Summary
Pre-cancerous and malignant cells can induce an immune response which results in destruction of transformed and/or malignant cells, a process known as immune surveillance. However, immune surveillance is not always successful, resulting in “edited” tumors that have escaped immune surveillance. Immunoediting is not simply the absence of anti-tumor immunity, but is due to pro-tumor immunity that blocks anti-tumor adaptive and innate responses, and promotes conditions that favor tumor progression. Several immune pro-tumor effector mechanisms are up-regulated by chronic inflammation, leading to the hypothesis that inflammation promotes carcinogenesis and tumor growth by altering the balance between pro-and anti-tumor immunity, thereby preventing the immune system from rejecting malignant cells, and providing a tumor-friendly environment for progressive disease.
Introduction
The concept that the immune system can be harnessed as a therapeutic agent to treat established tumors (immunotherapy) was first proposed in the early 1900’s by Paul Ehrlich. He suggested that molecules that we now know as antibodies, could deliver toxins directly to cancer cells. Ehrlich’s “magic bullet” strategy was expanded upon in the 1950’s by Burnet and Thomas. They hypothesized that the immune system may also protect against nascent cancers by destroying malignant cells before they developed into detectable tumors, a concept that has become the immune surveillance hypothesis [1,2]. Although enthusiasm for the validity of immunotherapy and immune surveillance waned in the 1970’s, subsequent studies demonstrated that the immune system can protect against tumor onset and be manipulated to reject established tumors. Revival of the immune surveillance hypothesis led to a re-working of the initial concept, to include the concept of “immunoediting.” During immunoediting, the immune system destroys many pre-cancerous and malignant cells; however, some cells escape the immune response and give rise to progressively growing tumors. Immunoediting is thought to continue throughout the life of the tumor so that the phenotype of an established tumor has been directed by the host’s immune response. It has also become apparent that both innate and adaptive immunity have a “dark” side and can promote tumor progression as well as mediate tumor destruction. Not surprisingly, chronic inflammation, which has long been associated with increased tumor risk, is involved in polarizing immunity towards those effectors that facilitate tumor growth. As a result, the immune system has the potential to either promote or delay tumor onset and progression, and the effectiveness of immune surveillance and the efficacy of immunotherapy depend on the balance between these diametric opposites (Figure 1). After a brief over-view of the observations supporting the concept of immune surveillance, this article will review the cells that mediate pro-and anti-tumor immunity including a discussion of how inflammation polarizes innate and adaptive immunity towards either a pro-tumor or anti-tumor phenotype.
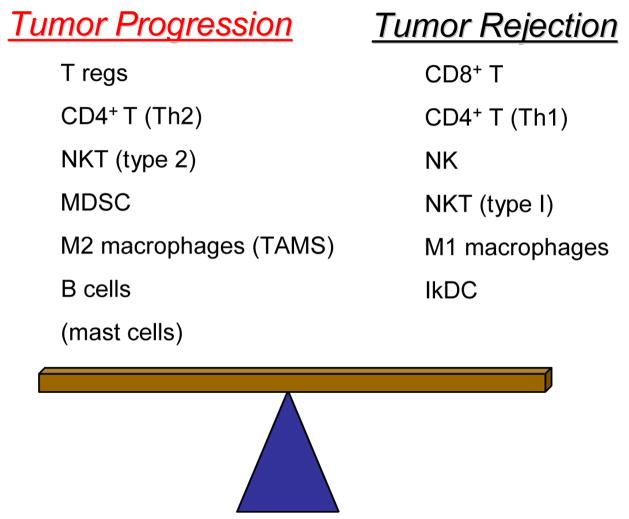
Figure 1.
Tumor immunity is a balance between immune mediators that promote tumor progression vs. mediators that promote tumor rejection. CD4+ T regulatory cells, Type 2 CD4+ T cells, Type 2 natural killer T cells, myeloid-derived suppressor cells, M2 or tumor-associated macrophages, B cells, and possibly mast cells promote tumor progression, while CD8+ T lymphocytes, type 1 CD4+ T lymphocytes, natural killer, type 1 natural killer T cells, M1 macrophages, and immune killer dendritic cells promote tumor destruction.
