Abstract
The microtubule (MT) cytoskeleton orchestrates the cellular plasticity and dynamics that underlie morphogenesis and cell division. Growing MT plus ends have emerged as dynamic regulatory machineries in which specialized proteins—called plus-end tracking proteins (+TIPs)—bind to and control the plus-end dynamics that are essential for cell division and migration. However, the molecular mechanisms underlying the plus-end regulation by +TIPs at spindle and astral MTs have remained elusive. Here, we show that TIP150 is a new +TIP that binds to end-binding protein 1 (EB1) in vitro and co-localizes with EB1 at the MT plus ends in vivo. Suppression of EB1 eliminates the plus-end localization of TIP150. Interestingly, TIP150 also binds to mitotic centromere-associated kinesin (MCAK), an MT depolymerase that localizes to the plus end of MTs. Suppression of TIP150 diminishes the plus-end localization of MCAK. Importantly, aurora B-mediated phosphorylation disrupts the TIP150–MCAK association in vitro. We reason that TIP150 facilitates the EB1-dependent loading of MCAK onto MT plus ends and orchestrates the dynamics at the plus end of MTs.
Keywords: microtubule, MAP, TIP150, MCAK, EB1
Introduction
Microtubules (MTs) are dynamic fibrous polymers that are required for maintaining the physical properties of cells, as well as having remarkable roles in numerous biological processes, such as cell division and migration. MTs undergo dynamic inter-conversion between growth and shrinkage both in vivo and in vitro. Direct regulation of MT dynamics is controlled by an array of MT-interacting proteins. An increasing number of proteins have recently been identified as the MT plus-end tracking proteins (+TIPs). Ever since cytoplasmic linker protein (CLIP) 170, the prototype for +TIPs, was discovered nearly a decade ago (Perez et al, 1999), the +TIP family has extended greatly, now including end-binding protein (EB)1/2/3, CLIP-115/170, cytoplasmic linker associated protein (CLASP) 1/2, p150Glued, adenomatous polyposis coli (APC), mitotic centromere-associated kinesin (MCAK), actin cross-linking factor (ACF) 7, lissencephaly (LIS) type 1, melanophilin and stromal interaction molecule (STIM) 1 (Vaughan et al, 1999; Mimori-Kiyosue et al, 2000; Nakagawa et al, 2000; Coquelle et al, 2002; Moore et al, 2005; Slep et al, 2005; Wu et al, 2005; Grigoriev et al, 2008). However, how these +TIPs regulate MT dynamics and how their specificity is controlled still eludes researchers.
Most of the +TIPs have been reported to interact directly with EB1 and their plus-end localization depends on their physical interaction with EB1. It is proposed that EB1 proteins might specify the loading of +TIPs onto MT plus ends. Furthermore, two types of EB1-binding domain have been identified within the sequences of +TIPs, one of which is the well-defined CAP–Gly domain that is found in CLIP-115/170 (Komarova et al, 2005) and p150Glued (Askham et al, 2002). One of the characteristics underlying the EB1-binding domains is the richness of serine, proline and basic residues, which is well-conserved in APC but is much less so in other EB-binding proteins such as ACF7, APC, CLASP1/2, melanophilin and STIM1 (Galjart, 2005; Wu et al, 2005; Akhmanova & Steinmetz, 2008). One outstanding question is whether there are any as yet unknown +TIPs showing structural features characteristic of the APC EB1-binding domain.
Here, we identified a new +TIP, TIP150, using computational analyses. Our characterization shows that TIP150 is an EB1-binding protein. Importantly, the loading of TIP150 to the MT plus ends requires its interaction with EB1, whereas TIP150 facilitates the loading of MCAK to MT plus ends. Our study sheds light on the mechanism of +TIP complex organization and MT dynamics regulation.
