Abstract
Background
Caspase-3, a pro-apoptotic enzyme, represents a class of proteins in which the active site contains reduced thiol (S-H) groups and is modulated by heavy metal cations such as Zn2+. We explored the effects of the thiol oxidant monochloramine (NH2Cl) on caspase-3 activity within cells of isolated rabbit gastric glands. In addition, we tested the hypothesis that NH2Cl-induced alterations of caspase-3 activity are modulated by oxidant-induced accumulation of Zn2+ within the cytoplasm.
Materials and Methods
Isolated gastric glands were prepared from rabbit mucosa by collagenase digestion. Caspase-3 activity was measured colorimetrically in suspensions of healthy rabbit gastric glands, following exposure to various concentrations of NH2Cl with or without the zinc chelator TPEN for 1 hour, and re-equilibration in Ringer's solution for 5 hours. Conversion of procaspase 3 to active caspase-3 was monitored by Western blot.
Results
Monochloramine inhibited caspase-3 activity in a dose dependent fashion. At concentrations of NH2Cl up to 100μM, these effects were prevented if TPEN was given concurrently and were partly reversed if TPEN was given one hour later. Caspase-3 activity was preserved by concurrent treatment with a thiol-reducing agent, dithiothreitol (DTT).
Conclusions
At pathologically relevant concentrations, NH2Cl impairs caspase-3 activity through oxidation of its thiol groups. Independently from its thiol oxidant effects on the enzyme, NH2Cl-induced accumulation of Zn2+ in the cytoplasm is sufficient to restrain endogenous caspase-3 activity. Our studies suggest that some bacterially generated oxidants such as NH2Cl impair host pathways of apoptosis through release of Zn2+ from endogenous pools.
Keywords: monochloramine, caspase-3, zinc, Helicobacter pylori, gastritis, oxidants, oxidative stress
Introduction
Infestation of the gastric mucosa by Helicobacter pylori leads to persistent mucosal injury. In Western countries, H. pylori infestation mucosal injury is often muted, and is associated with chronic inflammatory infiltrates, chronic peptic ulcer disease, and malignancy (1). In acute H. pylori gastritis, the intense inflammatory response is marked by neutrophil-enriched infiltrates that lead to severe erosions and bleeding (1, 2).
The acute inflammatory respose is characterized by the uncontrolled accumulation of a diverse array of oxidants, including monochloramine (2-4). Monochloramine (NH2Cl) is produced from the spontaneous reaction of hypochlorous acid (HOCl), generated by the activated neutrophil, and ammonia (NH3), produced through the action of H. pylori ureases (2, 5). It has been estimated that NH2Cl concentrations of up to 200μM may accumulate in acutely inflamed regions of gastrointestinal mucosa (5). Among the principal intracellular targets of monochloramine are cysteine-rich proteins containing reduced sulfhydryl (thiol) residues (6, 7).
Apoptosis, or programmed cell death, is activated in response to different forms of stress, including stimuli that are exogenous to the cell and signals that are generated within the cell (1, 8, 9). A key enzyme in the initiation of apoptosis is caspase-3. Activation of caspase-3 occurs through proteolytic cleavage of the Ile-Glu-Thr-Asp motif in the inactive procaspase-3, which after acidification dissipates the tri-aspartate residue “safety catch” that resists autocatalysis or premature cleavage by initiating caspases (10).
The active site of the caspase-3 molecule is composed of reduced cysteine residues that are potentially susceptible to oxidation and inactivation by thiol directed oxidants (10, 11). In addition, reversible modulation of caspase-3 activity by variation of intracellular zinc ([Zn2+]i) has been demonstrated in cell-free experimental systems (12-15). Recent studies in our laboratory indicate that exposure of gastric parietal cells to NH2Cl at pathologically relevant concentrations releases Zn2+ from thiol-rich intracellular pools in alimentary tract epithelial cells, including the gastric parietal cell (16, 17).
These considerations led us to hypothesize that caspase-3 activity is susceptible to oxidation by NH2Cl and modulated by the concurrent accumulation of Zn2+. The goals of this study were: first, to determine whether loading of gastric epithelial cells with oxidant-relevant concentrations of Zn2+ (nanomolar range (16)) alters endogenous caspase-3 activity; second, to determine whether exposure to NH2Cl leads to decreases in endogenous caspase-3 activity that are modulated by the concurrent accumulation of Zn2+; and, third, to determine whether these effects are attributable to oxidation of intracellular thiol groups.
Materials and Methods
Gland isolation
Anesthesia and euthanasia for New Zealand White rabbits were approved according to policies of Harvard Medical School. Gastric glands were harvested as described previously (17, 18). Rabbit gastric glands prepared by this method yield a homogenous gland preparation consisting of approximately 50% parietal cells and 40% chief cells (19-22).
