Abstract
Context
Immunological similarities have been noted between HIV-infected individuals and older HIV-negative adults. Immunologic alterations with aging have been noted in frailty in older adults, a clinical syndrome of high risk for mortality and other adverse outcomes. Using a frailty-related phenotype (FRP), we investigated in the Multicenter AIDS Cohort Study (MACS) whether progressive deterioration of the immune system among HIV positive individuals independently predicts onset of FRP.
Methods
FRP was evaluated semiannually in 1,046 HIV-infected men from 1994–2005. CD4 T-cell count and plasma viral load were evaluated as predictors of FRP by logistic regression (GEE), adjusting for age, ethnicity, educational level, AIDS status, and treatment era (pre-HAART (1994–1995) and HAART (1996–1999 and 2000–2005)).
Results
Adjusted prevalences of FRP remained low for CD4 T-cell counts >400 cells/mm3 and increased exponentially and significantly for lower counts. Results were unaffected by treatment era. After 1996, CD4 cell T-count, but not plasma viral load, was independently associated with FRP.
Conclusion
CD4 T-cell count predicted the development of a frailty-related phenotype among HIV infected men, independent of HAART use. This suggests that compromise of the immune system in HIV-infected individuals contributes to the systemic physiologic dysfunction of frailty.
Keywords: HIV, aging, frailty, CD4 T-cell count, Highly active antiretroviral therapy, prospective population-based cohort
Introduction
Similarities in immune dysfunction between aging and HIV infection have been noted 1–3. Aging is associated with lymphopenia and a progressive deficiency of CD4 T-cells 4, 5. An increase in the subset of CD8 T-cells not expressing CD28 (CD8+CD28−) and the shortening of telomeres of these cells are hallmarks of immunosenescence 6. Such changes have also been observed during HIV infection 7, 8. It has been hypothesized that a low total lymphocyte count, which is a marker of advancing HIV infection 9, may also be a marker of a general decline in physiological functions among healthy elderly individuals 10.
Although aging is associated with increased risk of specific diseases, it is also associated with development of frailty, often not in association with any specific disease 11–14. Rather, frailty is a syndrome 15–17 that involves enhanced vulnerability to stressors and is thought to be due to multisystem dysregulation 17–19. It has a clinical phenotype of loss of muscle mass and decreased energy and reserves, with resulting weight loss and declines in strength, exercise tolerance, performance speed and physical activity 16, 17, 19, and is predictive of mortality and loss of independence 15, 16. Potential etiologic factors of frailty such as inflammation, oxidative stress, and endocrine and immune dysfunctions 20–26 have also been encountered in HIV infection 27–31. It is not known if these factors are related to frailty in HIV positive individuals 32, as they are during aging in the absence of HIV. However, the phenotype of frailty is remarkably similar to the wasting syndrome that occurs in HIV infection 33–35 which is an AIDS-defining illness 36.
We recently showed that the prevalence and duration of HIV-1 infection were associated with a frailty-like phenotype in the absence of potent antiretroviral therapy 37. Further, the onset of a wasting syndrome indicates a poor prognosis among HIV positives 38, 39. These findings support the observation that people with HIV-infection and frail HIV-uninfected older adults can have similar clinical presentations 40 as well as similar risks for mortality 41. To the extent that the etiologies of frailty are also similar in both populations, it would provide new insights into the etiology of frailty associated with aging – or perhaps other associated diseases such as cancer or congestive heart failure – and potential therapeutic interventions, e.g., therapies targeted to improving anti-inflammatory or immune homeostasis.
In the present study, we sought to provide evidence for a causal relationship between physiologic dysfunction in HIV infection and a frailty-related phenotype. Specifically, we hypothesized that viral load and its adverse effects on CD4 T-cell count would predict the occurrence of frailty in HIV positive patients, and that the prevalence of both low CD4 counts and frailty would be reduced by highly active antiretroviral therapy (HAART). To address these hypotheses, we examined the prevalence of a frailty-related phenotype in the Multicenter AIDS Cohort Study (MACS), which includes HIV positive men followed prospectively, with both CD4 T-cell counts and viral load in both the pre-HAART and HAART eras.
Methods
Study population
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective study of the natural and treated histories of HIV infection. The MACS enrolled 4,954 HIV-negative and -positive homosexual men in 1984–1985, 668 in 1987–1991, and 1,350 in 2001–2003 in sites located in Baltimore, Chicago, Los Angeles and Pittsburgh. Participants were then followed every 6 months up to the present. Detailed descriptions of the MACS have been published 42–44. Informed consent was obtained from all participants in compliance with the appropriate ethical committee at each study site. Study design and questionnaires are available at http://www.statepi.jhsph.edu/macs/macs.html.
Study participants returned every 6 months for study evaluations that include a standardized interview, physical examinations, questionnaires (including SF-36 45 and items of the Centers for Epidemiologic Studies Depression Scale (CES-D) 46), and collection of blood for laboratory testing and storage in local and national repositories 47. T-lymphocyte subsets were measured at each MACS center using a standardized flow cytometry protocol. Levels of plasma HIV RNA (viral load) were measured using either the standard reverse transcription-polymerase chain reaction assay (level of detection = 400 copies/mL; Roche Diagnostics, Nutley, NJ) or with the Roche ultrasensitive assay (level of detection = 50 copies/mL; Roche Diagnostics). Retrospective testing of stored plasma was performed for selected men using the same assays. HIV seropositivity was determined by a positive ELISA confirmed by western blot. Highly active antiretroviral therapy (HAART) was defined according to the US Department of Health and Human Services (DHHS) Kaiser Panel guidelines 48 as (1) 2 or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least 1 protease inhibitor (PI) or 1 non-nucleoside reverse transcriptase inhibitor (NNRTI), (2) 1 NRTI in combination with at least 1 PI and at least 1 NNRTI, (3) a regimen containing ritonavir and saquinavir in combination with 1 NRTI and no NNRTIs; and (4) an abacavir- or tenofovir-containing regimen of 3 or more NRTIs in the absence of PIs and NNRTIs. Combinations of zidovudine (AZT) and stavudine (d4T) with a PI or a NNRTI were not considered HAART. Therapy regimens not classified as HAART were categorized as either monotherapy or combination antiretroviral therapy.