Results And Discussion
Identification of TIP150 as a new EB1-binding protein
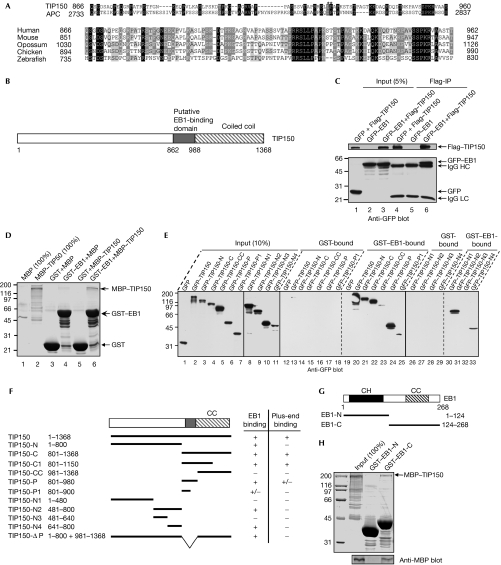
To identify potential +TIPs related to the EB1-binding domain of human APC, we used the EB1-binding domain of human APC (Su et al, 1995; Berrueta et al, 1998; Morrison et al, 1998) as the query sequence to carry out BLAST (basic local alignment search tool). However, no informative sequences were found in the search using default parameters. Therefore, we eliminated the compositional adjustments of the scoring parameters and obtained many more sequences. Sequences of the BLAST results were screened further by careful analysis of similarities of the residues essential for the EB1 interaction (Galjart, 2005). By using this approach, we identified an uncharacterized gene, KIAA0774, as a potential +TIP candidate. The predicted EB1-binding domain in KIAA0774 is rich in serines, proline and basic residues that are commonly conserved in the EB1-binding domain of other +TIPs (Galjart, 2005; Mimori-Kiyosue et al, 2005; Slep et al, 2005); these crucial residues are conserved in KIAA0774 (Fig 1A, asterisk). KIAA0774 also contains a putative carboxy-terminal coiled-coil domain (Fig 1B). Database searches showed the existence of KIAA0774 orthologues in vertebrates, but not in invertebrates, plants or fungi (Fig 1A). As KIAA0774 shows an MT plus-end tracking activity seen in other +TIPs, we refer to KIAA0774 as TIP150 (+TIP of 150 kDa).
Figure 1.
Identification and characterization of TIP150 as a new plus-end tracking protein. (A) Alignment of the end-binding protein 1 (EB1)-binding domains in tracking protein 150 (TIP150) and adenomatous polyposis coli (APC). Upper panel, dark and light shading indicate identical and conserved residues, respectively. Residue numbers at domain boundaries are indicated. Asterisks denote the isoleucine and proline residues from APC, which are required for interaction with EB1. Lower panel, alignment of EB1-binding domains of TIP150 from human, mouse (NCBI, Q3UHD3), opossum (NCBI, XP_001376437), chicken (NCBI, XP_417117) and zebrafish (NCBI, XP_684038). Dark and light shading indicate identical and conserved residues, respectively. Residue numbers at domain boundaries are indicated. (B) Schematic drawing of TIP150 structure; the putative EB1-binding domain and coiled-coil (CC) domain are illustrated. Residue numbers at domain boundaries are indicated. (C) Anti-Flag immunoprecipitates (Flag-IP) from lysates of human embryonic kidney 293T cells expressing Flag-TIP150 and green fluorescent protein (GFP)–EB1 were prepared, and the anti-Flag blotting verified co-precipitation of Flag–TIP150 (upper) and GFP–EB1 (lower). (D) Purified glutathione-S-transferase (GST)–EB1 was used to isolate bacterial recombinant maltose-binding protein (MBP)-TIP150. SDS–PAGE analysis indicates that MBP–TIP150 binds to GST–EB1 (lane 6, top arrow). (E) Purified GST–EB1 was used to absorb GFP–TIP150 and its fragments from the lysates of human embryonic kidney 293T cells. GST–EB1 bound materials were resolved by SDS–PAGE and immunoblotted with an anti-GFP antibody. (F) Schematic drawing of TIP150 deletion mutants. +, positive; +/−, weak; −, negative. Residue numbers at domain boundaries are indicated. (G) Schematic drawing of EB1 deletion mutants. Residue numbers at domain boundaries are indicated. (H) TIP150 binds to the carboxy-terminal EB1. Purified GST–EB1 proteins (N and C) were used to isolate bacterial recombinant MBP–TIP150 and fractionated by SDS–PAGE (upper) followed by anti-MBP blotting analysis (lower). CH, calponin-homology domain; HC, heavy chain; LC, light chain.
To test whether TIP150 interacts with EB1, we used the Flag antibody to isolate the Flag–TIP150 complex from lysates of human embryonic kidney (HEK)293T cells expressing Flag–TIP150 and green fluorescent protein (GFP)–EB1. Anti-GFP immunoblotting confirmed the presence of the GFP–EB1–TIP150 complex (Fig 1C, lane 6). The validity of the TIP150–EB1 interaction was supported further by the co-precipitation of Flag–TIP150 with EB1 (supplementary Fig S1 online). To test whether TIP150 interacts physically with EB1, we carried out a pull-down assay using glutathione-S-transferase (GST)–EB1 as an affinity matrix to isolate maltose-binding protein (MBP)–TIP150 protein. As shown in Fig 1D, MBP–TIP150 was absorbed by GST–EB1 (lane 6), but not by GST (lane 5), indicating that TIP150 is a new EB1-binding protein.