Preparation of Monochloramine
Monochloramine (NH2Cl) was prepared as described previously (16, 17). Briefly, a 200μl solution containing 500mM NaOCl in water was added dropwise to 10ml of 20mM NH4Cl and 5mM Na2HPO4 in water at 0°C. This procedure resulted in a 5mM NH2Cl solution. Use of concentrated NH2Cl solutions was completed within 6 hours of preparation. Over this time course we observed that NH2Cl remained stable in Ringer solution at concentrations ranging from 50 to 200μM, with <10% loss of absorbance at 242nm. The concentrated solution was kept on ice and then diluted to the final concentration just before each experiment. Taurine monochloramine (TaurNHCl) was generated under similar conditions by including taurine instead of NH4Cl in the reaction mixture (5, 16, 23). Concentrations were verified by measuring absorbance in a UV spectrophotometer at 242, 292, and 252nm for NH2Cl, HOCl, and TaurNHCl, respectively. [NH2Cl], [HOCl], and [TaurNHCl] were then quantified with molar extinction coefficients reported previously (23).
Caspase-3 activity
Caspase-3 activity was measured using assay systems obtained from Sigma-Aldrich, which are based on the hydrolysis of the peptide substrate Ac-DEVD-pNA (acetyl-Asp-Glu-Val-Asp p-nitroanilide). This leads to release of p-nitroaniline, which can be measured by absorbance at 405nm. Suspensions of healthy glands (∼800μl, which ultimately provides 3μg/ml to 5μg/ml protein by Bradford assay) were exposed to control and experimental Ringer's solutions for a total of 6 hours at 37°C, then lysed and subjected to brief centrifugation (16,100 × g for 5 minutes). Cytosol supernatants were assayed on 96 well plates per protocol (CASP3C, Sigma-Aldrich, St. Louis), with readout (Ex 485nm; Em 528nm) after 18 to 20 hours of incubation at 37°C with Ac-DEVD-pNA. In preliminary studies, we confirmed the stability of both Ac-DEVD-pNA alone and p-nitroaniline (after hydrolysis using purified caspase-3) in the presence of 200μM NH2Cl (data not shown). As NH2Cl appeared to interfere with the Bradford assay dose-dependently, results were normalized to control glands from the same aliquot as experimental groups.
Additional Reagents and Methods
TPEN (tetrakis-(2-pyridylmethyl)ethylene diamine) and BAPTA (tetramethyl1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra acetate) in its acetoxymethyl ester form were obtained from Molecular Probes (Eugene, OR). TPEN was dissolved directly in Ringer's, BAPTA was first dissolved in DMSO and then diluted 1:1000 in Ringer's solution. Dithiothreitol (DTT) and ascorbic acid (both from Sigma) were dissolved in Ringer's. All solutions were checked for changes in pH and adjusted if necessary to baseline pH. For Ringer's and intracellular buffer (ICB), calculations of free and bound concentrations of Ca2+, Zn2+, TPEN, BAPTA and EGTA were performed using the internet-based WEBMAXSTANDARD program (http://www.stanford.edu/∼cpatton/webmaxc/webmaxcS.htm).
Data Summary and Statistical Analysis
Luminescence intensities were monitored at discrete time intervals and measurements were summarized as means ± SE. Unless stated otherwise, comparisons were performed using analysis of variance for sequential or multiple measurements using standard statistical software (Sigma Stat, Version 2.0, Jandel Corp.).
Results
Modulation of endogenous caspase-3 activity though exogenous Zn2+ loading
Caspase-3 activity was monitored using a colorimetric method that monitors release of p-nitroaniline from hydrolysis of Ac-DEVD-pNA, a specific substrate of caspase-3. In preliminary studies, we confirmed the stability of both Ac-DEVD-pNA alone and p-nitroaniline (after hydrolysis using purified caspase-3) in the presence of 200μM NH2Cl (data not shown).
We then performed studies to determine whether endogenous caspase-3 activity in the gastric gland is susceptible to short-term increases in [Zn2+]i at concentrations (2.5nM, 25nM, 250nM). These concentrationsspan the range (24, 25) that is relevant to pathological release by oxidants such as NH2Cl (16). Glands were incubated at 37°C with Ringer's solutions containing EGTA (0.5mM) and the Zn2+ ionophore Na-pyrithione (50μM). Ringer's solutions containing EGTA (0.3mM free) and [Ca2+] (1.0mM free) were prepared with either no added Zn2+ or sufficient Zn2+ to achieve free concentrations of 2.5nM, 25nM or 250nM. The highest dose of Zn2+ administered represents an approximately 10-fold excess of the highest level of Zn2+ we would anticipate to have been released to the cytoplasm of a gastric gland. After 3 hours, glands were processed for caspase-3 activity. As shown in Figure 1, increases in [Zn2+]i led to dose-dependent decreases in activity, while exposure to TPEN (free concentration 10μM) elicited a modest, statistically insignificant activity increase in this experiment.
Figure 1.
