Abstract
Secreted frizzle-related protein 2 (SFRP2), a modulator of Wnt-signaling, has recently been found to be overexpressed in the vasculature of 85% of human breast tumors, however its role in angiogenesis is unknown. We found that SFRP2 induced angiogenesis in the mouse Matrigel plug assay and the chick chorioallantoic membrane assay. SFRP2 inhibited hypoxia induced endothelial cell apoptosis, increased endothelial cell migration, and induced endothelial tube formation. The canonical Wnt-pathway was not affected by SFRP2 in endothelial cells, however, a component of the non-canonical Wnt/Ca++ pathway was affected by SFRP2, as demonstrated by an increase in NFATc3 in the nuclear fraction of SFRP2-treated endothelial cells. Tacrolimus, a calcineurin inhibitor which inhibits dephosphorylation of NFAT, inhibited SFRP2-induced endothelial tube formation. Tacrolimus 3 mg/kg/daily inhibited the growth of SVR angiosarcoma xenografts in mice by 46% (p=0.04). In conclusion, SFRP2 is a novel stimulator of angiogenesis that stimulates angiogenesis via a calcineurin/NFAT pathway, and may be a favorable target for the inhibition of angiogenesis in solid tumors.
Keywords: Breast cancer, angiosarcoma, tumor endothelial marker, tacrolimus, endothelial cells
Introduction
Angiogenesis is the growth of new capillary blood vessels, and is a critical component of solid tumor growth(1). Limiting angiogenesis through blockade of vascular endothelial growth factor (VEGF) has resulted in an increase in overall or progression-free survival in patients with metastatic breast(2), colon(3), lung(4), and renal cell carcinoma(5). There is a need for new angiogenesis inhibitors because not all tumors respond equally well to anti-VEGF therapy, and most tumors eventually progress. Recognizing that tumor neovessels differ from normal vasculature, we developed a novel method for immuno-laser capture microdissection coupled with RNA amplification and genome-wide gene expression to profile tumor vasculature cells from human breast tumors with comparison to normal breast samples(6). In our analysis, the largest sample number to date of breast tumor vasculature, we identified 55 genes with greater than 4-fold increased expression compared to normal. Secreted frizzle related protein 2 (SFRP2) mRNA was increased more than 6-fold in breast cancer endothelium, and as shown by immunohistochemistry (IHC) 33/39 (85%) of breast tumors showed intense staining for SFRP2 in the neovasculature. Importantly, SFRP2 was highly expressed in the vasculature of luminal, Her2/neu, and basal tumors(6).
In our present study we evaluated the vascular expression of SFRP2 in angiosarcoma, colon cancer, prostate cancer, lung cancer, ovarian cancer, hepatocellular carcinoma, renal cell carcinoma, and pancreatic cancer, and found that SFRP2 is strongly expressed in all tumors, making SFRP2 a broad spectrum vascular target. Based on the expression of SFRP2 in vascular endothelium, we hypothesize that SFRP2 stimulates angiogenesis. We sought to associate a function with SFRP2 expression in endothelial cells using in vivo and in vitro angiogenesis models.
Materials and Methods
Chick chorioallantoic membrane (CAM) assay
Fertilized chickens eggs (NC State University Chicken Research Farm) were incubated at 100°F in an egg turner for 4 days and then cracked into sterile Petri dishes and incubated at 99°F 3%CO2 and 65% humidity. For application of drug onto the CAM, Whatman grade 1 filter paper was cut into circles with a 6mm diameter paper punch and autoclaved, soaked in 1ml of 3.0mg/ml cortisone acetate in absolute ETOH and air dried for 60 min in laminar flow hood. On day 8, 5 disks per egg were placed on outer third of CAM, 2–3 mm from a vessel. Control 0.1% BSA in PBS 7 μl was added to the discs for the control CAMs, and recombinant murine SFRP2 (US Biologicals, Swampscott, MA) 100 ng/7 μl 0.1% BSA in PBS was added to the disks for the treated CAMs (n=13 control disks and 23 SFRP2-treated disks). The CAMs were evaluated under stereomicroscope on day 3 after disk placement. Pictures of the area around the disk were taken with a Wild M-4 70 Macrosystem, and angiogenesis was quantified using Metamorph Software with an angiogenesis module.