Study outcome
The main outcome was the presence of a frailty-related phenotype (FRP), as previously defined among the HIV-negative men enrolled in the MACS 37 to approximate the phenotypic definition of frailty proposed by Fried et al. 16. This phenotype is based on measurements of five components: weakness (grip strength), slowness (time to walk 15 feet), exhaustion (self-reported), low physical activity level (measured through a weighted score of kilocalories expended per week), and weight loss (self-reported). To assess the frailty-related phenotype (see below), we utilized data collected in the MACS that reflect four of these components, including the SF-36 quality-of-life questionnaire 49 which was implemented in the MACS in April 1994 (visit 21). The FRP was identified using one item selected from the questionnaires for each out of the following four components: weight loss (answer “Yes” to “Since your last visit, have you had unintentional weight loss of at least 10 pounds”), exhaustion (answer “Yes” to “During the past 4 weeks, as a result of your physical health, have you had difficulty performing your work or other activities (for example, it took extra effort)?”), slowness (answer “Yes, limited a lot” to “Does your health now limit you in walking several blocks?”), and low physical activity level (answer “Yes, limited a lot” to “Does your health now limit you in vigorous activities, such as running, lifting heavy objects, participating in strenuous sports?”). The assessment of weakness (i.e., grip strength) was not incorporated into the MACS protocol until October 2005 and therefore could not be used in defining the FRP. A participant was considered as having the FRP at the visit if at least 3 out of the 4 components were present. The FRP thus defined had a prevalence of 4.4% among MACS HIV-uninfected men aged 65 years and older 37, which was similar to the prevalence of frailty observed in the Cardiovascular Health Study for men of similar ages 16.
Statistical Analysis
The study population was restricted to all HIV-seropositive men observed from April 1994 (visit 21) through April 2005 (visit 42), which included 1,046 men. Logistic regression models were performed using generalized estimating equations (GEE) to adjust for correlated repeated measurements within each individual 50. The outcome was FRP at each visit, and we specified an autoregressive working correlation matrix and logit-link function.
In a first analysis, we assessed the independent effects of CD4 T-cell count and plasma viral load on the prevalence of the FRP during the HAART era. Three multivariate models (models A–C) were performed. Besides potential confounders, model A included CD4 T-cell count, model B included plasma viral load, and model C included both variables. Potential confounders were age, ethnicity (white non Hispanic versus other), education (college or higher versus lower), and presence or absence of a clinical AIDS diagnosis. The values for CD4 T-cell count, plasma viral load, age, and clinical AIDS were the ones recorded at the previous visit, while the outcome was the FRP recorded at the current visit. Each visit was considered as an “AIDS-visit” if it occurred later than 6 months before the first AIDS-defining illness (i.e., if the visit occurred after the date of the first AIDS-defining illness minus 6 months), defined according to the 1993 CDC definition except that a CD4 T-cell count <200 cells/mm3 by itself was not considered AIDS 36. Fractional polynomials with a power of 1/2 were used for CD4 T-cell count to allow a flexible curve to be fitted to dose-response data 51. Since 50% of the viral load measurements were ≤ 400 copies/ml, it was not possible to analyze this variable as continuously distributed. Therefore, we categorized viral load into three indicator variables: ≤ 400 copies/ml (50% of the measurements), 401–50,000 copies/ml (39%), and > 50,000 copies/ml (11%).
In a second analysis, we evaluated the effect of HAART on the FRP by using a population-based approach using calendar time as an instrumental variable, rather than an individual-based approach. This approach has been used to assess the effectiveness of HAART on time to AIDS and death 52, 53. Three indicator variables were assigned according to calendar periods: pre-HAART (1994–1995), introduction of HAART (1996–1999), and established HAART (2000–2005) eras; the multivariate GEE model included the first two indicator variables, CD4 T-cell count modeled as in the first analysis, and the potential confounders cited above. Plasma viral load was not incorporated into the model because of insufficient data during the pre-HAART era.
To evaluate the effect of missing data on estimates of factors associated with FRP status, multiple imputations 54 were used to check the validity of the results. The Markov chain Monte Carlo method was used to impute values for each of 5 data sets (m = 5) with an arbitrary missing pattern, assuming a multivariate normal distribution for the data. SAS procedure MI was performed to impute values for each of the 4 FRP-related components when missing, using information at each visit on: income, employment status, education, smoking status, biological markers (viral load; and CD4, CD8 and CD3 T-cell counts), age, and current use of HAART.
The cut-off date of the study was the 30th of April 2005. Stata software V9.0 (Stata Corp, College Station, Texas) was used to determine the power of the fractional polynomials. SAS V9.1 (SAS Institute, Cary, North Carolina, USA) was used for the GEE models (procedure GENMOD) and multiple imputation analysis (procedure MIANALYZE).
Results
Description of the study population and person-visits
The study population consisted of 1,046 HIV positive men who were enrolled in the MACS before 1996 and who contributed 13,731 person-visits between April 1994 and April 2005. Nine hundred and forty one, 835, and 741 men contributed at least one person-visit in the 1994–1995, 1996–1999 and 2000–2005 eras, respectively. The vast majority of these men were white non-Hispanic and the majority were college-educated (Table 1). The median age in 1994 was 39 years, with an age range of 22 to 65, and the median seropositive time contributed in the analytical period (April 1994 to April 2005) was 9.2 years
Table 1.
Demographic characteristics of HIV seropositive men followed in the MACS between visit 21 (April–Sept 1994) through visit 42 (April–Sept 2005)
| Variables | N = 1,046 HIV+ men |
|---|---|
| White non Hispanic % (N) | 80% (835) |
| ≥ College % (N) | 52% (548) |
| Number of CD4 T-cell measurements*,1 | 13 (4–20) |
| Follow-up time (years)*,1 | 9.2 (3.0–10.5) |
| Age at 01/01/1994* | 39 (35–44) |
| Year of 1st clinical AIDS diagnosis | |
| Before 1996 | 25% (265) |
| 1996–1999 | 11% (115) |
| 2000–2004 | 3% (33) |
| Never | 61% (633) |
Median(IQR)
from first HIV+ visit (≥ V21, April 1994) through last visit
Of the 13,731 person-visits, the FRP could be evaluated in 12,530 (91%) person-visits. Of the 1,201 non evaluable cases, 95% resulted from missing quality-of-life data; these were evenly distributed across the three study eras (data not shown). Table 2 describes the 12,530 person-visits with evaluable FRP status according to the three eras defined above. The overall unadjusted prevalence of FRP decreased from 7.6% in 1994–1995, when approximately one quarter of visits were under monotherapy and the majority were not under treatment, to 4.5% in 2000–2005, when approximately 80% of the men were under treatment, almost all of which was HAART. The median number of CD4 T-cells increased from 323 to 494 cells/mm3, and the median age from 41 to 48 years.