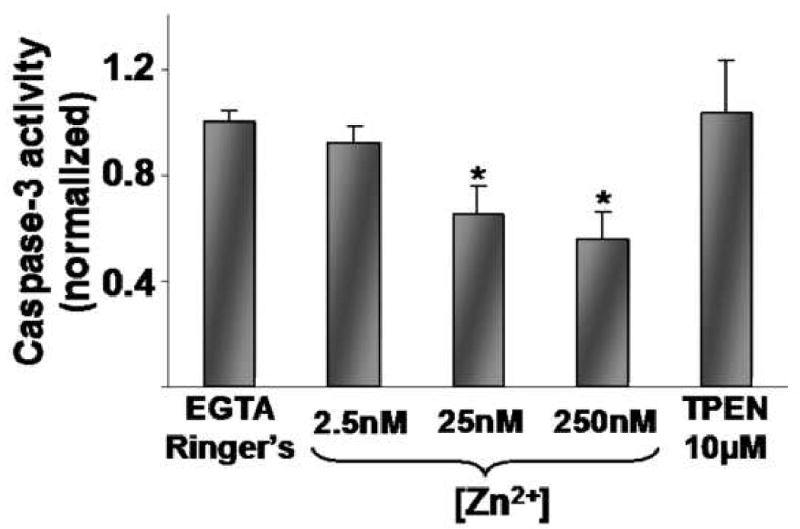
Caspase-3 activity in isolated gastric glands in response to [Zn2+] in the presence of pyrithione (50μM) at increasing doses (2.5nM, 25nM, 250nM) for 3 hrs. Results are normalized to Ca2+ and Zn2+ depleted Ringer's controls (EGTA 0.5mM, pyrithione 50μM; average value 1.0) run concurrently and expressed as means ± SE, n=5 wells, *p<0.05 compared to Ringer's alone (ANOVA).
Inactivation of caspase-3 by NH2Cl
To evaluate effects of NH2Cl on endogenous caspase-3 activity in glands, two protocols were used to determine responses to different doses and different intervals of exposure. In these studies, the goals were: first, to determine responses to short-term, but continuous exposures to NH2Cl; and, second, to determine whether responses were influenced by an equilibration in NH2Cl-free solution after exposure to NH2Cl. The first set of conditions might be considered as an in vitro test of acute toxicity of the oxidant, while the second set of conditions was intended to simulate disease in which oxidants reach a steady state.
In the first protocol, glands were exposed to NH2Cl at different concentrations (50μM, 100μM, or 200μM) for 3 hours and were then processed immediately for caspase-3 activity. In the second protocol, we measured generation of caspase-3 activity after isolated glands were incubated at different concentrations for varying intervals (1hr, 2hr, 3hr) and then allowed to equilibrate in Ringer's (5hr, 4hr, 3hr for each group respectively) to achieve a total experiment time of 6 hours. A maximum exposure to NH2Cl of 3 hours was chosen because exposure of glands to NH2Cl for longer time periods leads to complete destruction of the cells (data not shown.) In both protocols, concentration-dependent decreases in caspase-3 activity were observed, with severe inactivation during exposure to 200μM NH2Cl. (Figure 2) The findings of the first protocol (Figure 2A) indicate that the effects of NH2Cl are immediate. Those of the second protocol (Figure 2B) indicate that these effects are not reversed simply with removal of the oxidant, and that prolonged exposure beyond 1 hour increases inactivation at low and intermediate concentrations of NH2Cl.
Figure 2.
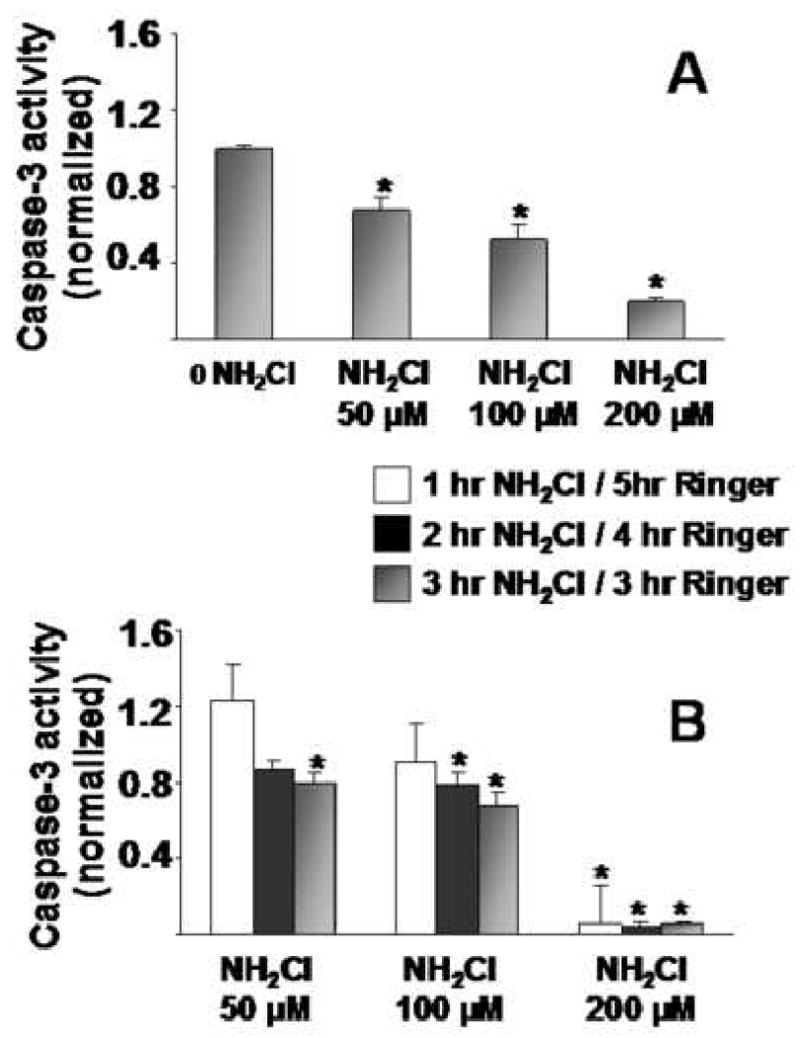