Mouse Matrigel plug angiogenesis assay
Mouse studies were approved by IACUC at UNC. The Matrigel plug assay was performed as previously described(7). Female C57BL/6 mice (8 weeks old) were injected s.c. with 0.5 ml of growth factor reduced basement membrane matrix (Matrigel) from BD Biosciences (San Jose, CA) containing either mouse recombinant SFRP2 (800 ng/ml) with 30 U/ml heparin (American Pharmaceutical Partners, Inc., Schaumburg, Illinois) or PBS with 30 U/ml heparin for negative control, were injected s.c. on the back. Two to three Matrigel plugs were injected per mouse. Seven days later the mice were sacrificed and the Matrigel plugs removed and evaluated for angiogenesis by hemoglobin concentration with the Drabkin’s reagent as previously described(8).
Antibodies
The following antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA): β-catenin antibody (sc-59893) (1:500 dilution), NFATc1 (sc-7294) (1:500 dilution), NFATc2 (sc-7296) (1:500 dilution), NFATc3 (sc- 8405) (1:250 dilution for Western blot, 1:100 dilution for IHC), H-Ras (sc-29) (1:500 dilution), FRP2 (sc-13940) (1:500 dilution for Western blot, 1:100 dilution for IHC). The β-Tubulin loading control (ab6046) was purchased from Abcam (Cambridge, MA.) and used in 1:1000 dilution. The following secondary antibodies were purchased from GE Healthcare Bio-Sciences Corp. (Piscataway, NJ): ECL anti-mouse IgG, HRP-Linked Whole antibody (NA931) and ECL anti-rabbit IgG, HRP-Linked Whole antibody (NA934) with 1:100,000 dilution.
Cell culture
Human coronary artery endothelial cells (HCAEC) were purchased from Clonetics (San Diego, CA). HCAEC were cultured in endothelial cell basal medium-2 (EGM-2) with BulletKit growth supplements (Clonetics, San Diego, CA). Mouse myocardial endothelial cells (MEC) from Robert Auerbach (University of Wisconsin) were grown in Low Glucose Dulbecco’s Modified Eagle Medium (Gibco, Langley, OK) with 10% Fetal Bovine Serum (Hyclone, Logan, UT). SVR angiosarcoma cells were obtained from Dr. Jack Arbiser (Emory University Medical School) and cultured in Glucose Dulbecco’s Modified Eagle Medium 10% Fetal Bovine Serum.
Endothelial cell apoptosis
HCAECs were used for apoptosis assays because we were not able to induce apoptosis with hypoxia in the MEC cells. HCAEC were grown in 10-cm dishes (Becton Dickinson, Franklin Lakes, NJ) with until 80% confluent. Media was replaced with EGM-2 without BulletKit growth supplements and 70 pM or 700 pM of SFRP2 was added to the cells. The plate was incubated in hypoxic conditions (37°C in a hypoxia chamber with an atmosphere of 5% CO2/94% N2 with an oxygen level of 1.0%) for 36 hours. Apoptosis was measured by the activity of cleaved Caspase 3 by using a caspase-specific fluorogenic substrate according to the protocol for the Caspase 3 Assay Kit (Sigma, St. Louis, MO).
Endothelial cell proliferation assay
MECs (2000 cells/well) were seeded on a 96 well plate in media for 24 hrs. The cells were quiesced in LG-DMEM with 0.1% FBS for 18 hours. Media was replaced with fresh LG-DMEM with 0.5% FBS and the cells were treated in triplicate with PBS alone, or SFRP2 (7 nM – 7 pM). After 24, 48, or 72 h, 10 μl of the colorometric compound 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) (5 mg/mL) was added to each well and allowed to incubate for 4 h at 37°C. All but 25 μl of media in each well was removed and 50 μl dimethyl sulfoxide (DMSO) was added. After 10 min at 37°C, the A540 was measured using a microplate reader.
Scratch wound assay
MECs were plated at 10,000 cells/well into a 96 well plate and allowed to become confluent. The cells were quiesced in DMEM without serum for 18 hours. The wound was formed using a 1 ml pipette tip and a 0.7pM-700pM dose curve of mouse recombinant SFRP2 was added to the wells. Migration distance was measured using a 1mm ocular ruler at time points between 16 and 32 hours.
Tube formation assay
ECMatrix (Chemicon, Temecula, CA) was thawed, diluted and solidified into wells of a 96 well plate according to the manufactures instructions. MEC cells were seeded onto the matrix at a concentration of 1×104 cells/well in 150 μl of DMEM with 10% FBS. A 7pM–7nM dose curve of mouse recombinant SFRP2 was added to the wells and the plates were returned to 37°C, 5% CO2 for 8 hours. Images were acquired using the Nikon Eclipse TS100 microscope at 4x magnification with a Nikon CoolPix 995 digital camera. Results were quantified by counting the number of branch points.