Table 2.
Description of person-visits by era
| Characteristics according to calendar period | ||||
|---|---|---|---|---|
| Variables | 1994–1995 (n=2,459) | 1996–1999 (n=4,891) | 2000–2005 (n=5,180) | Overall (n=12,530) |
| Overall person-visits | ||||
| FRP % (n) | 7.6 (186) | 5.2 (255) | 4.5 (232) | 5.4 (673) |
| Current age* | 41 (37–46) | 43 (39–48) | 48 (43–52) | 45 (40–50) |
| Clinical AIDS person-visits 1 % (n) | 20.7 (509) | 20.2 (986) | 21.7 (1,126) | 20.9 (2,621) |
| Current CD4 T-cell count (cells/mm3)* | 323 (140–503) | 415 (262–609) | 494 (329–704) | 431 (268–632) |
| Current viral load (log10 copies/ml) | NA | 3.2 (<1.6–4.3) | <1.6 (<1.6–3.6) | 3.0 (<1.6–4.3) |
| Type of current treatment % (n) | ||||
| Not treated | 57.7 (1,403) | 25.5 (1,226) | 19.8 (1,014) | 29.4 (3643) |
| Monotherapy | 26.1 (634) | 3.4 (181) | 0.9 (47) | 7.0 (862) |
| Combination therapy | 16.2 (393) | 18.8 (904) | 10.6 (545) | 14.9 (1842) |
| HAART | <0.1 (2) | 52.0 (2,526) | 68.7 (3,520) | 48.7 (6028) |
| Person-visits without clinical AIDS | ||||
| FRP % (n) | 3.3 (64) | 2.4 (95) | 2.9 (118) | 2.8 (277) |
| Current age* | 41 (36–46) | 43 (39–48) | 48 (43–52) | 45 (40–50) |
| Current CD4 T-cell count (cells/mm3)* | 390 (237–551) | 457 (306–650) | 521 (361–739) | 467 (314–667) |
| Person-visits with clinical AIDS | ||||
| FRP % (n) | 24.0 (122) | 16.2 (160) | 10.1 (114) | 15.1 (396) |
| Time from AIDS-diagnosis (years)* | 0.9 (0.2–2.3) | 2.6 (1.2–4.3) | 6.5 (4.3–8.8) | 3.6 (1.4–6.6) |
| Current age* | 41 (37–45) | 43 (40–48) | 48 (44–52) | 45 (41–50) |
| Current CD4 T-cell count (cells/mm3)* | 51 (16–173) | 251 (126–395) | 378 (235–582) | 265 (108–451) |
FRP, frailty-related phenotype
Median (interquartile range)
person-visits occurring within 6 months prior to a clinical AIDS diagnosis and thereafter
To determine the independent influence of AIDS on the prevalence of FRP, we excluded person-visits occurring later than 6 months before the first AIDS-defining illness (Table 2). Among AIDS-free person visits, the prevalence of the FRP was around 3% in 1994–1995, 1996–1999, and 2000–2005. However, among person-visits occurring later than 6 months before the first AIDS-defining illness, i.e., in the presence of AIDS, the prevalence of FRP decreased by more than half (from 24.0% to 10.1%) after the advent of HAART therapy.
Risk factors for the FRP in the HAART era (1996–2005) (Table 3)
Table 3.
Odds Ratios (OR) of having the frailty-related phenotype (FRP) among HIV seropositive men followed in the MACS cohort between January 1st, 1996 and April 30th, 2005
| OR [95% CI] | ||||
|---|---|---|---|---|
| Exposures | Unadjusted models | Adjusted model A | Adjusted model B | Adjusted model C |
| < College versus ≥ college | 1.54 (1.09 – 2.17)‡ | 1.75 (1.22 – 2.51) § | 1.74 (1.21 – 2.51) § | 1.73 (1.19 – 2.50) § |
| White non Hispanic versus other | 1.62 (1.06 – 2.49)‡ | 1.29 (0.83 – 2.00) | 1.28 (0.81 – 2.01) | 1.30 (0.82 – 2.06) |
| AIDS visit versus AIDS-free visits† | 4.83 (3.51 – 6.65) § | 3.63 (2.55 – 5.16) § | 4.97 (3.53–7.01) § | 3.62 (2.50 – 5.24) § |
| Age (×10 years) | 1.44 (1.19 – 1.75) § | 1.48 (1.21 – 1.80) § | 1.54 (1.25 – 1.90) § | 1.52 (1.24 – 1.87) § |
| Plasma viral load (in copies/ml) | ||||
| ≤400 | 1 | 1 | 1 | |
| 401–50,000 | 1.03 (0.78 – 1.37) | 1.21 (0.89 – 1.64) | 0.98 (0.71 – 1.35) | |
| >50,000 | 1.72 (1.26 – 2.34) § | 1.90 (1.37 – 2.63) § | 1.09 (0.74 – 1.61) § | |
| CD4 T-cell count (in cells/mm3) | ||||
| 100 | 3.65 (2.79 – 4.77) § | 2.84 (2.11 – 3.83) § | 2.80 (1.97 – 3.98) § | |
| 200 | 2.36 (1.98 – 2.83) § | 2.00 (1.64 – 2.44) § | 1.98 (1.57 – 2.50) § | |
| 350 | 1.47 (1.35 – 1.59) § | 1.36 (1.25 – 1.49) § | 1.36 (1.22 – 1.50) § | |
| 500 | 1 | 1 | 1 | |
| 750 | 0.59 (0.53 – 0.66) § | 0.65 (0.58 – 0.74) § | 0.66 (0.57 – 0.76) § | |
OR, Odds Ratio; CI, Confidence Interval
AIDS-visit = visit occurring later than 6 months before the first AIDS-defining illness
P ≤ 0.05
P ≤ 0.01
In univariate analysis (unadjusted models), having an AIDS-defining illness was significantly associated with an increased risk of FRP, as were older age, white non-Hispanic ethnicity, and low education (< college). Of note, after excluding visits where the AIDS-defining illness was the wasting syndrome, the OR for the association of FRP with AIDS was still significantly elevated at 4.23 [95% confidence interval (CI), 3.00–5.96; p<0.01]. A lower CD4 T-cell count was highly, and significantly, predictive of onset of FRP at the subsequent visit. Finally, men with a plasma viral load level >50,000 copies/ml were more likely to manifest the FRP than men with a plasma viral load level ≤400 copies/ml (p<0.01), whereas a plasma viral load level of 401–50,000 copies/ml was not significantly associated with a higher prevalence of the FRP (p=0.81).