Immune surveillance and immunoediting
Rejuvenation of the concept that the immune system protects against nascent malignant cells occurred with the demonstration that mice deficient for various components of the adaptive or innate immune systems were more likely to develop some types of tumors, specifically sarcomas as opposed to carcinomas, as compared to immune competent mice, when exposed to carcinogens or transplanted with syngeneic tumor cells. Immune deficiencies included the absence of B cells and αβ or γδ T cells due to deletion of the recombination-activating gene-2 (RAG2) required for immunoglobulin and T cell receptor gene rearrangements, and the absence of interferonγ (IFNγ) or the ability to respond to IFNγ, a key mediator of cellular immunity. Similarly, mice that were knocked-out for perforin, an essential molecule for cell-mediated cytotoxicity used by most effector cells of the innate and adaptive immune systems, or mice deficient for natural killer (NK) or NKT cells, effector cells of the innate immune system, were also more susceptible to spontaneous tumors or had more rapid growth rates of transplanted tumors as compared to wild type or immune competent mice [3,4].
Circumstantial evidence suggests that immune surveillance and immunoediting also occurs in cancer patients. Individuals with hereditary or acquired immunodeficiencies have higher incidences of some types of viral- and carcinogen-associated cancers. Organ transplant patients maintained on immune suppressive drugs are 3–8 fold more likely to develop cancer than normal controls, although tumors are not randomly distributed in all anatomical locations [1,2]. In contrast, ovarian, colorectal [5], and melanoma patients whose tumors have high levels of tumor-infiltrating lymphocytes have a better prognosis [1,2]. Collectively, experimental studies and the clinical observations in patients indicate that the immune system can foil carcinogenesis and mediate regression of established tumor.
CD4+ and CD8+ T lymphocytes
CD4+ and CD8+ T cells are the principal helper and effector cells, respectively, of adaptive cellular immunity, and many immunotherapy strategies are aimed at activating these cells to promote tumor cell destruction and long-term immune memory against recurrence of primary disease or outgrowth of metastases. Type 1 CD4+ T cells (Th1) facilitate tissue destruction and tumor rejection by providing help to cytotoxic CD8+ T cells, while Type 2 CD4+ T cells T(Th2) facilitate antibody production by B cells and polarize immunity away from a beneficial cell-mediated anti-tumor response (Figure 2). CD4+ T regulatory cells (T regs), which are naturally occurring or antigen-induced, promote tumor immunity by blocking the activation of CD8+ cytotoxic T cells. Although additional studies are needed to fully characterize the mechanism(s) by which CD4+ T regs block CD8+ T cell activation, T reg expression of cytotoxic T lymphocyte antigen 4 (CTLA4), an inhibitory signal for T cells, may be involved [6]. As for many pro-tumor mediators, inflammation enhances T reg function since prostaglandin E2 (PGE2) causes differentiation of T regs and increases their immune suppressive activity [7,8]. In addition to their inhibiting CD8+ T cell activation, CD4+ T regs block killing by natural killer cells [9], and thereby down-regulate both adaptive and innate anti-tumor immunity. Although most T regs are CD4+, CD8+ T regs induced by plasmacytoid DC have been identified in ovarian cancer patients [10].