For tacrolimus treatment experiments, MEC cells were treated as above with SFRP2 30nM with and without tacrolimus (LC Laboratories, Woburn, MA) at concentrations ranging from 1μM–100μM for 8 hours and branch points were determined as described above. Inhibition of SVR angiosarcoma tube formation by a polyclonal antibody to SFRP2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, catalog # SC-13940) was performed after removing sodium azide from the SFRP2 antibody using Zeba Desalt Spin Columns (Pierce Biotechnology, Rockford, IL). SVR cells were plated onto ECMatrix as above and a dilution curve of SFRP2 antibody (1:10-1:10,000) was added to the wells. Results were quantified by counting the number of branch points.
SiRNA to SFRP2 in SVR angiosarcoma cells
SVR cells were transfected with 72 μM siRNA for Sfrp2 [FRP-2 siRNA (sc-40001, Santa Cruz Biotechnology, Santa Cruz, CA)], which is a pool of 3 target-specific 20–25 nt siRNAs designed to knock down SFRP2 gene expression. The three sequences are 5′-GAGAUAACGUACAUCAACA-3′, 5′-CAAGCUGCAAUGCUAGUUU-3′, 5′-CCAUGUCAGGCGAAUUGUU-3′. The sham siRNA (sc-36869, Santa Cruz Biotechnology, Santa Cruz, CA) contains a scrambled sequence that does not lead to the specific degradation of any known cellular mRNA. SVR cells were maintained in DMEM with 10% fetal calf serum. After 72 h of transfection using Lipofectamine™ RNAiMAX Transfection reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol, cells were harvested for Western blot analyses and tube formation assay. Cells were seeded for a 4 hour tube formation assay as described above.
Western blot analyses
MECs were grown to 90% confluence followed by the change of media with and without mouse recombinant SFRP2 (700 pM). Cells were incubated for 1, 2, 4, 8 and 16 hours with SFRP2. MS1 and SVR cells were grown to 80–90% confluence. Nuclear and cytoplasmic proteins were extracted by using NE-PER™ nuclear and cytoplasmic extraction reagent (Pierce Biotechnology, Rockford, IL). The whole cell lysates were obtained using M-PER Mammalian Protein Extract reagent (Pierce Biotechnology, Rockford, IL) as described in the manufacturer’s manual. Protein concentration was measured using Bio-Rad Protein Assay at OD595 (Bio-Rad Laboratories, Inc., Hercules, CA). Equal amount of protein (20 μg) was loaded onto SDS-PAGE gels. Proteins were transferred to PVDF membrane, and Western blotting was carried out using the proper primary antibody, with horseradish peroxidase (HRP)-conjugated IgG as the secondary antibody. The ECL Advancetrade™substrate was used for visualization (GE Healthcare Bio-Sciences Corp. Piscataway, NJ cat# RPN2135).
Immunohistochemistry
Immunohistochemistry with antibodies to SFRP2 was performed on paraffin embedded human angiosarcoma, colon cancer, prostate cancer, lung cancer, ovarian cancer, pancreatic cancer, hepatocellular carcinoma, and renal cell carcinoma as previously described(6). Immunohistochemistry with antibodies to NFATc3 was performed on paraffin embedded human angiosarcoma. Tissues were sectioned at 8 μM, and 250ul of primary antibody (SFRP2 1:100 or NFATc3 1:100) was applied. A negative control without primary antibody was performed. Two pathologists (CL and NO) scored each tissue section for SFRP2 and NFATc3 expression based on intensity of stain in endothelium and percent positive endothelial cells staining.
Tumor Growth In Vivo
Mouse studies were approved by IACUC at Emory University. SVR cells (0.5×106) were injected into the flank of 6-week-old nude male mice obtained from Charles River Breeding Laboratories. Treatment was initiated on the day after inoculation. Mice received 3 mg/kg/daily tacrolimus or vehicle control suspended in 20% Intralipid (Baxter Healthcare, Deerfield, IL) in a total volume of 0.3 ml intraperitoneally (i.p.), and were treated daily for 19 days. Serial caliper measurements of perpendicular diameters were used to calculate tumor volume using the following formula: (shortest diameter)2 × (longest diameter) × 0.52.