To assess the independent associations of viral load and CD4 T-cell count with the presence of the FRP, we utilized three multivariate models incorporating either CD4 T-cell count (model A), viral load (model B), or both (model C). Both a low CD4 T-cell count and a high plasma viral load were significant predictors individually. However, viral load was not significantly associated with the FRP in the model that included CD4 T-cell count (model C), and the effect of the CD4 T-cell count was not attenuated. In model C, the effect of having an AIDS-defining illness, a low education, and an increasing age also remained essentially unchanged from the model with CD4 T-cell count (model A). The independent association of AIDS with FRP persisted even after excluding visits in which an individual manifested the wasting syndrome [adjusted OR (aOR), 3.34; 95%CI, 2.24–4.94; p<0.01]. Of note, there was no significant interaction between CD4 T-cell count and either age, AIDS, or viral load. Because of the potential for control of viremia without full reconstitution in CD4 T-cell count, we evaluated whether the association of CD4 T-cell count with FRP persisted among treated patients who controlled HIV replication. For this analysis, we selected person-visits where patients were treated with HAART and where HIV RNA < 400 copies/ml, and found that the association of CD4 T-cell count with the FRP was essentially unchanged (aOR of 2.69 for men with 100 cells/mm3 versus 500 cells/mm3; 95%CI, 1.71–4.24; p<0.01). Further adjustment for the presence of chronic hepatitis B or C or depressive symptoms (based on the CES-D scale) did not substantially affect the association of the CD4 T-cell count with the FRP (data not shown). Hepatitis B or C status was not associated with the FRP while men with moderate depressive symptoms (scores of 16–21; aOR, 2.40; 95%CI, 1.71–3.37; p<0.01) and men with severe depressive symptoms (scores ≥ 22; adjusted OR, 3.59; 95%CI, 2.55–5.05; p<0.01) had higher risks for manifesting the FRP than the other men (scores < 16).
Relationships between the FRP and CD4 T-cell count in pre- and current HAART eras (1994–2005)
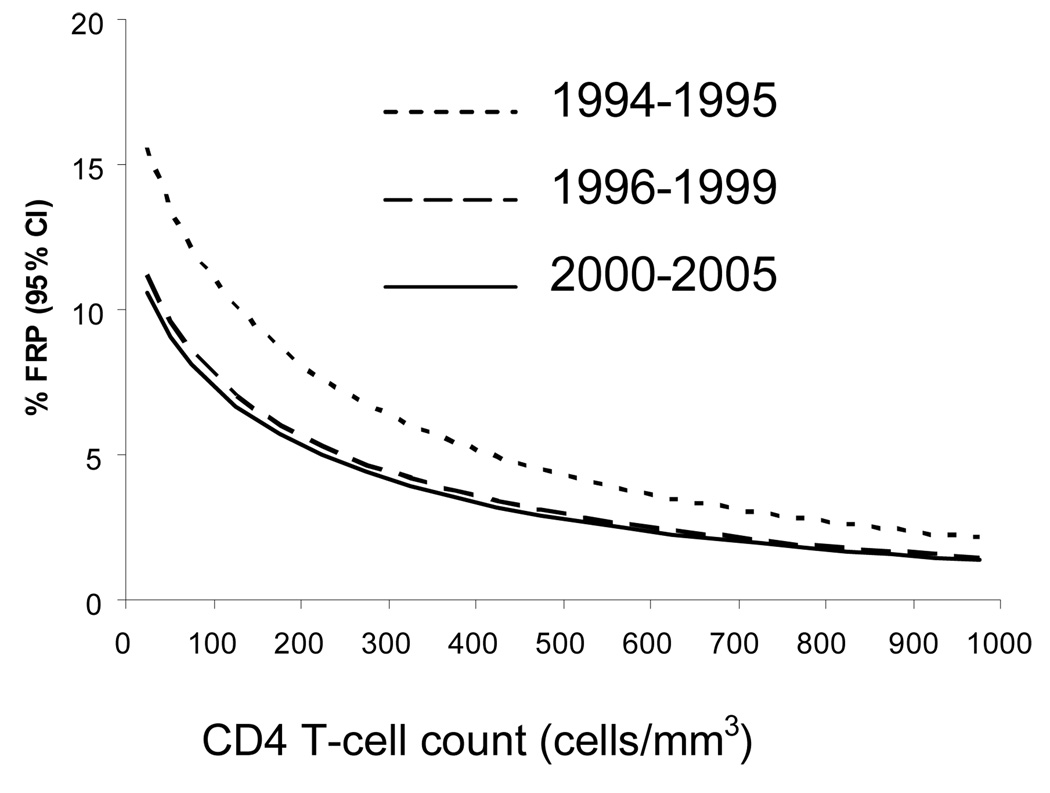
Figure 1 displays the relationship between CD4 T-cell count and the estimated prevalence of FRP according to the three calendar periods, for age fixed at 45 years and for values of ethnicity, education, and AIDS fixed at the average of the study population. There was no significant interaction between CD4 T-cell count and calendar period. Therefore, the shapes of the relationships between CD4 T-cell count and estimated prevalence of FRP were similar in all eras. However, after adjustment for age, CD4 T-cell count, clinical AIDS status, education, and ethnicity, the overall prevalence of FRP was higher in the pre-HAART era (1994–1995) than in both the introduction of HAART (1996–1999) (aOR, 1.47; 95%CI, 1.16–1.86; p<0.01) and established (2000–2005) HAART eras (aOR, 1.56; 95%CI, 1.15–2.11; p<0.01), while the prevalence of FRP was similar in the introduction of HAART era compared to established HAART era (aOR, 1.06; 95%CI, 0.82–1.37; p=0.64).
Figure 1.