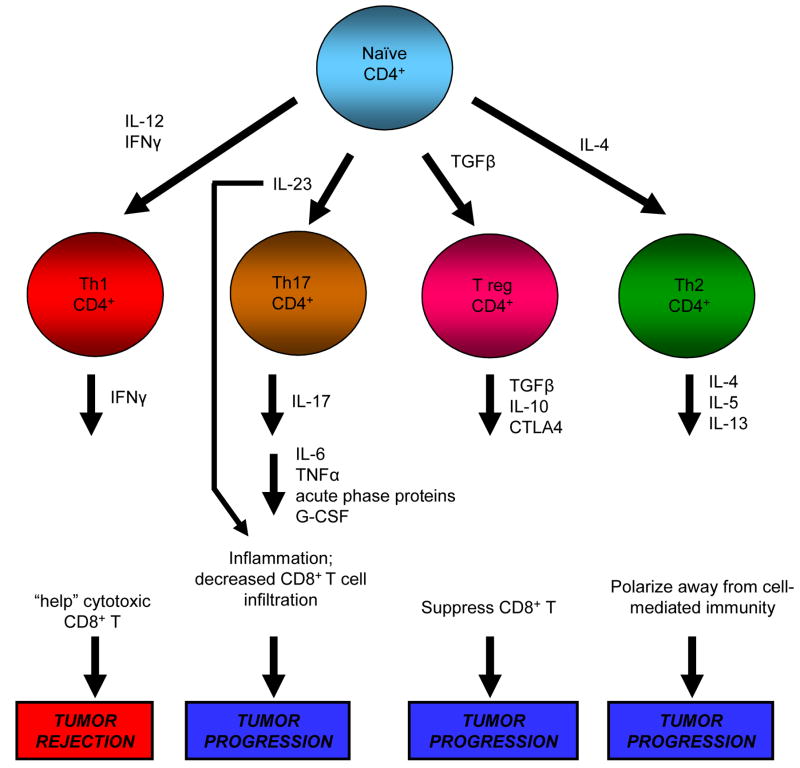
Figure 2.
CD4+ T lymphocytes are induced by cytokines to produce cytokines that either promote tumor progression or mediate tumor elimination. Type 1 CD4+ T cells are induced by IL-12 and IFNγ to produce IFNγ which promotes the differentiation and expansion of CD8+ T cells that are cytotoxic for tumor cells. In contrast, IL-4 polarizes CD4+ T cells towards a type 2 phenotype that produces IL-4, IL-5, and IL-13 which help B cells produce antibodies, thereby directing immunity away from a tumor-rejecting type 1 response. Under the influence of transforming growth factor β, CD4+ T cells develop into T regs that actively block tumor immunity by suppressing tumoricidal CD8+ T cells. Recently identified Th17 cells are induced by IL-23 to produce IL-17, which in turn induces cytokines and chemokines that promote inflammation. The resulting inflammatory mediators may contribute to tumor progression by up-regulating immune suppressive cells of the adaptive and innate immune systems (see figure 4)
Recently identified CD4+ Th17 cells [11,12], may also promote tumor progression. Th17 cells are induced by IL-23, a cytokine closely related to IL-12 and whose receptor shares the IL-12Rβ1 with IL-12 [13]. Upon activation by IL-23, Th17 cells produce IL-17 which exacerbates inflammation by inducing IL-6, TNFα, G-CSF, and other acute phase proteins [14]. IL-23 itself, has been shown to reduce CD8+ T cell infiltration into tumors, thereby promoting tumor growth [13,15] (Figure 2). Earlier experiments using IL-17-transfected tumor cells were inconclusive as to whether IL-17 promoted tumor growth or tumor rejection [16,17]. This ambiguity may be explained by a recent study showing that Th17-induced IL-6 blocks CD4+ T regs [18]. Additional experiments are clearly necessary to clarify the roles of IL-23, Th17 cells, IL-17, and regulatory T cells in tumor progression.
B lymphocytes
Tumor-reactive monoclonal antibodies can have significant anti-tumor efficacy when passively administered to cancer patients. In contrast, most cancer vaccines or other therapies that are aimed at inducing tumor-reactive antibodies are largely ineffective in promoting tumor rejection, although there are exceptions [19]. More recent experiments indicate that activated B cells and their soluble products, presumably antibodies, can also facilitate carcinogenesis. Using a transgenic mouse model in which the human keratin 14 promoter drives expression of early region genes of human papilomavirus 16, B cells were shown to promote a chronic inflammatory microenvironment that recruits innate immune cells and factors to the tumor site, thus establishing a stromal environment that supports de novo carcinogenesis. Thus, humoral immunity can enhance malignant transformation by activating the innate immune system [20,21].