Statistics
Statitistical differences for in vitro assays, Western blot analyses, CAM assay, and mouse Matrigel plug assay were performed with a two-tailed Student’s t-test, as the data from these experiments were normally distributed, with a p value < 0.05 being significant. For the in vivo mouse tumor growth experiment, differences in tumor volume over time were analyzed with a two way ANOVA. A P value of ≤ 0.05 indicated a statistically significant reduction in tumor growth of the treated group compared to the control group.
Results
SFRP2 vascular expression in tumors
We performed IHC with antibodies to SFRP2 in seven different tumor types and evaluated the intensity of staining in the vessels and the percent of endothelial staining. All tumors studies (prostate cancer n=7, angiosarcoma n=9, hepatocellular carcinoma n=7, colon cancer n=8, clear cell renal carcinoma n=8, lung cancer n=8, ovarian cancer n=8, and pancreatic cancer n=6) had 3+ vessel intensity staining in the tumor vasculature with the majority of endothelium staining positive in most tumors (Fig. 1, Supplemental Fig. 1), demonstrating that SFRP2 is a vascular target in a broad variety of tumors.
Figure 1.
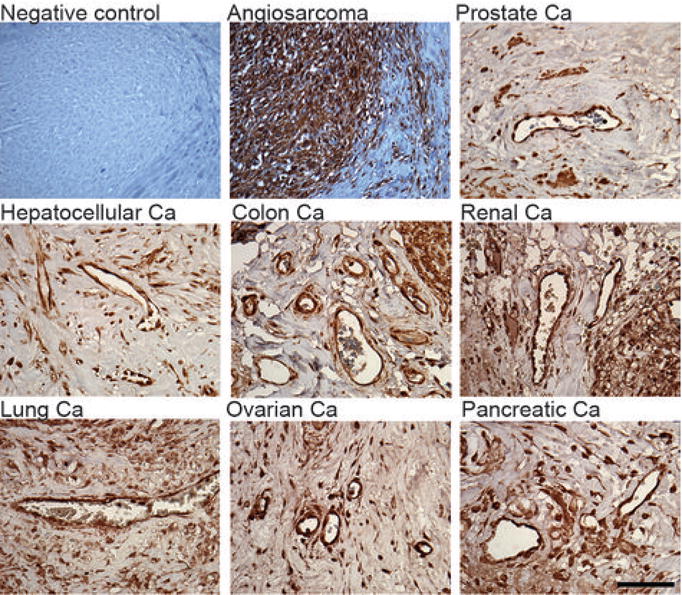
SFRP2 protein is present in the endothelium in a wide variety tumor types by immunohistochemistry. Paraffin-embedded sections of human tumors were stained with an antibody to SFRP2 as described in Material and Methods. Pictures are taken at 600 × magnification.
SFRP2 stimulates angiogenesis in vivo
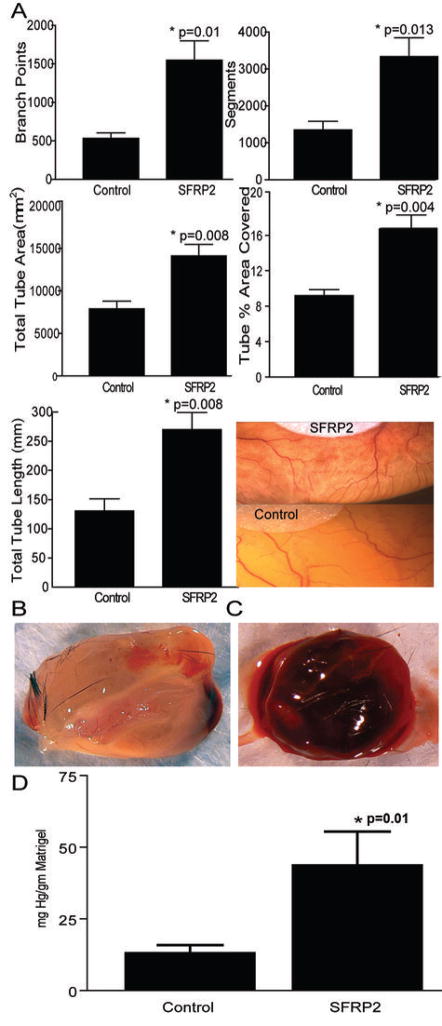
We implanted SFRP2 (n=23) impregnated filter paper or control (n=13) on the developing CAM on day 8. After 3 days, SFRP2 induced angiogenesis on the CAM with a statistically significant increase in number of branch points (0.010), segments (0.013), tube percent area covered (0.004), total tube area (0.008), and total tube length (0.008) (Fig. 2a). This observation on the CAM indicates that SFRP2 is a stimulator of angiogenesis.