Estimated prevalences of a frailty-related phenotype (FRP) as a function of CD4 T-cell count in the MACS for fixed values of age (45 years), fixed percentage for ethnicity (80% White non Hispanic), education (52% ≥ college), and prevalence of AIDS (20%), in the pre-HAART era (1994–1995; dotted line), introduction of HAART era (1996–1999; dashed line), and established HAART era (2000–2005; plain line). The curves for the two HAART eras do not differ significantly from each other, but both are significantly different from that of the pre-HAART era (see text for Odds Ratios and p-values comparing the three different eras).
To estimate the potential impact of missing data on predictors of FRP (8.8% of the 13,731 person-visits), a 5-set multiple imputation analysis was carried out. Estimates and standard errors calculated from the multiple imputation model remained essentially unchanged from those provided in the above analysis (data not shown).
Discussion
This study extends a previous report in which we defined a frailty-related phenotype (FRP) 37 to approximate the clinical definition of a frailty phenotype by Fried et al. 16, and showed it to be associated with HIV-1 infection 37. In the present study, a low CD4 T-cell count was an independent, significant predictor of the FRP in HIV-positive men, adjusting for AIDS, and also in the absence of having a wasting syndrome. Furthermore, the association between CD4 T-cell count and the FRP remained the same after restriction to HAART-treated men with a good virological response to HAART (whether they had a CD4 cell response or not), or after taking into account hepatitis B or C status and depressive symptoms. These findings provide evidence that the FRP is etiologically related to a compromised immune system as associated with HIV infection. Of note, the findings of the present study parallel other work in older, HIV-uninfected individuals which indicates that a compromised immune system is associated with frailty as well as predictive of mortality 26, 55–57. Taken together, these findings support the notion of a common etiology of frailty associated with aging and with HIV.
The finding that the prevalence of FRP declined by about 50% from the pre-HAART to the HAART era provides the first evidence that improvement of immune function can reduce the development of a frailty-like presentation. One explanation for this result might be decreased immune activation due to suppression of viral replication and viremia. Another explanation might be the psychological positive impact of the introduction of HAART in the HIV population 58, 59, which could have reduced the onset of frailty through an increase in mental health and/or physical activity 19. Whatever the mechanism, this observation has important implications for potential prevention of frailty in aging, as well as in HIV infection.
Of note, we estimated that in the current HAART era the impact of a 10-year increase in age was similar to that of a decrease of 250 CD4 T-cells/mm3 for average ages and CD4 T-cell counts. Even at relatively high CD4 T-cell counts, deterioration of the immune system conferred an effect similar to that associated with aging in increasing the risk of developing the FRP. These data are consistent with an important role of the immune system in the aging process, and specifically in frailty 24, although the specific relationship between CD4 T-cell counts and the aging-related outcome described here would not necessarily be expected to pertain to HIV-uninfected populations. Frailty is a late-stage clinical syndrome that in older adults is hypothesized to result from dysregulation or failure of many physiological systems (17–19 and Fried et al., Manuscript Submitted). It is of import that this report indicates that widespread use of HAART in HIV-infected persons was associated with an overall decrease in the prevalence of the FRP. This suggests that immunotherapy could have a role in the prevention or treatment of frailty in aging, even though it directly targets only one of the many systems likely to be dysregulated in frailty. To clarify the import of the decrease in FRP associated with HAART, it will now be important to determine whether frailty in HIV infection is a predictor of vulnerability to adverse outcomes, including mortality, as it is in aging in the absence of HIV infection, and if so whether this is independent of immune compromise and other consequences of HIV infection. Our preliminary analyses suggest that this is indeed the case (41, manuscript in preparation).
We observed that among AIDS-free men, the prevalence of FRP remained stable across calendar periods. One explanation for this finding could be that the negative impact of age is balanced by the positive impact of the increase in CD4 T-cell count due to HAART. Conversely, among men with clinically-defined AIDS, the prevalence of the FRP strongly decreased between 1994–1995 and 2000–2005. The difference between the responses of men with and without AIDS probably reflects the greater increase in CD4 T-cell count induced by HAART in the former group compared to the latter group (Table 2). After adjustment for ethnicity, education, and AIDS status, we found that the prevalence of the FRP decreased with the introduction of HAART but did not decrease further as the HAART era progressed. This result is consistent with the finding that the CD4 T-cell count increases primarily within the first two years after HAART initiation 60.
Several findings from the present study may be important for both HIV and frailty research. First, the non linear association between the prevalence of the FRP and CD4 T-cell count suggests that in HIV-infected men there may be a threshold CD4 T-cell level at which the FRP develops or becomes manifest. Second, in the HAART era, men with a high viral load (> 50,000 copies/ml) did not manifest the FRP more frequently than those with low viral load (< 400 copies/ml) after adjustment for age and CD4 T-cell count. This is consistent with the idea that the presence of FRP may be on the same causal pathway as viral load and its effect on CD4 T-cell count, but related more closely to the CD4 T-cell count itself, as discussed above. Alternatively, we may not have had the power to detect an association with viral load, since only 11% of viral load measurements were above 50,000 copies/ml. However, the large decrease in the point estimate for this category after adjusting for CD4 T-cell count argues against this explanation. Third, the prevalence of FRP decreased since the introduction of HAART independently of CD4 T-cell count, which implies that the effect of HAART is not fully mediated through the increase in CD4 T-cell count.
Anemia as well as co-morbidities such as chronic renal disorders have been shown to be associated with frailty in the elderly HIV-uninfected population 61, 62, and also with HIV infection 63, 64These factors were not included in this analysis because they are likely to be in the causal pathway for the frailty-related phenotype (FRP), i.e., anemia and chronic diseases might be consequences of the decrease in CD4 T-cell count. Analyzing these factors as if they were confounders would lead to bias, and may erroneously attenuate the association between CD4 cell count and frailty 65.
This study has some limitations. First, we studied a frailty-like phenotype rather than the exact frailty phenotype previously defined and validated 16. This approach was dictated by the data available in the study. Designed to approximate the frailty phenotype for 4 of 5 validated criteria for defining the frailty phenotype, its likely decreased specificity still yielded meaningful findings. Second, although we looked at the association between FRP at a given visit and CD4 T-cell count and/or viral load at the previous visit using longitudinal prospective data, we cannot conclude from our observational study that CD4 T-cell count (and not viral load) is a causal factor of the occurrence of the FRP. Third, a substantial proportion (8.8%) of person-visits could not be classified as having or not having the FRP. However, the vast majority of these were due to missing quality-of-life questionnaire data, and the consistency of the results obtained from multiple imputations argues that the amount of bias introduced by missing data, if any, was minimal. Fourth, we used calendar era as an instrumental variable for use of therapy. The close link between availability of HAART, its uptake in the cohort, and calendar time permits the effects of HAART to be examined on the population level as done by others 52, 53. However, in a given era not all infected persons utilized the same treatment, nor was HAART a uniform therapeutic regimen. Further analyses are necessary to study the effect of HAART on the manifestation of FRP at the individual level. Finally, our study population was composed of men only, so no inference can be drawn concerning occurrence of frailty in women.