To map the regions of TIP150 that bind to EB1, we generated full-length and deletion-mutant TIP150s tagged with GFP (Fig 1F). Surprisingly, both the predicted EB1-binding domain (TIP150-P; Fig 1E, lane 24) and a 160-amino-acid-fragment adjacent to the EB1-binding domain (TIP150-N4) were retained by the GST–EB1 (lane 33). To eliminate the possibility that the interactions were mediated by other GFP–TIP150 accessory proteins from HEK293T cells, we reconstituted the TIP150–EB1 interaction using bacterial recombinant proteins. As shown in supplementary Fig S2 online, both TIP150-P and TIP150-N4 fragments bind to EB1.
To map the domain of EB1 that is in physical contact with TIP150, we generated full-length GST–EB1 and its deletion-mutant proteins (Fig 1G). As shown in Fig 1H, the C-terminal EB1 binds directly to TIP150, in a manner similar to that observed for other +TIPs (Askham et al, 2002; Bu & Su, 2003; Komarova et al, 2005; Mimori-Kiyosue et al, 2005). Thus, we conclude that TIP150 interacts with EB1 through both typical and atypical EB-binding domains that are present in tandem.
TIP150 is a new MT plus-end tracking protein
The interaction between EB1 and TIP150 prompted us to examine their respective patterns of subcellular distribution. As our initial TIP150 antibody was insufficient in the immunocytochemistry, we carried out a double-labelling protocol using HeLa cells transfected with GFP–TIP150. To avoid MT bundling associated with the overexpression of GFP–TIP150, we selected a preparation in which cells express GFP–TIP150 to a level comparable with that of endogenous TIP150 (supplementary Fig S3 online). As shown in Fig 2A, EB1 marked the dorsal periphery of the cell in a comet-like structure, which is a typical appearance of a plus-end tracking protein (boxed area). Enlargement of the selected area is reminiscent of that observed at the distal (plus) ends of MTs (Morrison et al, 1998). Examination of the GFP–TIP50 distribution revealed a similar pattern and the merger of two channels projected the co-distribution of GFP–TIP150 with EB1 (Fig 2A, merge).
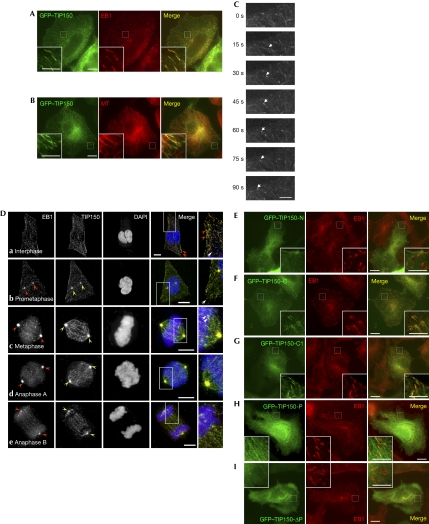
Figure 2.
TIP150 is a new plus-end tracking protein. (A,B) HeLa cells transfected with green fluorescent protein (GFP)–tracking protein 150 (TIP150) were fixed and counter-stained for (A) end-binding protein 1 (EB1; red), and (B) tubulin (red). Scale bar, 10 μm. (C) TIP150 tracks the plus end of growing microtubules (MTs) in live cells. The time-lapse image was taken for HeLa cells expressing a level of GFP–TIP150 similar to that of endogenous cells. The arrow indicates that GFP–TIP150 is associated with growing MT plus ends. (D) Triple labelling of endogenous TIP150, EB1 and DNA. HeLa cells were fixed and counter-stained for TIP150 (green), EB1 (red) and 4′,6-diamidino-2-phenylindole (DAPI; blue). TIP150 labelling is superimposed onto that of EB1, appearing as comet-like structures in the interphase cytoplasm (a, arrow) and membrane periphery (arrowheads). Both EB1 and TIP150 become concentrated at the centrosome in prophase (b, arrowheads) and remain throughout metaphase-anaphase transition (c,d, arrowheads). The centrosomal distribution of both EB1 and TIP150 becomes diminished as anaphase B. Note that EB1 and TIP150 remain localized to the plus-end of microtubules during mitosis (b,c inset, arrows). Scale bar, 10 μm. (E–I) HeLa cells transfected with various GFP–TIP150 combinations were fixed and counter-stained for EB1. Enlarged portions of the selected areas are shown in the insets. Note that the primary activity for the plus-end localization of TIP150 resides in its EB1-binding domain (TIP150-C and TIP150-C1). Scale bar, 5 μm.
