withdrew consent and one experienced docetaxel hypersensitivity. The objective response rate among the 26 assessable patients was 30.8% (exact 95% CI 14.3% to 51.8%); seven of these were PRs (27%) and one was a CR (4%). The median number of cycles delivered was five (range 1–9). The median time on study was 3.3 months (range 0.5–9.1). The most frequent reason for removal from the study was progression of disease (23 patients, 79%), but a patient with an unconfirmed radiographic CR was removed because of toxicity, one patient discontinued because of hypersensitivity, three at their own request, and one for an intercurrent illness not clearly related to therapy (cerebrovascular accident).
progression-free survival and OS.
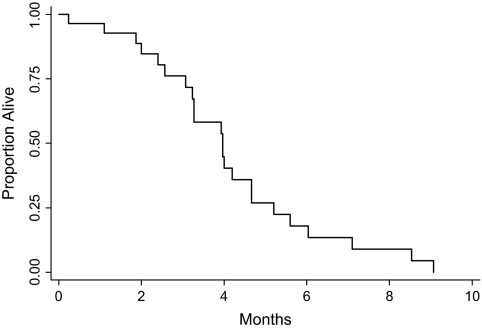
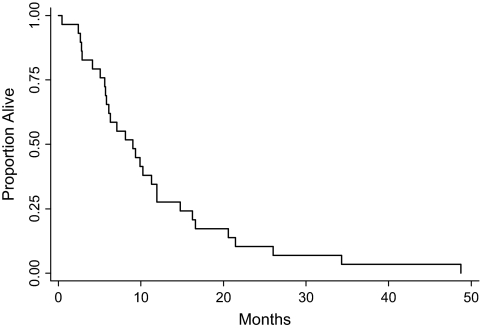
The median time to disease progression was 4.0 months (range 0.3–9.1, see Figure 1). The six patients who were removed for toxicity or other reasons without evidence of progression were censored at the time off study. The median survival was 9.0 months (range 0.4–48.8, see Figure 2).
Figure 1.
Progression-free survival for patients who had received no prior chemotherapy.
Figure 2.
Progression-free survival for patients who had received prior chemotherapy.
previously treated patients
patient characteristics.
Fifteen previously treated patients were accrued from October 2001 to May 2004. Fourteen were male and one female. The median age was 56 years (range 45–78). Thirteen had prior esophagectomy. Twelve had adenocarcinoma of the esophagus or gastroesophageal junction, and three had squamous cell carcinoma of the esophagus. All were eligible. The study was closed before full accrual of this stratum because enrollment was significantly slower than projected for previously treated patients.
toxicity.
The principal toxic effects seen in this stratum were hematologic and gastrointestinal. The worst grade of neutropenia was grade 3 in six patients (40%) and grade 4 in two patients (13%), of whom one also developed neutropenic fever. Neither grade 3 anemia nor grade 3 thrombocytopenia was seen (Table 2). Grade 3 diarrhea was seen in four patients (27%); no patient in this stratum experienced grade 4 diarrhea. Grade 3/4 hyperglycemia (three patients, 20%) and grade 3 hypophosphatemia (two patients, 13%) were also seen in this cohort and were likely related to the use of dexamethasone. Dose reductions were required in eight patients (53%). Six patients required one dose reduction and two patients required two dose reductions. Four patients who did not require dose reduction had one dose delay.
response.
Objective responses were seen in 3 (20%) (95% CI 0% to 31.0%) of the 15 previously treated patients; two of these responses were partial (13%) and one was complete (7%). The median time on study was 3.3 months (range 0.3–7.7). The most frequent reason for removal from the study was progression of disease (13 patients, 87%), but two patients discontinued therapy without evidence of progression: one died of a myocardial infarction while on study, and one was removed from treatment because of severe post-treatment inflammation in the arm.
progression-free survival and OS.
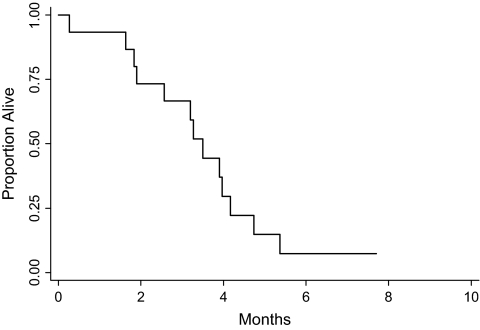
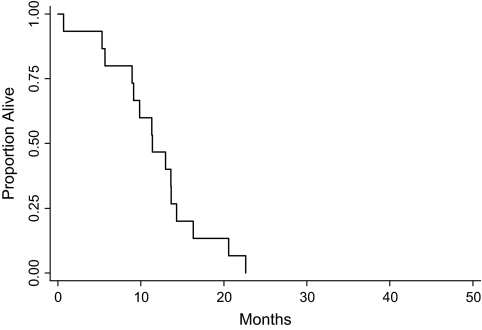
The median time to disease progression in previously treated patients was 3.5 months (range 0.3–5.4, see Figure 3). The two patients who were removed for death or toxicity without evidence of progression were censored at the death or time off study. The median survival was 11.4 months (range 0.7–22.6, see Figure 4); no patients survived.