Aging is associated with a decline in immune function and increased inflammatory activity, as well as with increased levels of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) 24. In the elderly, elevated IL-6 levels have been reported to be associated with physical decline, age-related diseases (such as Alzheimer disease, osteoporosis, cognitive decline), and frailty 20, 66–69, while elevated TNF-α levels are believed to be associated with mortality 70, 71. Several studies have also reported elevated levels of those cytokines in HIV infection 72–75. It has also recently been shown that the number of CD8 T-cells that have lost expression of the co-stimulatory receptor CD28 (CD8+CD28−) was greater among frail elderly women than in their non frail counterparts 76. This T-cell subset is believed to be a good biological marker of intrinsic aging of the immune system 77; it is also considered as a marker of immunosenescence resulting from chronic activation of the immune system 78, 79, where chronic infections such as cytomegalovirus 80 or HIV 81 may play a important role; indeed, markers of chronic CMV infection are associated with frailty 82. In addition to our previous results that suggested HIV infection might accelerate some manifestations of aging 37, the present study provides biologic plausibility for the hypotheses that frailty is a final common pathway from a variety of processes (e.g., chronic diseases and aging), where the secondary immunologic dysfunction appears to be the driver more than the ultimate cause 83, 84. The evidence of decreased rates of a frailty-like phenotype in the HAART era provides the first evidence of the potential to prevent this predictor of mortality.
ACKNOWLEDGMENTS
The Multicenter AIDS Cohort Study (MACS) is funded by the National Institute of Allergy and Infectious Diseases, with additional supplemental funding from the National Cancer Institute and the National Heart, Lung and Blood Institute (UO1-AI-35042, 5-MO1-RR-00722 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613,UO1-AI-35041).
Data in this manuscript were collected by the MACS with centers (Principal Investigators) at The Johns Hopkins University Bloomberg School of Public Health (Joseph B. Margolick, Lisa Jacobson), Howard Brown Health Center and Northwestern University Medical School (John Phair), University of California, Los Angeles (Roger Detels), and the University of Pittsburgh (Charles Rinaldo). The MACS website located at http://www.statepi.jhsph.edu/macs/macs.html.
Footnotes
Some of the results were presented at: XVI International AIDS Conference 2006, Toronto 2006 [Poster].
Contributor Information
Loic Desquilbet, Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University; 615 North Wolfe Street, Room E7644; Baltimore, Maryland MD 21205; +1 (410) 955-4320; Email: desquilbet@agroparistech.fr.
Joseph B. Margolick, Department of Molecular Microbiology and Immunology, Bloomberg School of Public Health, Johns Hopkins University; 615 North Wolfe Street; Baltimore, Maryland 21205; Tel: +1 (410) 283-6517; Email: jmargoli@jhsph.edu.
Linda P. Fried, Division of Geriatric Medicine and Gerontology and Center on Aging and Health, Johns Hopkins School of Medicine; 2024 E Monument Street, Suite 2-700; Baltimore, Maryland 21205; +1 (410) 955-0491; Email: lfried@jhmi.edu.
John P. Phair, Howard Brown Health Center and Department of Medicine, Northwestern University; 676 N Street Clair, Suite 200, Chicago, Illinois 60611; Tel: +1 (312) 695-5065; Email: j-phair@northwestern.edu.
Beth D. Jamieson, Department of Medicine, Geffen School of Medicine, UCLA Medical Center, University of California; 10833 LeConte Avenue; Los Angeles, California 90095; Tel: +1 (310) 794-9491; Email: bjamieso@ucla.edu.
Marcy Holloway, Department of Infectious Diseases and Microbiology, University of Pittsburgh; A419 Crabtree Hall; Pittsburgh, Pennsylvania 15261; Tel: +1 (412) 383-1673; mnh75@hotmail.com.
Lisa P. Jacobson, Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University; 615 North Wolfe Street, Room E7646; Baltimore, Maryland 21205; +1 (410) 502-9770; Email: ljacobso@jhsph.edu.