Caspase-3 activity in Isolated gastric glands in response to monochloramine at increasing doses (50 μM, 100 μM, 200 μM). Panel 2A: Exposure of glands to different concentrations of NH2Cl for 2 hr, with immediate processing for caspase-3 activity. Panel 2B: Exposure of glands to NH2CI at different doses for different periods of time (1hr, 2hr, 3 hr), after which glands were equilibrated with Ringer's for 5hr, 4hr, or 3hr, respectively, before processing. Each column represents 10 to 12 wells of glands from 3 to 4 separate preparations. Results are normalized to protein content (Bradford assay) and then to Ringer's controls (first white column in each group = 1.00) and expressed as means ± SE, *p<0.05 compared to Ringer's alone (ANOVA).
Modulation of NH2Cl-induced inactivation of caspase-3 by the concurrent release of Zn2+ into the cytoplasm
We next sought to determine whether NH2Cl-induced alterations in caspase-3 activity are influenced by the concurrent accumulation of Zn2+ in the cytoplasm. In these studies, the strategy was to expose glands to NH2Cl, either alone or in the presence of a heavy metal chelator, TPEN (20μM). First, we evaluated the effects of TPEN on acute administration of NH2Cl. In these studies, TPEN was present from the beginning of the 1 hour exposure to NH2Cl that was followed by immediate processing of glands for caspase-3 activity. As shown in Figure 3A, in the absence of NH2Cl, exposure to TPEN alone did not alter baseline caspase-3 activity. However, TPEN enhanced caspase-3 activity during exposure to NH2Cl at all concentrations tested, suggesting that endogenous release of free Zn2+ contributes to the inactivation of caspase-3.
Figure 3.
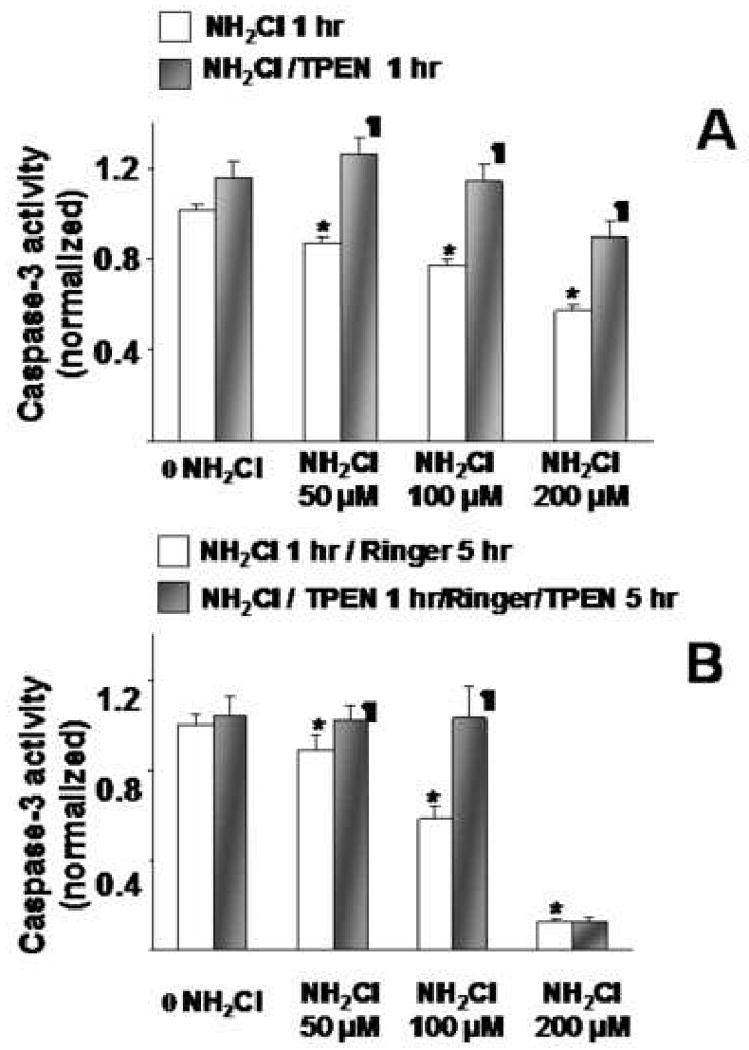
Caspase-3 activities in glands exposed to NH2Cl at different concentrations, in the presence or absence of TPEN 20μM. Panel 3A: Glands were exposed to NH2Cl alone (white bars) or TPEN (shaded bars) for 1 hr. Panel 3B: Glands were exposed to NH2Cl for 1 hr, followed by equilibration in Ringer's, alone (white bars) or in the presence of TPEN (shaded bars). Each column represents 5-6 wells of glands from 3 separate preparations. Results are normalized to protein content (Bradford assay) and then to Ringer's controls (first white column in each group = 1.00) and expressed as means ± SE, *p<0.05 compared to Ringer's alone (ANOVA), ¶ indicates p<0.05 compared to effects without TPEN at the corresponding concentration.