Figure 3.
Overall survival for patients who had received no prior chemotherapy.
Figure 4.
Overall survival for patients who had received prior chemotherapy.
joint analysis of previously untreated and previously treated patients
A joint analysis of the results in previously treated and previously untreated patients was not part of the original statistical plan for the study. However, because of the small size of the previously treated cohort and the similarity of results in the two cohorts, such an analysis was undertaken. The response rate for the entire enrolled cohort, including inassessable patients, was 25% (exact 95% CI 13% to 40%). The median time to disease progression was 3.9 months (range 0.2–9.1). The median survival was 9.8 months (range 0.4–48.8). The difference in response rates between previously treated and untreated patients was not statistically significant (P = 0.722 by Fisher's exact test). Such analyses were not specified in the protocol and thus do not account for the sequential study design.
discussion
This study met the prespecified criteria for activity in treatment-naive patients based on the objective response rate of 28% (31% among assessable patients). The objective response rate of 20% in previously treated patients was also promising, but the stratum for previously treated patients did not accrue fully, and thus the question of activity is not formally answered in this stratum. The combination of docetaxel and irinotecan in patients with metastatic or recurrent adenocarcinoma or squamous cell carcinoma of the esophagus has been studied previously [23]. A prior phase II trial of the combination given on an every 3 weekly schedule resulted in a response rate of 30%. The study was halted after 15 patients were enrolled because 43% of patients experienced febrile neutropenia and one patient died of cecal perforation. We observed comparable efficacy and much greater tolerability using a weekly schedule at doses we had previously studied in a phase I trial. In another trial investigating weekly administration, with irinotecan given at 55 mg/m2 over 90 min followed by docetaxel 25 mg/m2 on days 1, 8, and 15 of a 28-day cycle [24]. In a small cohort of cisplatin-refractory patients, a response rate of 12.5% was observed, and the hematologic toxicity and diarrhea were somewhat less than we observed, despite the use of a higher dose of irinotecan and a more intensive schedule. The reverse order of administration used in that study is not predicted to produce optimal pharmacologic synergy, perhaps explaining both lesser toxicity and lesser efficacy.
tolerability
The present study used a lower dose of irinotecan than that achieved at the maximum tolerated dose in our phase I study, specifically to permit inclusion of patients with prior esophageal surgery or prior chemotherapy and radiation and a lesser tolerance of gastrointestinal toxicity. The incidence of grade 3 or 4 diarrhea we observed was 35%, greater than the 10% incidence seen for the cohort treated at this dose and schedule in our phase I experience [16]. The higher rate of diarrhea may reflect the extent of prior therapy, including surgery, or longer time on therapy with this particular combination than in the more heavily pretreated phase I population. Additionally, 45% of patients required dose modification, suggesting that a higher starting dose would not have resulted in much greater dose intensity across subsequent cycles. A 10% rate of grade 4 neutropenia was also observed; however, we did not test patients for UGT1A1 polymorphism, which has been associated with a higher risk of severe toxicity in irinotecan-treated patients in some studies, albeit meta-analysis suggests this effect is relevant predominantly at higher dose every 2- or 3-week schedules [25].
efficacy
The objective response rate we observed was comparable to rates reported for the classic 5-fluorouracil and cisplatin combination, but somewhat lower than those reported for epirubicin- or oxaliplatin-containing regimens in gastric and gastroesophageal junction cancers [7, 26, 27]. Both agents studied here, docetaxel and irinotecan, have been incorporated into cisplatin-based combinations, with good efficacy. The combination of docetaxel with cisplatin and 5-fluorouracil was reported in the V325 phase III study to have a response rate of 37%, with a median survival of 9.2 months that represented a significant advance over cisplatin–5-fluorouracil combination in gastric cancer [7]. Cisplatin and irinotecan doublet therapy has been studied, in a multicenter phase II trial, in patients with metastatic or unresectable esophageal cancer of both histologies [11]. The objective response rate was 57% and similar in patients with squamous cell carcinoma or adenocarcinoma. The median time to progression was not reported; however, the median number of cycles administered was four, suggesting a favorable duration of disease control. A randomized superiority/noninferiority trial comparing irinotecan–5-fluorouracil combination with cisplatin–5-fluorouracil in gastric and gastroesophageal junction cancers demonstrated nonsignificant improvements for the irinotecan-containing arm, with a median OS in these unselected patients of 9.0 months [12].