References
- 1.Appay V, Rowland-Jones SL. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 2002 Dec;23(12):580–585. doi: 10.1016/s1471-4906(02)02338-4. [DOI] [PubMed] [Google Scholar]
- 2.Kalayjian RC, Landay A, Pollard RB, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis. 2003 Jun 15;187(12):1924–1933. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 3.van Baarle D, Tsegaye A, Miedema F, Akbar A. Significance of senescence for virus-specific memory T cell responses: rapid ageing during chronic stimulation of the immune system. Immunol Lett. 2005 Feb 15;97(1):19–29. doi: 10.1016/j.imlet.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Rea IM, Stewart M, Campbell P, Alexander HD, Crockard AD, Morris TC. Changes in lymphocyte subsets, interleukin 2, and soluble interleukin 2 receptor in old and very old age. Gerontology. 1996;42(2):69–78. doi: 10.1159/000213775. [DOI] [PubMed] [Google Scholar]
- 5.Calderon E, Sanchez B, Medrano FJ, Stiefel P, Leal M. CD4+ T-lymphocytopenia in the elderly. Eur J Clin Microbiol Infect Dis. 1995 Jan;14(1):75–77. doi: 10.1007/BF02112629. [DOI] [PubMed] [Google Scholar]
- 6.Effros RB. Long-term immunological memory against viruses. Mech Ageing Dev. 2000 Dec 20;121(1–3):161–171. doi: 10.1016/s0047-6374(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 7.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005 Jun;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 8.Effros RB, Allsopp R, Chiu CP, et al. Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996 Jul;10(8):F17–F22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Margolick JB, Munoz A, Donnenberg AD, et al. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. The Multicenter AIDS Cohort Study. Nat Med. 1995 Jul;1(7):674–680. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 10.Izaks GJ, Remarque EJ, Becker SV, Westendorp RG. Lymphocyte count and mortality risk in older persons. The Leiden 85-Plus Study. J Am Geriatr Soc. 2003 Oct;51(10):1461–1465. doi: 10.1046/j.1532-5415.2003.51467.x. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP. Conference on the physiologic basis of frailty. April 28, 1992, Baltimore, Maryland, U.S.A. Introduction. Aging (Milano) 1992 Sep;4(3):251–252. [PubMed] [Google Scholar]
- 12.Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing. 1997 Jul;26(4):315–318. doi: 10.1093/ageing/26.4.315. [DOI] [PubMed] [Google Scholar]
- 13.Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998 Jan;53(1):S9–S16. doi: 10.1093/geronb/53b.1.s9. [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L, Cavazzini C, Corsi A, et al. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25(10 Suppl):10–15. [PubMed] [Google Scholar]
- 15.Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of Frailty: Characterization in the Women's Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006 Mar;61(3):62–66. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Walston J. Frailty and Failure to Thrive. In: Hazzard WR, Blass JP, Ettinger WH Jr, Halter JB, Ouslander J, editors. Principles of Geriatric Medicine and Gerontology. New York: McGraw Hill; 1998. pp. 1387–1402. [Google Scholar]
- 18.Lipsitz LA. Physiological complexity, aging, and the path to frailty. Sci Aging Knowledge Environ. 2004 Apr 21;2004(16):pe16. doi: 10.1126/sageke.2004.16.pe16. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005 Aug 3;2005(31):pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 20.Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003 Feb;23(1):15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 21.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007 Jun;55(6):864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 22.Ershler WB. A gripping reality: oxidative stress, inflammation, and the pathway to frailty. J Appl Physiol. 2007 Jul;103(1):3–5. doi: 10.1152/japplphysiol.00375.2007. [DOI] [PubMed] [Google Scholar]
- 23.Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999 Sep;83(5):1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Patel DD, Manton KG. The immune system in aging: roles of cytokines, T cells and NK cells. Front Biosci. 2005 Jan 1;10:192–215. doi: 10.2741/1521. [DOI] [PubMed] [Google Scholar]
- 25.Walston J. Frailty--the search for underlying causes. Sci Aging Knowledge Environ. 2004 Jan 28;2004(4):pe4. doi: 10.1126/sageke.2004.4.pe4. [DOI] [PubMed] [Google Scholar]
- 26.Paganelli R, Di Iorio A, Cherubini A, et al. Frailty of older age: the role of the endocrine--immune interaction. Curr Pharm Des. 2006;12(24):3147–3159. doi: 10.2174/138161206777947533. [DOI] [PubMed] [Google Scholar]
- 27.Israel N, Gougerot-Pocidalo MA. Oxidative stress in human immunodeficiency virus infection. Cell Mol Life Sci. 1997 Dec;53(11–12):864–870. doi: 10.1007/s000180050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gil L, Martinez G, Gonzalez I, et al. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res. 2003 Mar;47(3):217–224. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 29.Connolly NC, Riddler SA, Rinaldo CR. Proinflammatory cytokines in HIV disease-a review and rationale for new therapeutic approaches. AIDS Rev. 2005 Jul–Sep;7(3):168–180. [PubMed] [Google Scholar]
- 30.Grinspoon SK, Bilezikian JP. HIV disease and the endocrine system. N Engl J Med. 1992 Nov 5;327(19):1360–1365. doi: 10.1056/NEJM199211053271906. [DOI] [PubMed] [Google Scholar]
- 31.Corcoran CP, Grinspoon S. Diagnosis and treatment of endocrine disorders in the HIV-infected patient. J Int Assoc Physicians AIDS Care. 1998 Feb;4(2):10–14. 34. [PubMed] [Google Scholar]
- 32.Margolick JB, Chopra RK. Relationship between the immune system and frailty: pathogenesis of immune deficiency in HIV infection and aging. Aging (Milano) 1992 Sep;4(3):255–257. doi: 10.1007/BF03324101. [DOI] [PubMed] [Google Scholar]
- 33.Coodley GO, Loveless MO, Merrill TM. The HIV wasting syndrome: a review. J Acquir Immune Defic Syndr. 1994 Jul;7(7):681–694. [PubMed] [Google Scholar]
- 34.Smit E, Skolasky RL, Dobs AS, et al. Changes in the incidence and predictors of wasting syndrome related to human immunodeficiency virus infection, 1987–1999. Am J Epidemiol. 2002 Aug 1;156(3):211–218. doi: 10.1093/aje/kwf039. [DOI] [PubMed] [Google Scholar]
- 35.Shikuma CM, Valcour VG, Ratto-Kim S, et al. HIV-associated wasting in the era of highly active antiretroviral therapy: a syndrome of residual HIV infection in monocytes and macrophages? Clin Infect Dis. 2005 Jun 15;40(12):1846–1848. doi: 10.1086/430376. [DOI] [PubMed] [Google Scholar]
- 36.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992 Dec 18;41(RR17):1–19. [PubMed] [Google Scholar]
- 37.Desquilbet L, Jacobson LP, Fried LP, et al. HIV-1 Infection Is Associated With an Earlier Occurrence of a Phenotype Related to Frailty. J Gerontol A Biol Sci Med Sci. 2007 Nov;62(11):1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler DA, Gibert CL, Launer CA, et al. Weight loss as a predictor of survival and disease progression in HIV infection. Terry Beirn Community Programs for Clinical Research on AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1998 May 1;18(1):80–85. doi: 10.1097/00042560-199805010-00012. [DOI] [PubMed] [Google Scholar]
- 39.Palenicek JP, Graham NM, He YD, et al. Weight loss prior to clinical AIDS as a predictor of survival. Multicenter AIDS Cohort Study Investigators. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Nov 1;10(3):366–373. [PubMed] [Google Scholar]
- 40.O'Dell MW, Levinson SF, Riggs RV. Focused review: physiatric management of HIV-related disability. Arch Phys Med Rehabil. 1996 Mar;77(3 Suppl):S66–S73. doi: 10.1016/s0003-9993(96)90247-6. [DOI] [PubMed] [Google Scholar]