We then evaluated the effects of TPEN on the steady state model of NH2Cl exposure. In these studies, glands were exposed to escalating concentrations of NH2Cl for 1 hour and subsequently equilibrated for 5 hours in Ringer's, with or without 20μM TPEN, which was present from the start of the experiment. As shown in Figure 3B, caspase-3 activity was preserved when glands were exposed to NH2Cl and TPEN concurrently, at low (50μM) and intermediate (100μM) concentrations of NH2Cl. In control studies (not shown), we added 20μM TPEN directly to purified caspase-3, at levels observed in our experiments (dilution of pure enzyme 1:500 to 1:1000). There was no change in activity (data not shown), confirming that the observed effect is not attributable simply to chemical (i.e., non-biological) enhancement of caspase-3 activity by TPEN.
Finally we tested whether a reversal of caspase-3 inactivation is observed if TPEN is administered following the exposure to NH2Cl (100μM) rather than concurrently. In these experiments, one group of glands was exposed to Ringer's for 1 hour while another group was exposed to Ringer's plus 100μM NH2Cl for 1 hour; both groups were then equilibrated with Ringer's for 5 hours. We then compared caspase-3 activity generated in wells that had TPEN present from the beginning of the experiment and in wells in which TPEN was added 1 hour later. As summarized in Figure 4, delayed administration of TPEN achieved a response similar to that when TPEN was present concurrently. Additional experiments showed that the effects of monocloramine are almost immediate, with cell viability decreasing as early as 30 minutes after monochloramine exposure (data not shown.) These findings indicate that Zn2+ released to the cytoplasm by NH2Cl continues to elicit its inhibitory effect on caspase-3 activity, well past the period of exposure to the oxidant.
Figure 4.
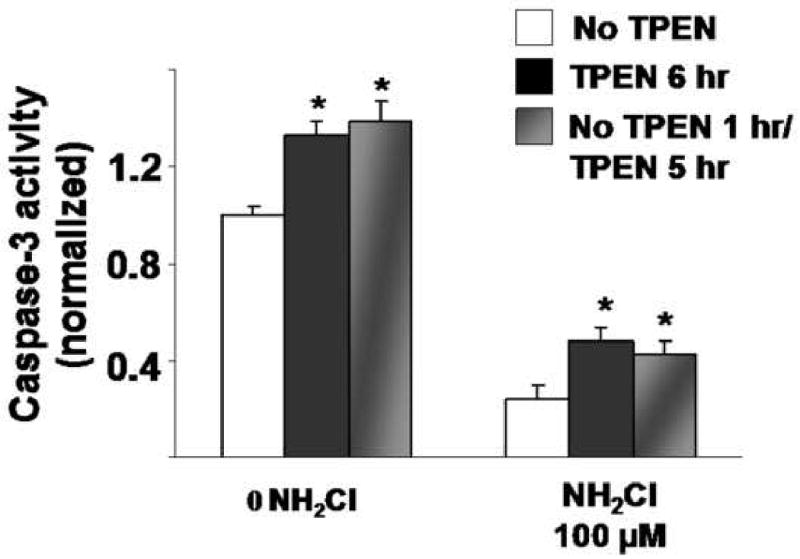
Effects of delayed TPEN treatment after exposure to NH2CI. Glands were perfused with Ringer's alone or Ringer's plus NH2CI (100μM) for 1 1hr. Glands were perfused under similar conditions with TPEN present for the entire 6 hr incubation, or only for the 5 hr following exposure to NH2CI. Each column represents 15 wells of glands from 3 separate preparations. Results are normalized to protein content (Bradford assay) and then to Ringer's controls (first white column in each group = 1.00) and expressed as means ± SE, *p<0.05 compared to Ringer's alone (ANOVA).