Thus, the median survival times on this study of 9.0 months for CN and 11.4 months for chemotherapy-exposed (CE) patients are comparable to the results in phase II and III studies testing platinoid-based combinations, as outlined above, as well as to the results for irinotecan–5-fluorouracil combination. The interesting finding of longer survival for CE than CN patients likely reflects a different underlying biology in patients who initially presented with locally advanced disease and had a period of disease control afforded by chemotherapy, chemoradiation, or trimodality therapy from those who presented with metastatic disease at diagnosis; however, we did not study tissue from these patients for putative biomarkers of prognosis, such as epidermal growth factor receptor or p53 [28].
Addition of biologic agents to multidrug chemotherapy regimens may increase response rates and is associated with encouraging progression and survival statistics. For example, Pinto et al. [29] have reported a median time to progression of 8 months, with median survival not reached at median follow-up of 11 months, for the combination of 5-fluorouracil, folinic acid and irinotecan with cetuximab. Bevacizumab has also been studied in combination with chemotherapy in metastatic esophagogastric cancer, with a reported objective response rate of 65%, median time to progression of 8.3 months, and median survival slightly exceeding a year [30].
The addition of biologic agents may perhaps permit less toxic backbone regimens, but this hypothesis remains to be proved. The regimen described here may be an attractive backbone for future study in combination with a biologic agent, especially in patients who received a platinum compound in initial definitive therapy or who are otherwise poor candidates for cisplatin therapy. Recent data also indicate that cytotoxic therapy can be individualized by analysis of predictive biomarkers, particularly in the case of predicting benefit from cisplatin based on levels of ERCC1 expression [31–33]. For such patients, an active, alternative, non-cisplatin-containing regimen would be worthy of further study.
conclusion
Docetaxel and irinotecan is an active combination in the treatment of advanced esophageal cancer. In this study, we demonstrated the safety of this regimen on a weekly schedule and its activity in patients who did not have prior chemotherapy exposure. A very promising response rate was also observed in previously treated patients, albeit in a smaller sample than initially planned. Response in previously treated patients is particularly relevant and valuable given the proportion of patients now receiving preoperative chemotherapy with epirubicin, cisplatin, and continuous infusion 5-fluorouracil combination or 5-fluorouracial, cisplatin, and external beam radiation, and who are thus platinum exposed at the time they initiate therapy for metastatic/recurrent disease. Its ultimate role in the management of metastatic esophageal cancer may depend on demonstrating its activity in patients who are predicted to derive little benefit from a platinoid and on testing its worth in combination with biologic agents such as bevacizumab and cetuximab.
funding
The study was supported with funds from Aventis Pharmaceuticals, Pharmacia Upjohn, and US National Cancer Institute support of the Yale Cancer Center, NCI 2 P30 CA016359.
References
- 1.Cancer Statistics. American Cancer Society; 2008. www.cancer.org/docroot/stt/stt_0.asp (12 October 2008, date last accessed) [Google Scholar]
- 2.Karim-Kosa HE, de Vriesa E, Soerjomatarama I, et al. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–1389. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Bleiberg H, Conroy T, Paillot B, et al. Randomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell esophageal cancer. Eur J Cancer. 1997;33:1216–1220. doi: 10.1016/s0959-8049(97)00088-9. [DOI] [PubMed] [Google Scholar]
- 4.Mooney MM. Neoadjuvant and adjuvant chemotherapy for esophageal adenocarcinoma. J Surg Oncol. 2005;92:230–238. doi: 10.1002/jso.20364. [DOI] [PubMed] [Google Scholar]
- 5.Riou JF, Naudin A, Lavelle F. Effects of Taxotere on murine and human tumor cell lines. Biochem Biophys Res Commun. 1992;187:164–170. doi: 10.1016/s0006-291x(05)81474-3. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, Fodor MB, Tjulandin SA, et al. Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol. 2005;23:5660–5667. doi: 10.1200/JCO.2005.17.376. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 8.Ajani JA, Moiseyenko VM, Tjulandin S, et al. V-325 Study Group. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25(22):3210–3216. doi: 10.1200/JCO.2006.08.3956. [DOI] [PubMed] [Google Scholar]