Macrophages
Macrophages are part of the innate immune system and play important roles in all aspects of immunity. They are an exceptionally heterogeneous population of cells. Similar to CD4+ T cells, macrophages can contribute to tumor destruction or facilitate tumor growth and metastasis, depending on their phenotype (Figure 3).
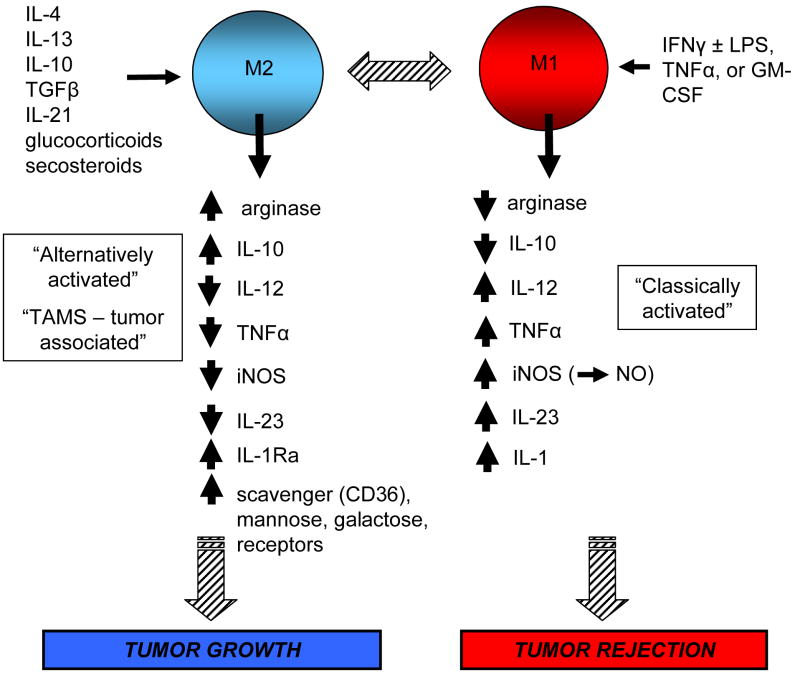
Figure 3.
Macrophages are differentially activated by different cytokines or other factors and become either tumor-promoting or tumoricidal. Classically-activated or M1 macrophages produce high levels of type 1 cytokines that promote a tumor-rejecting type 1 response as well as factors such as inducible nitric oxide synthase which are cytotoxic for tumor cells, and low levels of type 2 cytokines. In contrast, alternatively-activated or M2 macrophages produce high levels of cytokines that polarize immunity towards a tumor-promoting type 2 response, and low levels of cytokines that promote a tumor-destructive type 1 response. Some of the molecules produced by M2 macrophages attract additional pro-inflammatory mediators to the tumor site, thereby amplifying the inflammatory microenvironment.
Macrophages that are “classically activated” by IFNγ and bacterial lipopolysaccharides destroy tumor cells through their production of nitric oxide and type 1 cytokines and chemokines. These macrophages also function as antigen presenting cells to activate cytotoxic CD8+ T [22]. In contrast, macrophages activated through the “alternative” pathway with IL-4, IL-13 and/or TGFβ promote tumor progression by enhancing angiogenesis and producing type 2 cytokines and chemokines [23]). Because of the similarities in cytokine profiles, Mills coined the terminology “M1/M2” after the Th1/Th2 paradigm for classically-activated and alternatively-activated macrophages, respectively [24]. This jargon was further developed by Mantovani and colleagues, although they are careful to point out that macrophages are a continuum of phenotypes with M1 and M2 being the polarized extremes [25,26].