DTT preserves caspase-3 activity during exposure to thiol oxidant NH2Cl
We next performed studies to confirm that the effects of NH2Cl are due to its targeting of thiol groups, by determining whether a thiol-specific reducing agent, dithiothreitol (DTT) would preserve endogenous caspase-3 activity during exposure of glands. Glands were exposed for 2 hours to different concentrations of NH2Cl, in the presence or absence of 1mM DTT. A comparison group of glands was incubated with NH2Cl in the presence of 20μM TPEN. As shown in Figure 5, dose-dependent inactivation of caspase-3 activity were observed in response to NH2Cl. In the presence of the metal chelator, TPEN, this activity was partially restored. However, in the presence of DTT, caspase-3 activity was fully preserved, indicating that thiol oxidation is a critical determinant of the effects of NH2Cl.
Figure 5.
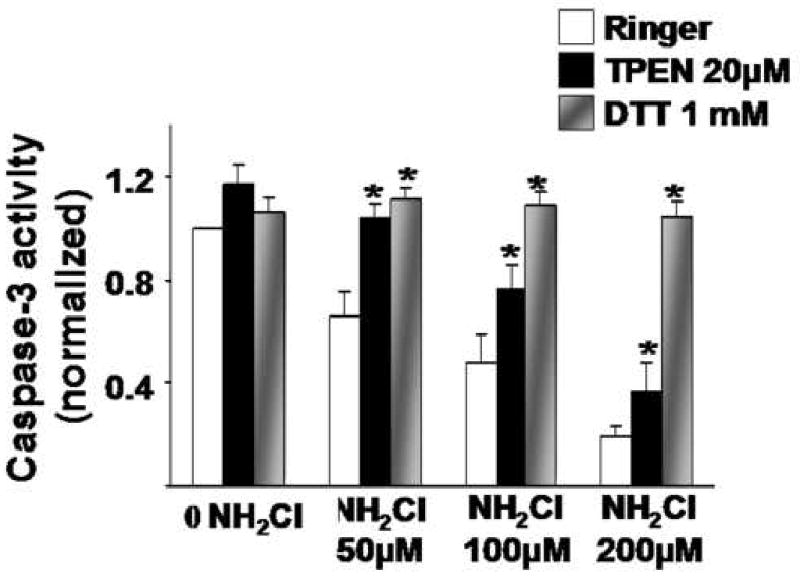
Comparison of effects of TPEN and DTT on inactivation of caspase-3 activity by NH2CI. Glands were incubated for 2 hours at different concentrations of NH2CI, alone or in the presence of the metal chelator TPEN or thiol reducing agent diothiothreitol (DTT). Results are normalized to protein content (Bradford assay) and then to Ringer's controls (first white column in each group = 1.00) and expressed as means ± SE, *p<0.05 compared to Ringer's or Ringer's plus NH2Cl (ANOVA).
Discussion
Our studies indicate that exposure of the gastric gland to pathologically relevant concentrations of NH2Cl leads to inactivation of caspase-3, a cysteine-rich intracellular protein (10, 11) that play a key role in cell death. This inactivation is greatly dependent on the concentration of the oxidant and, to a much lesser extent, on the duration of exposure. These effects are completely prevented by concurrent exposure to the thiol-reducing agent DTT. This observation supports the hypothesis that the effects of NH2Cl are due to its targeting of thiol residues in cysteine-rich structures (6, 7, 26).
In addition, we find that controlled increases in [Zn2+]I, within the limits of known physiologic conentration, dose-dependently inhibit activation and activity of caspase-3, a key step in programmed cell death (13-15). Moreover, our studies indicate that application of the heavy metal chelator TPEN unmasks a component of endogenous caspase-3 activity that is otherwise be suppressed by endogenous Zn2+. Thus TPEN maintains or even elevates Caspase-3 activity, even in the presence of NH2Cl-induced Zn2+ release. For caspase-3, the apparent binding constant for Zn2+ has been reported in the range of 0.1μM to 10μM, although in ultra-pure systems 50% inhibition at concentrations as low as 1.7nM has been reported (12). In addition, it has been proposed that the activation of caspase-3 from procaspase-3 occurs in response to [Zn2+]i in the range of 1 to 10nM (14). Thus, it is plausible that NH2Cl-induced increases in [Zn2+]i above this level are sufficient to inhibit endogenous activity of caspase-3 and to modulate other intracellular proteins that depend on Zn2+ for proper function or structure. The continued activity of Caspase-3 even at very high doses of Zn2+, however, suggests that Zn is not the sole modulator of Caspase-3 activity. Our studies provide novel evidence for this kind of modulation, in situ, in a primary epithelial structure such as the gastric gland.
These findings raise three additional questions for discussion. The first is technical and involves the quantitation of caspase-3 activity and whether it simply is proportional to viability of glands. In this study, valid comparison of caspase-3 activity between groups depends on the equivalence of the mass of viable glands allotted to each well. We controlled for equivalence of loading by monitoring cell protein levels (Bradford assay). In addition, the comparison between groups exposed to different concentrations of NH2Cl might depend on overall viability of the glands. Since NH2Cl is a known oxidant and can cause cell death at concentrations >200μM (4, 5, 27), it is possible that NH2Cl-induced decreases in caspase-3 activity, particularly at our highest concentration, are due to cell death as well as the postulated inactivation of the enzyme.