Figure 2.
SFRP2 induces angiogenesis in vivo. (a) SFRP2 induces angiogenesis on the CAM. Filter disks with and without SFRP2 100 nm were placed on the CAM on day 8 and angiogenesis was quantitated as described in Materials and Methods. N= 13 control disks and n=23 SFRP2-impregnated disks. SFRP2 induced statistically significant increase in number of branch points, segments, tube percent area covered, and total tube length, as calculated with a two tailed t-test. (b–d) SFRP2 induces angiogenesis in the mouse Matrigel plug assay. b) Control Matrigel plug. c) SFRP2-impregnated matrigel plug. d) SFRP2 plugs had a 3.3 fold increase in hemoglobin concentration compared to control plugs (n=25 SFRP2 plugs, n=26 control plugs, *p=0.01, two-tailed t-test).
The angiogenic capacity of SFRP2 was confirmed by the mouse Matrigel plug assay. Seven days after injection into mice, the control Matrigel plugs containing PBS and 30 U/ml heparin and SFRP2 (800ng) + 30 U/ml heparin Matrigel containing plugs were removed. Evaluation of the angiogenic response by measurement of hemoglobin content showed a 3.3 fold increase in SFRP2 plugs compared with the vehicle control (n=25 SFRP2 plugs, n= 26 control plugs, p=0.01, Fig. 2b–d).
SFRP2 promotes cellular processes required for angiogenesis
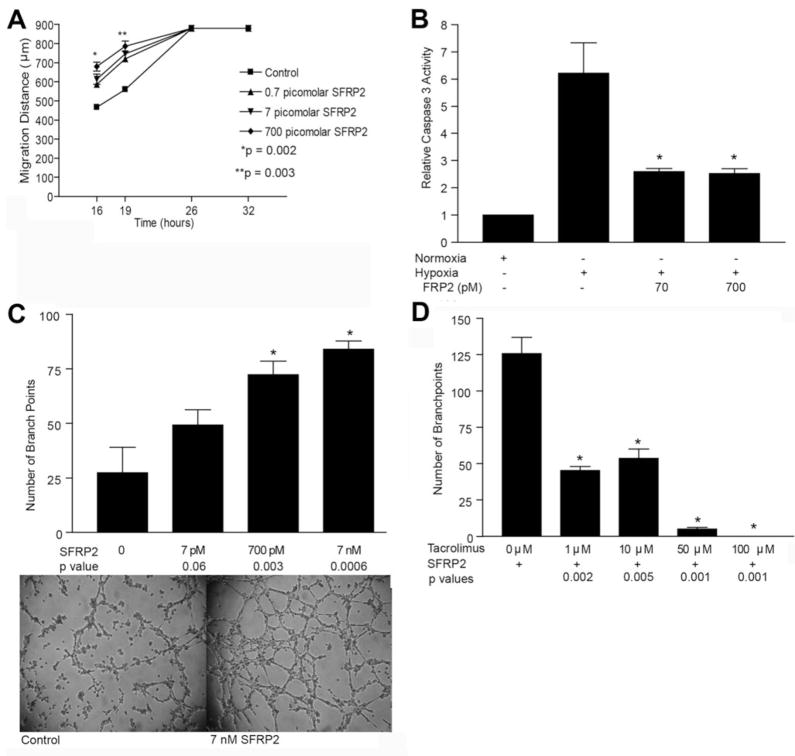
To explore the cellular mechanism through which SFRP2 promotes angiogenesis, we evaluated the effects of SFRP2 on endothelial cell migration, proliferation, apoptosis, and tube formation; which are all important steps in the angiogenesis cascade(9). The effect of SFRP2 on the migration properties of endothelial cells was evaluated using a scratch wound assay. At 16 hours, control mouse endothelial cells (MEC) migrated 480 μm ± 23, and SFRP2 (700pM)-treated MEC cells migrated 680 μm ± 40, p < 0.01, n=3 per group (Fig. 3a).
Figure 3.
Effects of SFRP2 on cellular processes involved in angiogenesis: (a) SFRP2 increases endothelial cell migration in a wound scratch assay. The rate of wound closure is shown for controls and increasing concentrations of mouse recombinant SFRP2. The values are the means ± SEM, n=3 for each concentration. SFRP2 increased MEC cell migration at low concentrations ranging from 0.7pM–700pM. (b) SFRP2 inhibits hypoxia induced apoptosis in HCAEC cells. HCAEC’s were incubated in a hypoxic chamber for 24 hours with and without the addition of SFRP2. Relative Caspases 3 activity was measured as described in “Material and Methods” Section. SFRP2 inhibited hypoxic induced apoptosis at 70pM and 700pM (*p<0.02). (c) Mouse endothelial cell tube formation assay. SFRP2 induces endothelial tube formation at 8 hours in a concentration-dependent manner. (d) Tacrolimus inhibits SFRP2 induced tube formation in mouse endothelial cells.