- 9.Hsiang YH, Hertzberg R, Hecht S, Liu L. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260:14873–14878. [PubMed] [Google Scholar]
- 10.Wu J, Yin MB, Hapke G, et al. Induction of biphasic DNA double strand breaks and activation of multiple repair protein complexes by DNA topoisomerase I drug 7-ethyl-10-hydroxy-camptothecin. Mol Pharmacol. 2002;61:742–748. doi: 10.1124/mol.61.4.742. [DOI] [PubMed] [Google Scholar]
- 11.Ilson DH, Saltz L, Enzinger P, et al. Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol. 1999;17:3270–3275. doi: 10.1200/JCO.1999.17.10.3270. [DOI] [PubMed] [Google Scholar]
- 12.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 13.Bissery MC, Vrignaud P, Lavelle F. In vivo evaluation of the docetaxel-irinotecan combination. Proc Am Assoc Ca Res. 1996;37:378. (Abstr 2578) [Google Scholar]
- 14.Pei XH, Nakanishi Y, Takayama K, et al. Effect of CPT-11 in combination with other anticancer agents in lung cancer cells. Anticancer Drugs. 1997;8:231–237. doi: 10.1097/00001813-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kano Y, Akutsu M, Tsunoda S, et al. In vitro schedule-dependent interaction between paclitaxel and SN-38 (the active metabolite of irinotecan) in human carcinoma cell lines. Cancer Chemother Pharmacol. 1998;42:91–98. doi: 10.1007/s002800050790. [DOI] [PubMed] [Google Scholar]
- 16.Bleickardt E, Argiris A, Rich R, et al. Phase I dose escalation trial of weekly docetaxel plus irinotecan in patients with advanced cancer. Cancer Biol Ther. 2002;1:646–650. doi: 10.4161/cbt.314. [DOI] [PubMed] [Google Scholar]
- 17.Adjei AA, Klein Cheri E, Kastrissios H, et al. Phase I and pharmacokinetic study of irinotecan and docetaxel in patients with advanced solid tumors: preliminary evidence of clinical activity. J Clin Oncol. 2000;18:1116–1123. doi: 10.1200/JCO.2000.18.5.1116. [DOI] [PubMed] [Google Scholar]
- 18.Couteau C, Risse M-L, Ducreux M, et al. Phase I and pharmacokinetic study of docetaxel and irinotecan in patients with advanced solid tumors. J Clin Oncol. 2000;18:3545–3552. doi: 10.1200/JCO.2000.18.20.3545. [DOI] [PubMed] [Google Scholar]
- 19.Abigerges D, Armand JP, Chabot GG, et al. Irinotecan (CPT-11) high-dose escalation using intensive high-dose loperamide to control diarrhea. J Natl Cancer Inst. 1994;86:446–449. doi: 10.1093/jnci/86.6.446. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Geneva, Switzerland: World Health Organization Offset Publication No. 48; 1979. [Google Scholar]
- 21.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 22.Koyama T, Chen H. Proper Inference from Simon's Two-Stage Designs. Stat Med. 2008;27(16):3145–3154. doi: 10.1002/sim.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govindan R, Read W, Faust J, et al. Phase II study of docetaxel and irinotecan in metastatic or recurrent esophageal cancer: a preliminary report. Oncology (Williston Park) 2003;17(9 Suppl 8):27–31. [PubMed] [Google Scholar]
- 24.Lordick F, von Schilling C, Bernhard H, et al. Phase II trial of irinotecan plus docetaxel in cisplatin-pretreated relapsed or refractory oesophageal cancer. Br J Cancer. 2003;89(4):630–633. doi: 10.1038/sj.bjc.6601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoskins JM, Goldberg RM, Qu P, et al. UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst. 2007;99:1290–1295. doi: 10.1093/jnci/djm115. [DOI] [PubMed] [Google Scholar]
- 26.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 28.Gibson MK, Abraham SC, Wu TT, et al. Epidermal growth factor receptor, p53 mutation, and pathological response predict survival in patients with locally advanced esophageal cancer treated with preoperative chemoradiotherapy. Clin Cancer Res. 2003;9:6461–6468. [PubMed] [Google Scholar]
- 29.Pinto C, Di Fabio F, Siena S, et al. Phase II study of cetuximab in combination with FOLFIRI in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma (FOLCETUX study) Ann Oncol. 2007;18:510–517. doi: 10.1093/annonc/mdl459. [DOI] [PubMed] [Google Scholar]
- 30.Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201–5206. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 31.Wei J, Zou Z, Qian X, et al. ERCC1 mRNA levels and survival of advanced gastric cancer patients treated with a modified FOLFOX regimen. Br J Cancer. 2008;98(8):1398–1402. doi: 10.1038/sj.bjc.6604317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsubara J, Nishina T, Yamada Y, et al. Impacts of excision repair cross-complementing gene 1 (ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth factor receptor on the outcomes of patients with advanced gastric cancer. Br J Cancer. 2008;98:832–839. doi: 10.1038/sj.bjc.6604211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]