To ascertain whether the comet-like distribution of TIP150 is the distal (plus) end of the MTs, we carried out a double-labelling of GFP–TIP150 and tubulin (red). As shown in Fig 2B, GFP–TIP150 localizes predominantly to the distal (plus) end of MTs. Higher magnification of the selected membrane periphery confirmed that the comet-like distribution of TIP150 is consistent with the distal end of MTs. If TIP150 is an authentic +TIP, it should show the typical molecular dynamics associated with MT plus-end growth. Indeed, real-time imaging of GFP–TIP150 revealed a typical +TIP motion (Fig 2C, arrow). The average velocity of movement of the GFP–TIP150 complex was 0.11±0.02 μm/s (n=50 MTs), which is consistent with that observed for other +TIPs (Mimori-Kiyosue et al, 2000). Our preliminary co-sedimentation assay indicated that TIP150 binds to MTs (supplementary Fig S4 online). Thus, we conclude that TIP150 is a new +TIP.
To show the distribution of endogenous TIP150 relative to EB1, we carried out immunofluorescent staining using a newly available peptide antibody. The labelling of TIP150 is superimposed onto that of EB1 and appears as comet-like structures in the interphase cytoplasm (Fig 2Da, arrow) and membrane periphery (arrowheads). Interestingly, a portion of EB1–TIP150 relocated to the centrosome during prophase (Fig 2Db, arrowheads), in addition to their plus-end tip localization (arrow). TIP150 remained co-localized with EB1 to the plus-end tip and centrosome from prometaphase to anaphase B (Fig 2Dc–e). Western blotting analyses show that TIP150 is expressed throughout the cell cycle (supplementary Fig S5 online).
To define the structural elements responsible for localizing TIP150 to the MT plus ends in vivo, we assessed the ability of TIP150 deletion mutants to localize to the MT plus ends. Western blotting analyses indicated that various TIP150 proteins were expressed at comparable levels in HeLa cells (data not shown). An examination of positively transfected HeLa cells revealed that TIP150 deletion mutants containing the EB1-binding domain (TIP150-P) show a superimposition with that of EB1 labelling (Fig 2F–H, merges). Although deletion of the typical EB1-binding domain disrupted the plus-end tip localization of TIP150 (Fig 2E,I), the typical EB1-binding activity alone is not sufficient to target TIP150 at the plus ends (Fig 2H). An inclusion of the C-terminal coiled-coil domain together with the typical EB1-binding domain, allows a marked localization of TIP150 at the MT plus ends, which is readily apparent by the co-distribution of GFP–TIP150 and EB1 (Fig 2F,G). However, the coiled-coil domain of TIP150 neither interacts with EB1 nor localizes to the MT plus ends (data not shown); instead, it contributes to TIP150 dimerization (supplementary Fig S6 online). Thus, we conclude that TIP150 is a +TIP and that typical EB1-binding is essential for its plus-end tracking.
TIP150 associates with MCAK
Besides identifying TIP150 orthologues in vertebrates, database searches also revealed several TIP150 homologues, such as the inner centromere KinI stimulator (ICIS) in Xenopus. ICIS was observed at the inner centromere during mitosis and has been shown to be able to stimulate the polymerization activity of Xenopus MCAK (Ohi et al, 2003). However, no significant localization of TIP150 was found at the centromere of mitotic human cells (data not shown). Our recent study characterized human MCAK as a +TIP (Moore et al, 2005); thus, we sought to test whether MCAK interacts with TIP150. As shown in Fig 3A, Flag–TIP150 and the deletion mutant, which is able to localize to the MT plus ends, pull down Myc–MCAK (Fig 3A, lanes 7 and 10), suggesting that MCAK forms a cognate complex with TIP150. Indeed, the EB1-binding domain TIP150-P absorbs GFP–MCAK from HEK293T cell lysates (Fig 3B). An additional in vitro binding assay validated a direct contact between TIP150 and MCAK (Fig 3C).