SFRP2 (7pM–7nM) had no effect on MEC or HCAEC proliferation at 24, 48, or 72 hours (data not shown). However, SFRP2 protected against hypoxia-induced HCAEC cell apoptosis. Treatment with hypoxia alone in HCAEC cells resulted in a 6-fold increase in apoptosis compared to cells in normoxia. Hypoxia does not induce apoptosis in MEC cells and therefore the effect of SFRP2 on MEC cell apoptosis was not evaluable. Addition of SFRP2 (70pM and 700pM) to hypoxic HCAEC cells decreased apoptosis by 55% ± 9 (n=4 per group, p<0.02, Fig. 3b).
MEC tube formation was induced by SFRP2 in a concentration-dependent manner at 8 hours (p=0.0006 at 7nM, n=4 per group) (Fig. 3c). Taken together, these in vitro experiments demonstrate that SFRP2 induces migration, anti-apoptosis, and tube formation, which are important cellular processes necessary for new blood vessel formation.
To study whether loss of function of SFRP2 would inhibit tube formation we used a model of a highly vascularized malignant endothelial tumor, the SVR angiosarcoma tumor model, which was derived from the transfection of Ras into MS1 endothelial cells. MS1 cells were previously generated by immortalizing murine endothelial cells by expressing the temperature-sensitive large T antigen(10). Upon implantation into mice, these cells form dormant hemangiomas(10). MS1 cells were then transfected with Ras (SVR), and this cell line forms angiosarcomas when injected into nude mice(10).
We collected protein lysates from MS1 and SVR cells and, using Western blot analyses probing for SFRP2, found that SFRP2 was increased in SVR cells compared to MS1 cells (Fig. 4a), which correlated with H-RAS overexpression. We then transfected SVR angiosarcoma cells with siRNA to SFRP2 or sham control and evaluated tube formation in a Matrigel assay. siRNA trasnsfected SVR cells had 70% fewer branch points compared to sham transfected cells (sham control =267 ± 33 branch points, siRNA SFRP2 transfected cells = 65 ± 10 branch points, p=0.001, n=4 per group, Fig. 4b). We then treated SVR cells in a tube formation assay with a polyclonal antibody to SFRP2 and found inhibition of SVR tube formation in a concentration dependent manner (Fig. 4c).
Figure 4.
SFRP2 is required for angiosarcoma tube formation. (a) Western blot analyses detects an increase in SFRP2 protein in SVR angiosarcoma cell line compared to MS1 cells, which is related to increased of H-ras protein in SVR cells. (b) siRNA to SFRP2 in SVR angiosarcoma cells reduces tube formation compared to sham transfected controls, *p=0.001, n=4 per group. (c) A polyclonal antibody to SFRP2 inhibits SVR tube formation in vitro.