- 41.Desquilbet L, Margolick JB, Fried LP, et al. Frailty and time from HAART initiation to AIDS or death. Paper presented at XVI International AIDS Conference; Toronto. 2006. [Google Scholar]
- 42.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR., Jr The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987 Aug;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson LP, Kirby AJ, Polk S, et al. Changes in survival after acquired immunodeficiency syndrome (AIDS): 1984–1991. Am J Epidemiol. 1993 Dec 1;138(11):952–964. doi: 10.1093/oxfordjournals.aje.a116815. [DOI] [PubMed] [Google Scholar]
- 44.Dudley J, Jin S, Hoover D, Metz S, Thackeray R, Chmiel J. The Multicenter AIDS Cohort Study: retention after 9 1/2 years. Am J Epidemiol. 1995 Aug 1;142(3):323–330. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 45.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994 Jan;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 47.Jacobson LP, Phair JP, Yamashita TE. Update on the Virologic and Immunologic Response to Highly Active Antiretroviral Therapy. Curr Infect Dis Rep. 2004 Aug;6(4):325–332. doi: 10.1007/s11908-004-0055-9. [DOI] [PubMed] [Google Scholar]
- 48.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002 Sep 3;137(5 Pt 2):381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 49.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]
- 50.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42(1):121–130. [PubMed] [Google Scholar]
- 51.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999 Oct;28(5):964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 52.Detels R, Munoz A, McFarlane G, et al. Effectiveness of potent antiretroviral therapy on time to AIDS and death in men with known HIV infection duration. Multicenter AIDS Cohort Study Investigators. JAMA. 1998 Nov 4;280(17):1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- 53.Schneider MF, Gange SJ, Williams CM, et al. Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984–2004. AIDS. 2005 Nov 18;19(17):2009–2018. doi: 10.1097/01.aids.0000189864.90053.22. [DOI] [PubMed] [Google Scholar]
- 54.Rubin DB. Multiple Imputation After 18+ Years. J Am Stat Assoc. 1996;91(434):473–489. [Google Scholar]
- 55.Ginaldi L, De Martinis M, Monti D, Franceschi C. Chronic antigenic load and apoptosis in immunosenescence. Trends Immunol. 2005 Feb;26(2):79–84. doi: 10.1016/j.it.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Roberts-Thomson IC, Whittingham S, Youngchaiyud U, Mackay IR. Ageing, immune response, and mortality. Lancet. 1974 Aug 17;2(7877):368–370. doi: 10.1016/s0140-6736(74)91755-3. [DOI] [PubMed] [Google Scholar]
- 57.Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998 May 15;102(2–3):187–198. doi: 10.1016/s0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 58.Rabkin JG, Ferrando S. A 'second life' agenda. Psychiatric research issues raised by protease inhibitor treatments for people with the human immunodeficiency virus or the acquired immunodeficiency syndrome. Arch Gen Psychiatry. 1997 Nov;54(11):1049–1053. doi: 10.1001/archpsyc.1997.01830230091013. [DOI] [PubMed] [Google Scholar]
- 59.Rabkin JG, Ferrando SJ, Lin SH, Sewell M, McElhiney M. Psychological effects of HAART: a 2-year study. Psychosom Med. 2000 May–Jun;62(3):413–422. doi: 10.1097/00006842-200005000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Tarwater PM, Margolick JB, Jin J, et al. Increase and plateau of CD4 T-cell counts in the 3(1/2) years after initiation of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2001 Jun 1;27(2):168–175. doi: 10.1097/00126334-200106010-00012. [DOI] [PubMed] [Google Scholar]
- 61.Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005 Jun;60(6):729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 62.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004 May;43(5):861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 63.Kreuzer KA, Rockstroh JK. Pathogenesis and pathophysiology of anemia in HIV infection. Ann Hematol. 1997 Nov–Dec;75(5–6):179–187. doi: 10.1007/s002770050340. [DOI] [PubMed] [Google Scholar]
- 64.Krawczyk CS, Holmberg SD, Moorman AC, Gardner LI, McGwin G., Jr Factors associated with chronic renal failure in HIV-infected ambulatory patients. AIDS. 2004 Nov 5;18(16):2171–2178. doi: 10.1097/00002030-200411050-00009. [DOI] [PubMed] [Google Scholar]
- 65.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002 Jan 15;155(2):176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 66.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999 Jun;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 67.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 68.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002 Dec;50(12):1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 69.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002 Aug 13;59(3):371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 70.Bruunsgaard H. Effects of tumor necrosis factor-alpha and interleukin-6 in elderly populations. Eur Cytokine Netw. 2002 Oct–Dec;13(4):389–391. [PubMed] [Google Scholar]
- 71.Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin Exp Immunol. 2003 Apr;132(1):24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birx DL, Redfield RR, Tencer K, Fowler A, Burke DS, Tosato G. Induction of interleukin-6 during human immunodeficiency virus infection. Blood. 1990 Dec 1;76(11):2303–2310. [PubMed] [Google Scholar]
- 73.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990 Jan 15;144(2):480–484. [PubMed] [Google Scholar]
- 74.Grimaldi LM, Martino GV, Franciotta DM, et al. Elevated alpha-tumor necrosis factor levels in spinal fluid from HIV-1-infected patients with central nervous system involvement. Ann Neurol. 1991 Jan;29(1):21–25. doi: 10.1002/ana.410290106. [DOI] [PubMed] [Google Scholar]
- 75.Aukrust P, Liabakk NB, Muller F, Lien E, Espevik T, Froland SS. Serum levels of tumor necrosis factor-alpha (TNF alpha) and soluble TNF receptors in human immunodeficiency virus type 1 infection--correlations to clinical, immunologic, and virologic parameters. J Infect Dis. 1994 Feb;169(2):420–424. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- 76.Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Exp Gerontol. 2005 Jan–Feb;40(1–2):81–87. doi: 10.1016/j.exger.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004 Mar;10(3):119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Globerson A, Effros RB. Ageing of lymphocytes and lymphocytes in the aged. Immunol Today. 2000 Oct;21(10):515–521. doi: 10.1016/s0167-5699(00)01714-x. [DOI] [PubMed] [Google Scholar]
- 79.Tarazona R, Solana R, Ouyang Q, Pawelec G. Basic biology and clinical impact of immunosenescence. Exp Gerontol. 2002 Jan–Mar;37(2–3):183–189. doi: 10.1016/s0531-5565(01)00182-6. [DOI] [PubMed] [Google Scholar]
- 80.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005 Jun;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 81.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infectionq. Exp Gerontol. 2007 May;42(5):432–437. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 82.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005 May;53(5):747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 83.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006 Jun;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 84.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008 Jan;214(2):231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]