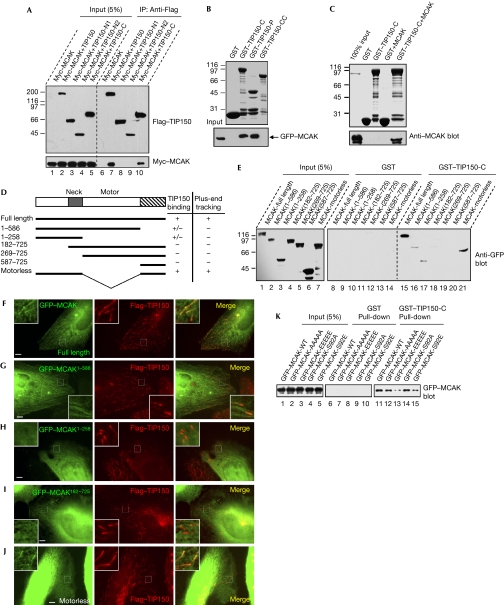
Figure 3.
TIP150 interacts with mitotic centromere-associated kinesin in vitro and in vivo. (A) Various constructs of Flag–tracking protein 150 (TIP150) and Myc–mitotic centromere-associated kinesin (MCAK) were isolated from human embryonic kidney (HEK)293T cell lysates with Flag antibodies. Immunoblotting verified co-precipitation of TIP150 (upper) and MCAK (lower). (B) Glutathione-S-transferase (GST)–TIP150 deletion proteins were used to isolate green fluorescent protein (GFP)–MCAK from HEK293T cell lysates followed by fractionation on SDS–PAGE (upper), and bound MCAK was confirmed by anti-GFP blotting (lower). Note that TIP150-P pulls down GFP–MCAK. (C) GST–TIP150-C protein was tested to bind recombinant His-MCAK. Anti-MCAK blotting (bottom) indicates that the C-terminal TIP150 physically binds to MCAK. (D) Schematic representation of MCAK deletions is shown; +, positive; +/−, weak; −, negative. Residue numbers at domain boundaries are indicated. (E) GST–TIP150-C protein was used to isolate GFP–MCAK deletion mutant proteins from HEK293T cell lysates. Anti-GFP blotting indicates that motorless MCAK binds to TIP150. (F–J) HeLa cells transfected with GFP–MCAK deletion mutants and Flag–TIP150 were processed for Flag–TIP150 (red) and (F) GFP–MCAK, (G) MCAK1−586, (H) MCAK1−258, (I) MCAK182−725 and (J) motorless. Scale bar, 5 μm. (K) GST–TIP150-C protein was used to isolate MCAK and various mutants from HEK293T lysates. Anti-GFP blotting indicates that MCAKS92E binds to TIP150 poorly.
To map the TIP150-binding activity on MCAK, we used the GST–TIP150-C protein to isolate GFP–MCAK from the lysates of HEK293T cells expressing various GFP–MCAK deletion mutants (Fig 3D). As shown in Fig 3E, the amino-terminal of MCAK binds weakly to TIP150 (Fig 3D, lane 17). However, the TIP150-binding activity was greatly enhanced when the C-terminal coiled-coil domain of MCAK was present (Fig 3D, lane 21).
To correlate TIP150 binding to the plus-end tracking of MCAK, we assessed the distribution profiles of MCAK and TIP150 in HeLa cells expressing Flag–TIP150 and GFP–MCAK deletion mutants. As shown in Fig 3F–J, both N- and C-termini of MCAK are required for a stable plus-end tracking of MCAK, which is consistent with our earlier observations (Moore et al, 2005).
Our recent study showed that aurora B phosphorylation abolishes the MT tracking activity (Moore et al, 2005). However, it has remained elusive as to how this phosphorylation prevents the plus-end tracking of MCAK. If MCAK localization depends on its interaction with TIP150, the phospho-mimicking mutant that failed to localize to the plus end could disrupt the MCAK–TIP150 interaction. Indeed, although wild-type and non-phosphorylatable MCAK proteins bind to TIP150-C (Fig 3K, lanes 11, 12 and 14), the MCAKS92E protein binds to TIP150-C weakly (Fig 3K, lanes 13 and 15). The weak binding observed between MCAKS92E and TIP150 (Fig 3K, lane 15) coincided well with the poor localization of the MCAKS92E mutant at the tip of MTs (Moore et al, 2005), suggesting that a stable interaction between TIP150 and MCAK might facilitate the plus-end localization of MCAK.