Most progressively growing tumors are infiltrated by large numbers of macrophages. These tumor-associated macrophages (TAMS) are a key component of the tumor stroma and are essential for the angiogenesis and matrix remodeling that supports progressively growing neoplasms. Using a spontaneous mouse mammary tumor model, the transition from pre-malignant to malignant phenotype was associated with increased blood vessel formation, and that the elimination of TAMS blocked the neoangiogenesis, while early infiltration of TAMS enhanced angiogenesis [27]. Metastasis is also enhanced by TAMS when they promote the intravasation of tumor cells into local blood vessels, as graphically shown by intravital multiphoton imaging of live mammary tumors in situ [28]. As shown in human ovarian cancer, TAMS also promote tumor progression by blocking the activation of tumor-specific T cells by their expression of B7-H4, a negative regulator of T cell activation [29]
Since TAMS promote tumor progression, they are often called M2 macrophages. Gene profiling of TAMS and alternatively-activated peritoneal macrophages (M2) has confirmed that TAMS and M2 macrophages express many of the same molecules; however, TAMS also express some IFN-inducible genes that are characteristic of M1 macrophages, indicating that they are intermediate in the continuum of macrophage phenotypes [30,31].
Natural killer (NK) cells
NK cells are components of the innate immune system that interact with adaptive immunity through their production of cytokines that modulate dendritic cell (DC) and cytotoxic T cell maturation. They are well recognized for their ability to directly lyse MHC class I-deficient tumor cells through the engagement of their activating receptors and lack of engagement of their inhibitory receptors. However, a subset of NK cells are also cytotoxic for activated CD8+ T cells [32] and DC [33], and thereby can reduce CD8-mediated anti-tumor immunity. In addition, NK cells have been shown to inhibit DC-mediated antigen presentation through a non-cytotoxic mechanism [34], and elimination of NK cells increases activation of tumor-specific CD8+ T cells following immunization [35].
NKT cells
NKT cells, which express both NK and TCR, bridge the innate and adaptive immune systems. They are usually CD4+ and respond to lipid and glycolipid antigens as presented by non-classical MHC class I CD1d molecules. Until recently there was confusion as to whether NKT cells promote tumor rejection or enhance immune surveillance. NKT cells prevent the spread of B16 melanoma metastases and promote immune surveillance in mice treated with the carcinogen 3-methyl-cholanthrene. However, CD1d knockout mice, which lack CD1d-restricted NKT cells, reject recurrent fibrosarcomas and are resistant to the 4T1 mammary carcinoma. These apparently conflicting findings were resolved when it was found that type I NKT cells, which express the invariant Vα14Jα18 TCR Vβ chain, mediate tumor rejection, while type II NKT cells, which express a non-Vα14Jα18 TCR Vβ chain, promote tumor growth [36].
Myeloid-derived suppressor cells (MDSC)
MDSC are a morphologically and functionally heterogenous population of cells of myeloid origin that are elevated in almost all patients and experimental mice with cancer [37]. They suppress both innate and adaptive anti-tumor immunity by inhibiting CD8+ and CD4+ T cells, NK and NKT cells, and by blocking DC maturation [38–41]. MDSC suppress T cells through their production of arginase and/or reactive oxygen species (ROS); however, there is variability in which mediator(s) is used depending on the tumor model [38,42,43]. MDSC heterogeneity is further demonstrated by the requirement for CD80 expression for suppression by some MDSC [44] and the absence of CD80 on other MDSC [45,46]. Likewise, the IL-4Rα is required for the IL-13-induced activation of some MDSC [47]; however, equally suppressive MDSC have been isolated from IL-4R-deficient and wild type mice [40]. Suppression requires MDSC to T cell contact, and for suppression of CD8+ T cells, MDSC nitrate tyrosines of the CD8+ T cells’ TCRs, thereby rendering the T cells incapable of activation by peptide-MHC I complexes of antigen presenting cells [48].
In addition to inhibiting anti-tumor immunity by blocking T cell activation, MDSC also induce CD4+ T regs through an IL-10 and IFNγ-dependent process that is ROS-independent [49]. They also polarize immunity towards a tumor-promoting type 2 phenotype by secreting high levels of IL-10 and shutting down macrophage production of the Type 1 cytokine, IL-12. Macrophages in turn, up-regulate MDSC production of IL-10 further favoring tumor progression [50].