The major goal of this study was to evaluate the hypothesis that oxidant-induced accumulation of Zn2+ modulates the activity of caspase-3 within a cell exposed to pathologically relevant concentrations of NH2Cl for time periods sufficient to cause cell injury without immediate cellular destruction (Figure 2). Our studies show that, for each level of exposure to NH2Cl (50μM, 100μM, 200μM) an unmeasured component of caspase-3 inhibition was unmasked by chelation of Zn2+ by the high affinity chelator TPEN (Figure 3). Moreover, we observed this unmasking of caspase-3 inhibition even when glands were exposed to the chelator after the oxidant had been removed from the system (Figure 4). These findings suggest that, within each concentration of NH2Cl, the effects of TPEN cannot be attributed solely to effects on gland viability.
The second question is also technical and involves whether the effects of TPEN might be due not to the Zn2+ released by NH2Cl, but to chelation of heavy metal cations already bound to caspase-3 by the higher-affinity chelator. As shown in Figures 3 and 5, significant effects of TPEN were not observed in groups of glands that were not exposed to NH2Cl, making it unlikely that the endogenous pool of caspase-3 was already “loaded” with Zn2+.
The third question is the relationship between Zn2+-induced suppression of caspase-3 activity and viability of the gastric gland. In other cell systems, it has been suggested that release of Zn2+ by reactive nitrogen species, such as nitric oxide, may be anti-apoptotic and therefore protective (13-15, 28). In our experimental system, the reactive nitrogen species, NH2Cl, is likely to be toxic to the cell. In addition, Zn2+ accumulation in response to oxidant stress may be protective if brief and controlled or toxic if high and sustained. Thus, low-level, oxidant-induced increases in intracellular Zn2+ may have an overall protective effect when necrotic cell death is rare and apoptosis is the dominant pathway for cell demise. When oxidant stress is high, however, the concurrent Zn2+-induced suppression of caspase-3 activity may accelerate chaotic cell death (necrosis) by suppressing pathways that lead the gland to a more orderly and controlled demise (apoptosis). Further studies are underway to clarify the distinction between these two types of cell death in a complex epithelial unit such as the gastric gland, and to determine how oxidant-induced release of Zn2+ may influence the balance between apoptosis and necrosis in the setting of acute mucosal inflammation.
In summary, our results suggest that caspase-3, a zinc-depdendent protein present in the cytoplasm of rabbit gastric glands, is inhibited by the rise in intracellular Zn2+ associated with thiol oxidative stress. This effect can be prevented by treatment with DTT, a thiol oxidant, and can be both arrested and reversed using the selective zinc chelator TPEN. Though the role of Zn2+ as a pro-apoptotic signal has not been established, these experiments serve as a reminder of the many essential physiologic roles played by this cation.
Acknowledgments
This work was funded by NIH R01 DK069929 (DIS), NIH T32 DK 007754 (JM, JEK) and by an American College of Surgeons Resident Research Fellowship (JEK). We thank Melissa Beshire for technical assistance with the assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suerbaum S, Michetti P. Helicobacter pylori Infection. New England Journal of Medicine. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa T, Naito Y. The role of neutrophils and inflammation in gastric mucosal injury. Free Radical Research. 2000;33:785–794. doi: 10.1080/10715760000301301. [DOI] [PubMed] [Google Scholar]
- 3.Dekigai H, Murakami M, Kita T. Mechanism of Helicobacter pylori-associated gastric mucosal injury. Digestive Diseases and Sciences. 1995;40:1332–1339. doi: 10.1007/BF02065547. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M, Miura S, Suematsu M, Fukumura D, Kurose I, Suzuki H, Kai A, Kudoh Y, Ohashi M, Tsuchiya M. Helicobacter pylori-associated ammonia production enhances neutrophil dependent gastric mucosal injury. American Journal of Physiology. 1992;263:G719–G725. doi: 10.1152/ajpgi.1992.263.5.G719. [DOI] [PubMed] [Google Scholar]
- 5.Grisham MB, Gaginella TS, von Ritter C, Tamai H, Be RM, Granger DN. Effects of neutrophil-derived oxidants on intestinal permeability, electrolyte transport, epithelial cell viability. Inflammation. 1990;14:531–542. doi: 10.1007/BF00914274. [DOI] [PubMed] [Google Scholar]
- 6.Peskin AV, Winterbourn CC. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radicals in Biology and Medicine. 2001;30:572–579. doi: 10.1016/s0891-5849(00)00506-2. [DOI] [PubMed] [Google Scholar]