A hierarchical interaction in TIP150 complex
The direct interactions between TIP150–EB1 and TIP150–MCAK, and the correlation between the localization of MCAK deletion mutants and their interaction with TIP150 led us to speculate that these proteins might associate at the MT tips cooperatively. To investigate this, we knocked down EB1, TIP150 and MCAK using RNA-mediated interference (RNAi) in HeLa cells. Quantitative Western analyses showed that the levels of EB1, TIP150 and MCAK proteins were reduced by about 82%, 87% and 88%, respectively, by the corresponding short interfering RNA (siRNA) 48 h after the treatment. Suppression of EB1, TIP150 and MCAK did not alter the expression levels of the other proteins, and yielded no changes in tubulin level (Fig 4A) and no gross change in MT stability (supplementary Fig S7 online).
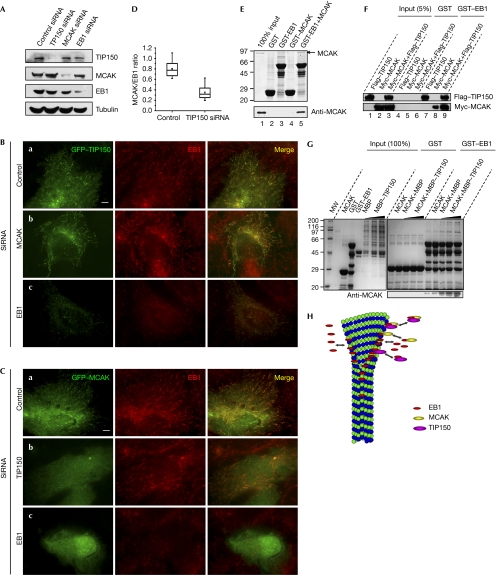
Figure 4.
A hierarchy of interaction between mitotic centromere-associated kinesin, tracking protein 150 and end-binding protein 1. (A) HeLa cells treated with indicated short interfering RNA (siRNA) oligonucleotides were collected for analyses of specificity and efficiency. (B) HeLa cells transfected with green fluorescent protein (GFP)–tracking protein 150 (TIP150) and siRNA oligonucleotides were fixed, stained and examined for end-binding protein 1 (EB1; red) and GFP–TIP150. Note that TIP150 localization to the plus end is a function of EB1. Scale bar, 5 μm. (C) HeLa cells transfected with GFP-mitotic centromere-associated kinesin (MCAK) and siRNA oligonucleotides were fixed and stained for EB1 (red) and MCAK (green). Note that MCAK localization to the plus end requires EB1 and TIP150. Scale bar, 5 μm. (D) Statistics of the MCAK/EB1 ratio at the plus ends in control and TIP150-repressed cells. The box boundaries indicate the 25th and the 75th percentiles; whiskers indicate the 95th and 5th percentiles; black square indicates the mean; straight line indicates the median. (E) Purified glutathione-S-transferase (GST)–EB1 was used to absorb recombinant His-MCAK. Western blotting shows that MCAK binds to GST–EB1 but not GST. (F) EB1 forms a complex with TIP150 and MCAK. Purified GST–EB1 protein was used to isolate MCAK and TIP150 complex from lysates of human embryonic kidney 293T cells expressing Myc–MCAK and Flag–TIP150. (G) Identification of the ternary complex of TIP150, EB1 and MCAK. GST–EB1-bound glutathione Sepharose beads were used as an affinity matrix to absorb MCAK and MCAK plus TIP150. After appropriate washes, GST–EB1-bound protein complex was solubilized in SDS–PAGE sample buffer and fractionated by SDS–PAGE, followed by Western blotting analyses of TIP150, MCAK and EB1 antibodies. An anti-MCAK antibody was also included to show the specificity of the TIP150 ternary complex. (I) Working model accounting for the functions of TIP150, EB1 and MCAK in regulating microtubule plus-end dynamics.
Next, we examined the localizations in siRNA-treated cells. Depletion of EB1 markedly reduced the MT-tip localizations of both GFP–TIP150 (Fig 4Bc) and GFP–MCAK (Fig 4Cc). Conversely, no change in the GFP–EB1 signal was observed in cells with either TIP150 (Fig 4Cb) or MCAK (Fig 4Bb) knockdown. Interestingly, the suppression of TIP150 resulted in a moderate decrease in MCAK signal at the MT plus ends (Fig 4Cb). Quantitative analysis showed that the ratio of MCAK to EB1 was reduced by 56.2% in TIP150 siRNA-treated cells when compared with the control cells (Fig 4D). By contrast, knockdown of MCAK caused no significant change in the localization of TIP150 at the MT plus ends (Fig 4Bb).