MDSC are similar to other immune system cells in that chronic inflammation heightens their pro-tumor activity. IL-1β and IL-6 increase the accumulation and suppressive activity of MDSC [46,51,52], while reductions in these cytokines reduce MDSC levels [52]. PGE2 is one of the inflammatory inducers of MDSC, since co-cultures of c-kit+ mouse bone marrow stem cells with PGE2 produce immune suppressive Gr1+CD11b+ MDSC [53], and cyclooxygenase-2 (COX-2) produced by human lung cancer cells up-regulates arginase expression in human MDSC [54].
Conclusions
The immune system has the capacity to either block tumor development and deter established tumors, or to promote carcinogenesis, tumor progression, and metastasis. Which of these conditions prevails depends on the balance between the pro- and anti-tumor mediators of both innate and adaptive immunity. Presumably, there are unifying mechanisms that orchestrate immunity towards tumor promotion vs. tumor destruction. Since many of the tumor-promoting elements of the immune system are induced by, or themselves cause, inflammation, chronic inflammation may be a key process that polarizes immunity towards a tumor-promoting phenotype [55]. Accordingly, chronic inflammation would produce an immune suppressive, tumor-friendly environment that would negate immune surveillance and be permissive for carcinogenesis. As tumor growth progressed and tumors themselves produced pro-inflammatory molecules, innate and adaptive immunity would be further polarized towards a tumor-promoting phenotype, creating an ideal environment for further tumor growth and metastasis (Figure 4). Chronic inflammation has long been associated with increased risk of tumor onset and progression, and is known to enhance angiogenesis and tissue remodeling, and promote protein and DNA damage through oxidative stress, processes that are integral to tumor progression [55–57]. By polarizing immunity towards a tumor-promoting phenotype, inflammation not only promotes the genetic and histological changes that facilitate carcinogenesis, but it also deters immune surveillance, thereby functioning as both an initiator and a protector for neoplastic cells.
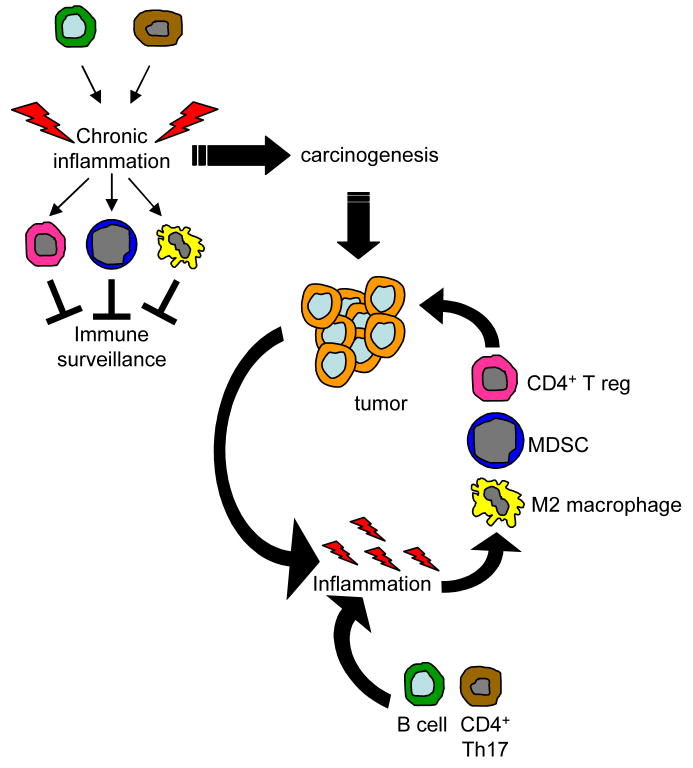
Figure 4.
Inflammation may regulate the balance between pro- and anti-tumor immunity by inducing the development of immune mediators that promote carcinogenesis and tumor progression. Activated B cells or possibly CD4+ Th17 cells can contribute to an existing state of chronic inflammation or de facto induce inflammation which results in the increase and activation of M2 macrophages, CD4+ T regulatory cells, and myeloid-derived suppressor cells. These immune suppressive cells then block immune surveillance, preventing the host’s immune system from rejecting pre-malignant cells. In the presence of established tumor, the inflammatory environment is maintained by B cell-secreted factors and possibly CD4+ Th17 cells, and by additional factors produced by the tumor cells and by host cells attracted to the tumor site. This increased inflammation induces the accumulation and activation of additional M2 macrophages and myeloid and T suppressor cells which fuel tumor progression.