- 7.Prutz WA. Reactions of hypochlorous acid with biological substrates are activated catalytically by tertiary amines. Archives of Biochemistry and Biophysics. 1998;357:265–273. doi: 10.1006/abbi.1998.0822. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore AP. Anoikis. Cell Death and Differentiation. 2005;12:1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 9.Maeda S, Mentis AF. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2007;12 1:10–14. doi: 10.1111/j.1523-5378.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 10.Roy S, Bayly CI, Gareau Y, Houtzager VM, Kargman S, Keen SL, Rowland K, Seiden IM, Thornberry NA, Nicholson DW. Maintenance of caspase-3 proenzyme dormancy by an intrinsic “safety catch” regulatory tripeptide. Proceedings of the National Academy of Sciences U S A. 2001;98:6132–6137. doi: 10.1073/pnas.111085198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porter AG. Flipping the safety catch of procaspase-3. Nature Chemical Biology. 2006;2:509–510. doi: 10.1038/nchembio1006-509. [DOI] [PubMed] [Google Scholar]
- 12.Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. Journal of Biological Chemistry. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- 13.Perry DK, Smyth MJ, Stennicke HR, Salvesen GS, Duriez P, Poirier GG, Hannun YA. Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. Journal of Biological Chemistry. 1997;272:18530–18533. doi: 10.1074/jbc.272.30.18530. [DOI] [PubMed] [Google Scholar]
- 14.Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14:315–330. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- 15.Truong-Tran AQ, Grosser D, Ruffin RE, Murgia C, Zalewski PD. Apoptosis in the normal and inflamed airway epithelium: role of zinc in epithelial protection and procaspase-3 regulation. Biochemical Pharmacology. 2003:1459–1468. doi: 10.1016/s0006-2952(03)00498-2. [DOI] [PubMed] [Google Scholar]
- 16.Cima RR, Dubach JM, Wieland AM, Walsh BM, Soybel DI. Intracellular Ca2+ and Zn2+Signals During Monochloramine Induced Oxidative Stress in Isolated Rat Colon Crypts. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005 doi: 10.1152/ajpgi.00501.2004. [DOI] [PubMed] [Google Scholar]
- 17.Walsh BM, Naik HB, Dubach JM, Wieland AM, Soybel DI. Thiol-Oxidant Monochloramine Mobilizes Intracellular Ca2+ in Parietal Cells of Rabbit Gastric Glands. American Journal of Physiology- Cell Physiology. 2007;293 doi: 10.1152/ajpcell.00189.2006. [DOI] [PubMed] [Google Scholar]
- 18.Gerbino A, Hofer AM, McKay BM, Lau BW, Soybel DI. Divalent Cations Regulate Acidity within the Lumen and Tubulovesicle Compartment of Gastric Parietal Cells. Gastroenterology. 2004;126:182–195. doi: 10.1053/j.gastro.2003.10.068. [DOI] [PubMed] [Google Scholar]
- 19.Berglindh T, Helander H, Sachs G. Secretion at the parietal cell level--a look at rabbit gastric glands. Scand J Gastroenterol Suppl. 1979;55:7–20. [PubMed] [Google Scholar]
- 20.Berglindh T, Obrink KJ. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol Scand. 1976;96:150–159. doi: 10.1111/j.1748-1716.1976.tb10184.x. [DOI] [PubMed] [Google Scholar]
- 21.Ghoshal NG, Bal HS. Comparative morphology of the stomach of some laboratory mammals. Lab Anim. 1989;23:21–29. doi: 10.1258/002367789780886911. [DOI] [PubMed] [Google Scholar]
- 22.Koelz HR, Hersey SJ, Sachs G, Chew CS. Pepsinogen release from isolated gastric glands. Am J Physiol. 1982;243:G218–225. doi: 10.1152/ajpgi.1982.243.3.G218. [DOI] [PubMed] [Google Scholar]
- 23.Thomas EL, Grisham MB, Jefferson MM. Preparation and characterization of chloramines. Methods in Enzymology. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- 24.Cima RR, Dubach JM, Wieland AM, Walsh BM, Soybel DI. Intracellular Ca(2+) and Zn(2+) signals during monochloramine-induced oxidative stress in isolated rat colon crypts. Am J Physiol Gastrointest Liver Physiol. 2006;290:G250–261. doi: 10.1152/ajpgi.00501.2004. [DOI] [PubMed] [Google Scholar]
- 25.Haase H, Hebel S, Engelhardt G, Rink L. Flow cytometric measurement of labile zinc in peripheral blood mononuclear cells. Anal Biochem. 2006;352:222–230. doi: 10.1016/j.ab.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Maret W, Jacob C, Vallee BL, Fischer EH. Inhibitory sites in enzymes: zinc removal and reactivation by thionein. Proceedings of the National Academy of Sciences USA. 1999;96:1936–1940. doi: 10.1073/pnas.96.5.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamai H, Kachur JF, Baron DA, Grisham MB, Gaginella TS. Monochloramine, a neutrophil-derived oxidant, stimulates rat colonic secretion. Journal of Pharmacology and Experimental Therapeutics. 1991;257:887–894. [PubMed] [Google Scholar]
- 28.St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. American Journal of Physiology. 2002;282:L185–L192. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]