Given the recent finding of the EB1–MCAK interaction (Lee et al, 2007), we sought to examine whether TIP150 links MCAK to EB1, by which MCAK tracks MT plus ends. We confirmed the interaction between EB1 and MCAK by pull-down assay (Fig 4E). Next, we sought to test whether GST–EB1 can isolate both MCAK and TIP150 from HEK293T cells expressing Myc–MCAK, Flag–TIP150 or both. As predicted, the GST–EB1 protein absorbed abundant levels of the Myc–MCAK protein from both the MCAK and TIP150 transfected HEK293T cells (Fig 4F, lane 9). Lesser amounts of MCAK were pulled down from Myc–MCAK-transfected HEK293T cells, suggesting the possibility of a ternary complex formation of TIP150–EB1–MCAK. Indeed, our in vitro pull-down assay using GST–EB1 as an affinity matrix confirmed the presence of an endogenous complex of TIP150–EB1–MCAK (Fig 4G). Thus, we conclude that MCAK localizes to the MT plus end through its physical association with the TIP150 that binds to EB1.
In summary, our results indicate that EB1 is required for the efficient targeting of both TIP150 and MCAK at MT plus ends, which regulates the dynamics at the plus end of MTs (Fig 4H). We speculate that TIP150, similar to other +TIPs, could form a dimer through its C-terminal coiled-coil domain (Slep & Vale, 2007). This dimerization can perhaps facilitate its interaction with EB1 and other cellular proteins to orchestrate the dynamics of +TIPs. Interestingly, TIP150 contains two tandem EB1-binding domains. Further experimentation will be required to determine how these two domains coordinate their functions in orchestrating MT plus-end tracking in stationary and migratory cells. It is noteworthy that MCAK depolymerase activity is influenced by both its NH2 and COOH domains (Ems-McClung et al, 2007). Our biochemical characterization revealed that both its NH2 and COOH domains bind to TIP150 cooperatively, independently of the motor domain (Fig 3E). It would be of great interest to test whether the binding of TIP150 to MCAK modulates the conformation and depolymerase activity of MCAK. The interactions between TIP150 and MCAK, and between TIP150 and EB1 established here will provide a framework for studying molecular dynamics in great detail.
Methods
Molecular cloning. Human TIP150 complementary DNA (cDNA) was obtained from the Kazuza DNA Research Institute (Chiba, Japan). We carried out PCR using genomic DNA as a template to obtain the first exon that included the missing 5′ end of the cDNA. Those two segments were inserted sequentially into pMD-T vector (Takara, Dalian, China) to form a full cDNA of TIP150. GFP–TIP150, MBP–TIP150 and Flag–TIP150 were constructed by subcloning TIP150 from pMD-TIP150 into enhanced GFP-C1, pMAL-c2x and p3 × Flag, respectively. EB1 was PCR amplified from the human testicle cDNA library (Clontech, Mountain View, CA, USA). All TIP150, EB1 and MCAK deletions were generated using PCR.
Protein expression and purification. MBP–TIP150, GST–TIP150 deletions, GST–EB1 deletions and HIS–EB1 were expressed in Rosetta (DE3) pLysS at 30°C and purified using amylose resin, glutathione-Sepharose 4B and Ni-NTA agarose, respectively, as described previously (Yao et al, 1997). MCAK was expressed and purified as described previously (Maney et al, 1998).
Antibodies. Mouse serum against TIP150 was generated using HIS-TIP150-P in accordance with the standard protocol. Rabbit peptide antibodies against the 15 C-terminal amino acids of TIP150 were developed in Yanzyme (San Francisco, CA, USA) and affinity purified against peptide-conjugated Sepharose beads as described previously (Yao et al, 1997).
Cell culture, transfections and immunocytochemistry. HeLa and HEK293T cells were maintained in DMEM (Invitrogen, Carlsbad, CA, USA), transfected and processed for immunocytochemistry as described previously (Yao et al, 2000).