Effects of SFRP2 on molecular signaling pathways in endothelial cells
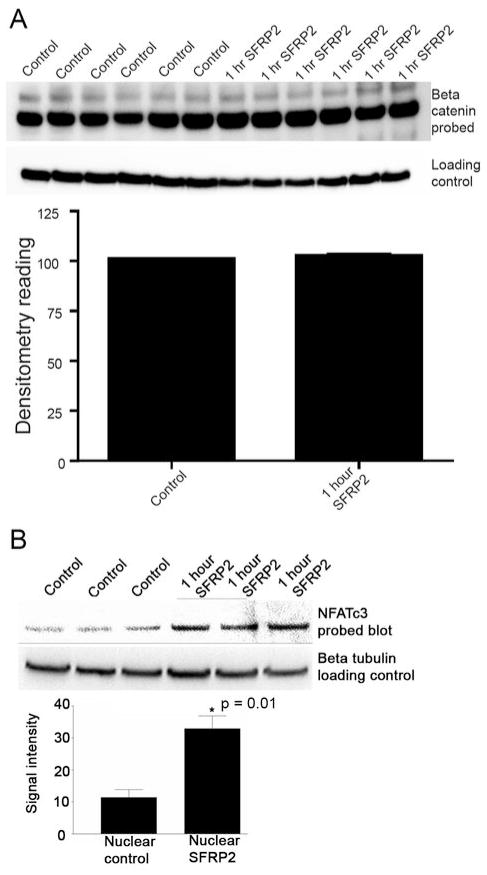
To begin to elucidate the molecular mechanism of SFRP2 in angiogenesis, we first investigated whether SFRP2 inhibited the canonical Wnt pathway, since SFRP2 has been reported to inhibit this signaling pathway in other cell types(11). We compared protein levels of dephosphorylated nuclear β-catenin in control and SFRP2 treated MEC cells. SFRP2 (30nM) decreased nuclear β-catenin in SFRP2- stimulated endothelial cells at 1 hour (Supplementary Fig. 2). However, at 700pM, which is the dose of SFRP2 that induced migration and tube formation in MEC cells, there was no statistically significant change in nuclear β-catenin in the SFRP2- stimulated endothelial cells at 1 hour (n=6 per group) (Fig. 5a), or at 2, 4, 8 and 16 hours (data not shown). This suggests that although SFRP2 may inhibit the canonical-Wnt pathway in endothelial cells, it is likely that the angiogenic effects of SFRP2 are being mediated through a non-canonical pathway, because the canonical pathway was not inhibited at the lower concentrations of SFRP2 which induce angiogenesis.
Figure 5.
Western blot analysis for nuclear β-catenin and nuclear NFATc3 protein in mouse endothelial cells treated with SFRP2. (a) There was no statistically significant change in nuclear β-catenin after treatment with SFRP2 (700pM), the dose that effects endothelial cell tube formation, migration, and apoptosis. (b) In contrast, nuclear NFATc3 was increased in SFRP2 treated MEC cells at 1 hour, *p=0.01, n=3 per group.
We next evaluated the role of the non-canonical Wnt/Ca++ pathway in SFRP2 induced angiogenesis. When the non-canonical Wnt/Ca++ pathway is activated, calcineurin dephosphorylates NFAT in the cytoplasm, allowing NFAT to translocate into the nucleus and act as a transcription factor(12). We found that NFATc3 was increased at 1 hour in the nuclear fraction of SFRP2-treated MEC cells (Fig. 5b). There was no change in NFATc1 or NFATc2 in SFRP2-treated endothelial cells (data not shown). This suggests that SFRP2 is signaling through the Wnt/Ca++ pathway and induces NFAT nuclear translocation in endothelial cells.
NFATc3 expression in human angiosarcoma
To correlate our in vitro finding of increased NFATc3 expression in SFRP2-treated endothelial cells with in vivo data, we performed immunohistochemistry with antibodies to NFATc3 on paraffin-embedded human angiosarcomas, and found that 6/8 tumors stained positive for NFATc3 (Supplementary Fig. 3).
Tacrolimus inhibits SFRP2-induced tube formation and tumor growth in vivo
If SFRP2 stimulates angiogenesis via the calcineurin/NFAT pathway, then pharmacologic inhibition of NFAT should inhibit SFRP2 induced angiogenesis. To test this hypothesis, we studied whether tacrolimus would inhibit SFRP2 mediated angiogenesis. Tacrolimus (FK506) binds to the immunophlin FKBP12. The FK506-FKBP12 complex associates with calcineurin and inhibits its phosphatase activity, which inhibits nuclear translocation of NFAT(13). Therefore we treated MEC cells in a tube formation assay with SFRP2 with and without tacrolimus. Tacrolimus (1μM) inhibited SFRP2 induced MEC tube formation by 64%, p=0.002, n=3 per group) (Fig. 3d). Tacrolimus was not cytotoxic to MEC cells, as only 5% of tacrolimus-treated cells took up trypan blue dye (data not shown). This provides further support for the role of NFAT in SFRP2 stimulated tube formation.
We then treated mice with SVR angiosarcoma xenografts with tacrolimus. Treatment with tacrolimus (n = 12) at 3 mg/kg/day daily i.p. for 19 days was effective at suppressing the growth of SVR angiosarcoma tumor in nude mice as compared with control (n = 12). Treatment with tacrolimus reduced mean tumor volume by 46% at day 19 (589 ± 129 mm3 vs 315 ± 93 mm3, two-way ANOVA, p=0.04, Fig. 6a, b). There were no signs of toxicity (i.e., no diarrhea, infection, lethargy, or weight loss) after 19 days of treatment.
Figure 6.