Acknowledgments
The author’s laboratory is supported by National Institute of Health grants R01CA118550 and R01CA84232, and Susan G. Komen Foundation for the Cure BCTR0503885.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reiman JM, Kmieciak M, Manjili MH, Knutson KL. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol. 2007;17:275–287. doi: 10.1016/j.semcancer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 4•.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. This is a comprehensive review of the concepts of cancer immunosurveillance and immunoediting by the investigators who resurrected the immunosurveillance hypothesis. [DOI] [PubMed] [Google Scholar]
- 5.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 6.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 7•.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. This paper demonstrates that immune suppressive T regulatory cells are induced and activated by PGE2, demonstrating another mechanism by which inflammation suppresses adaptive anti-tumor immunity and promotes tumor progression. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Zhu L, Yang SC, Zhang L, Lin J, Hillinger S, Gardner B, Reckamp K, Strieter RM, Huang M, et al. Cyclooxygenase 2 inhibition promotes IFN-gamma-dependent enhancement of antitumor responses. J Immunol. 2005;175:813–819. doi: 10.4049/jimmunol.175.2.813. [DOI] [PubMed] [Google Scholar]
- 9.Ralainirina N, Poli A, Michel T, Poos L, Andres E, Hentges F, Zimmer J. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol. 2007;81:144–153. doi: 10.1189/jlb.0606409. [DOI] [PubMed] [Google Scholar]
- 10.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 11.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu Rev Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 12.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 13••.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. Using a mouse model for skin papillomas this paper demonstrates that the cytokine IL-23, which activates CD4+ Th17 cells to produce IL-17, up-regulates numerous pro-inflammatory mediators and reduces CD8+ T cell infiltration into tumors, thereby promoting tumor progression. Taken together with reports on CD4+ Th17 cells (see reviews. #12 and #15), this report identifies a novel cytokine pathway that polarizes immunity towards a tumor-promoting phenotoype in response to inflammation. [DOI] [PubMed] [Google Scholar]
- 14.Bi Y, Liu G, Yang R. Th17 cell induction and immune regulatory effects. J Cell Physiol. 2007;211:273–278. doi: 10.1002/jcp.20973. [DOI] [PubMed] [Google Scholar]
- 15.Langowski JL, Kastelein RA, Oft M. Swords into plowshares: IL-23 repurposes tumor immune surveillance. Trends Immunol. 2007;28:207–212. doi: 10.1016/j.it.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 17.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 18.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 19.Nanni P, Nicoletti G, De Giovanni C, Landuzzi L, Di Carlo E, Cavallo F, Pupa SM, Rossi I, Colombo MP, Ricci C, et al. Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice. J Exp Med. 2001;194:1195–1205. doi: 10.1084/jem.194.9.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. Using an in vivo skin carcinogenesis mouse system, this article demonstrates that B lymphocytes produce soluble factors that support an inflammatory environment that leads to de novo carcinogenesis. In addition to identifying a pro-tumor role for B cells, this report directly links tumor induction to inflammation. [DOI] [PubMed] [Google Scholar]
- 21.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Ostrand-Rosenberg S, Sinha P. Macrophages and tumor development. In: Gabrilovich D, Hurwitz A, editors. Tumor-induced immune suppression: Mechanisms and therapeutic reversal. Springer; 2007. in press. [Google Scholar]
- 23.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 24.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 26.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 27.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 28.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 29.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 31.Ghassabeh GH, De Baetselier P, Brys L, Noel W, Van Ginderachter JA, Meerschaut S, Beschin A, Brombacher F, Raes G. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood. 2006;108:575–583. doi: 10.1182/blood-2005-04-1485. [DOI] [PubMed] [Google Scholar]