Other standard procedures are described in the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figs S1–7
Acknowledgments
This work is supported by grants from the Chinese 973 project (2002CB713700; 20076B914503), Chinese Academy of Science (KSCX1-YW-R65; KSCX2-YW-H-10) China National Key Projects for Infectious Disease (2008ZX10002-021), Chinese Natural Science Foundation (30270654, 30070349, 90508002, and 30121001); a Georgia Cancer Coalition Breast Cancer Research grant, and a grant from Atlanta Clinical & Translation Science Institute, and National Institutes of Health (DK56292 and CA132389 to X.Y.). X.Y. is a Georgia Cancer Coalition Eminent Scholar.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akhmanova A, Steinmetz MO (2008) Tracking the ends: a dynamic protein network controls the fate of MT tips. Nat Rev Mol Cell Biol 9: 309–322 [DOI] [PubMed] [Google Scholar]
- Askham JM, Vaughan KT, Goodson HV, Morrison EE (2002) Evidence that an interaction between EB1 and p150Glued is required for the formation and maintenance of a radial MT array anchored at the centrosome. Mol Biol Cell 13: 3627–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrueta L et al. (1998) The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle MTs. Proc Natl Acad Sci USA 95: 10596–10601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu W, Su LK (2003) Characterization of functional domains of human EB1 family proteins. J Biol Chem 278: 49721–49731 [DOI] [PubMed] [Google Scholar]
- Coquelle FM et al. (2002) LIS1, CLIP-170′s key to the dynein/dynactin pathway. Mol Cell Biol 22: 3089–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ems-McClung SC et al. (2007) The interplay of the N- and C-terminal domains of MCAK control MT depolymerization activity and spindle assembly. Mol Biol Cell 18: 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galjart N (2005) CLIPs and CLASPs and cellular dynamics. Nat Rev Mol Cell Biol 6: 487–498 [DOI] [PubMed] [Google Scholar]
- Grigoriev I et al. (2008) STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol 18: 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova Y, Lansbergen G, Galjart N, Akhmanova A (2005) EB1 and EB3 control CLIP dissociation from the ends of growing MTs. Mol Biol Cell 16: 5334–5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Langford KJ, Askham JM, Morrison EE (2007) MCAK associates with EB1. Oncogene 27: 2494–2500 [DOI] [PubMed] [Google Scholar]
- Maney T, Hunter AW, Wagenbach M, Wordeman L (1998) Mitotic centromere-associated kinesin is important for anaphase chromosome segregation. J Cell Biol 142: 787–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y et al. (2005) CLASP1 and CLASP2 bind to EB1 and regulate MT plus-end dynamics at the cell cortex. J Cell Biol 168: 141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Shiina N, Tsukita S (2000) The dynamic behavior of the APC-binding protein EB1 on the distal ends of MTs. Curr Biol 10: 865–868 [DOI] [PubMed] [Google Scholar]
- Moore AT et al. (2005) MCAK associates with the tips of polymerizing MTs. J Cell Biol 169: 391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison EE et al. (1998) EB1, a protein which interacts with the APC tumour suppressor, is associated with the MT cytoskeleton throughout the cell cycle. Oncogene 17: 3471–3477 [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Koyama K, Murata Y, Morito M, Akiyama T, Nakamura Y (2000) EB3, a novel member of the EB1 family preferentially expressed in the central nervous system, binds to a CNS-specific APC homologue. Oncogene 19: 210–216 [DOI] [PubMed] [Google Scholar]
- Ohi R, Coughlin ML, Lane WS, Mitchison TJ (2003) An inner centromere protein that stimulates the MT depolymerizing activity of a KinI kinesin. Dev Cell 5: 309–321 [DOI] [PubMed] [Google Scholar]
- Perez F, Diamantopoulos GS, Stalder R, Kreis TE (1999) CLIP-170 highlights growing MT ends in vivo. Cell 96: 517–527 [DOI] [PubMed] [Google Scholar]
- Slep KC, Rogers SL, Elliott S, Ohkura H, Kolodziej P, Vale RD (2005) Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the MT plus end. J Cell Biol 168: 587–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slep KC, Vale RD (2007) Structural basis of MT plus end tracking by XMAP215, CLIP-170, and EB1. Mol Cell 27: 976–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LK, Burrell M, Hill DE, Gyuris J, Brent R, Wiltshire R, Trent J, Vogelstein B, Kinzler KW (1995) APC binds to the novel protein EB1. Cancer Res 55: 2972–2977 [PubMed] [Google Scholar]
- Vaughan KT, Tynan SH, Echeverri CJ, Vallee RB (1999) Colocalization of cytoplasmic dynein with dynactin and CLIP-170 at MT distal ends. J Cell Sci 112: 1437–1447 [DOI] [PubMed] [Google Scholar]
- Wu XS, Tsan GL, Hammer JA III (2005) Melanophilin and myosin Va track the MT plus end on EB1. J Cell Biol 171: 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Anderson KL, Cleveland DW (1997) CENP-E is an integral component of kinetochore corona fibers that Link centromeres to spindle microtubules. J Cell Biol 139: 435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Abrieu A, Zheng Y, Sullivan KF, Cleveland DW (2000) CENP-E forms a link between attachment of spindle MTs to kinetochores and the mitotic checkpoint. Nat Cell Biol 2: 484–491 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs S1–7