- 32.Rabinovich BA, Li J, Shannon J, Hurren R, Chalupny J, Cosman D, Miller RG. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol. 2003;170:3572–3576. doi: 10.4049/jimmunol.170.7.3572. [DOI] [PubMed] [Google Scholar]
- 33.Ferlazzo G, Morandi B, D’Agostino A, Meazza R, Melioli G, Moretta A, Moretta L. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur J Immunol. 2003;33:306–313. doi: 10.1002/immu.200310004. [DOI] [PubMed] [Google Scholar]
- 34.Barber MA, Zhang T, Gagne BA, Sentman CL. NK cells negatively regulate antigen presentation and tumor-specific CTLs in a syngeneic lymphoma model. J Immunol. 2007;178:6140–6147. doi: 10.4049/jimmunol.178.10.6140. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa Y, Screpanti V, Yagita H, Grandien A, Ljunggren HG, Smyth MJ, Chambers BJ. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol. 2004;172:123–129. doi: 10.4049/jimmunol.172.1.123. [DOI] [PubMed] [Google Scholar]
- 36.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 39•.Bunt SK, Hanson EM, Sinha P, Srivastava MK, Clements VK, Ostrand-Rosenberg . Tumor-associated myeloid-derived suppressor cells. In: Prendergast GC, Jaffee E, editors. Cancer immunotherapy: immune suppression and tumor growth. Elsevier; 2007. pp. 309–331. This article comprehensively reviews the development, function, activation, and mechanisms of suppression of MDSC, [Google Scholar]
- 40.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 41.Yanagisawa K, Exley MA, Jiang X, Ohkochi N, Taniguchi M, Seino K. Hyporesponsiveness to natural killer T-cell ligand alpha-galactosylceramide in cancer-bearing state mediated by CD11b+ Gr-1+ cells producing nitric oxide. Cancer Res. 2006;66:11441–11446. doi: 10.1158/0008-5472.CAN-06-0944. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez PC, Ochoa AC. T cell dysfunction in cancer: role of myeloid cells and tumor cells regulating amino acid availability and oxidative stress. Semin Cancer Biol. 2006;16:66–72. doi: 10.1016/j.semcancer.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang R, Cai Z, Zhang Y, Yutzy WHt, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 45.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 46•.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. This paper and ref. #51 are the first reports demonstrating that inflammation, in these cases induced by IL-1β, regulate the accumulation and retention of MDSC. This report also is the first to propose that inflammation promotes tumor progression by inducing MDSC which block immune surveillance. [DOI] [PubMed] [Google Scholar]
- 47.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. This elegant study demonstrates that when myeloid-derived suppressor cells contact CD8+ T cells they produce reactive oxygen species and peroxynitrite that nitrate the T cell receptor and thus prevent the CD8+ T cell from receiving activating peptide-MHC I signals from normal antigen presenting cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 50.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 51•.Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol. 2005;175:8200–8208. doi: 10.4049/jimmunol.175.12.8200. (See ref. #46) [DOI] [PubMed] [Google Scholar]
- 52.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. Using a mouse mammary tumor, this paper from the author’s laboratory demonstrated that the inflammatory agent PGE2 induces the differentiation of myeloid-derived suppressor cells from bone marrow stem cells and that tumor progression is delayed in mice that are deficient for the E-prostanoid receptor 2 for PGE2. This paper, together with ref. #54, provide direct evidence that induction of this important population of suppressor cells that is found in most cancer patients and experimental animals with tumors, is directly regulated by inflammation. [DOI] [PubMed] [Google Scholar]
- 54•.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. This paper demonstrated that a mouse lung tumor produces COX-2 which up-regulates the immunosuppressive enzyme arginase I in myeloid-derived suppressor cells via the E-prostanoid receptor 4, thereby promoting immune suppression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. This comprehensive review article describes the studies demonstrating a linkage between infection, inflammation, and cancer, and proposed that NF-κB is a key regulatory molecule in inflammation-induced tumor progression. It also reviews the various immune mediators that are activated by inflammation. [DOI] [PubMed] [Google Scholar]
- 56.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 57.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]