Tacrolimus inhibits the growth of SVR angiosarcoma xenograft in nude mice. Nude mice were injected with 0.5 × 106 SVR tumor cells as described in “Material and Methods”. a) Tacrolimus 3 mg/kg (n=12) or control buffer (n=12) was injected i.p. daily starting the day after tumor implantation. Treatment with tacrolimus reduced mean tumor volume by 46% at day 19 (589 ± 129 mm3 vs 315 ± 93 mm3, two-way ANOVA, *p=0.04) b) Representative control mouse tumor and representative tacrolimus treated mouse tumor on day 19 of treatment.
Discussion
SFRP2 belongs to a large family of secreted frizzle-related proteins which are related to the Wnt-signaling cascade, and has been previously implicated in cancer. Some studies suggest that SFRP2 is an inhibitor of the Wnt-beta catenin pathway(11), and this data taken together with reports of epigenetic inactivation of SFRP2 in breast cancer cell lines(14), oral squamous cell carcinoma(15), and hepatocellular carcinoma(16) suggest that SFRP2 may be a tumor suppresser gene. In contrast, several studies provide evidence that suggest that SFRP2 may induce tumor growth. For example, SFRP2 has been found to be highly induced in canine mammary gland tumors but not in normal mammary glands(17), and overexpression of transfected SFRP2 in breast adenocarcinoma cells increased their resistance to apoptotic signals (18). Additionally, SFRP2 has also been found to be produced by the majority of malignant glioma cell lines, and SFRP2 overexpressing intracranial glioma xenografts were significantly larger than xenografts consisting of control cells in nude mice(19). Although these studies suggest contrasting views of the role for SFRP2 on tumor epithelium, no study to date has evaluated the contribution of SFRP2 to angiogenesis.
We recently reported that SFRP2 is a novel tumor endothelial marker for breast cancer, and is overexpressed in the endothelium of approximately 85% of human breast tumors, including luminal A, basal, and Her2/neu subtypes(6). In this paper we now show that SFRP2 is expressed in the vasculature of a wide variety of tumors, including angiosarcoma, renal cell carcinoma, prostate cancer, lung cancer, ovarian cancer, pancreatic cancer and colon cancer, making SFRP2 a broad spectrum vascular target. Based on its overexpression in tumor endothelium, we hypothesized that SFRP2 stimulates angiogenesis. We report that SFRP2 is a novel stimulator of angiogenesis in vivo in the mouse Matrigel plug and CAM assays. SFRP2 modulates the cellular processes involved in angiogenesis, including endothelial cell migration, tube formation, and protection against hypoxia-induced endothelial cell apoptosis, and is required for angiosarcoma tube formation.
SFRP2 contains a cysteine-rich domain which is homologous to the putative Wnt binding domain. The Wnt-signaling network influences biological processes ranging from developmental cell fate to cell adhesion and apoptosis(11). Wnt proteins have been grouped into two classes – canonical and noncanonical – on the basis of their activity in cell lines or in vivo assays. The core of the Wnt pathway is the stability of beta-catenin. Although SFRPs have been regarded as inhibitors of the canonical Wnt-beta catenin pathway(11), four recent studies have shown that SFRP2 can act as a Wnt agonist rather than an antagonist. SFRP2 has been found to increase nuclear beta-catenin levels in: 1) cardiomyocytes exposed to hypoxia and treated with mouse recombinant SFRP2(20), 2) MCF7 cells with stable transfectants of SFRP2(21), 3) mammary canine tumors with increased expression of SFRP2(17), and hypoxic adipose tissue derived stem cells(22). Our study in endothelial cells show that at the dose of SFRP2 that induces angiogenesis, there is no change nuclear beta-catenin, suggesting that beta-catenin is not responsible for the angiogenic actions of SFRP2. This led us to investigate whether the angiogenic effects of SFRP2 are mediated through a noncanonical Wnt pathway.
Noncanonical Wnts activate other signaling pathways, such as the Wnt/Ca++ pathway(23) The Wnt/Ca++ pathway is a beta-catenin-independent pathway for which signaling is mediated through Wnt5a binding to the frizzled 5 transmembrane receptor, which leads to a transient increases in cytoplasmic free calcium. This subsequently activates the phosphatase calcineurin. Activated calcineurin dephosphorylates NFAT, which then translocates to from the cytoplasm to the nucleus. The NFAT family consists of four members (NFATc1-c4), which exist as transcriptionally inactive, cytosolic phosphoproteins(12). NFAT nuclear localization is dependent on a dynamic import-export balance between the activity of the Ca++/calmodulin-dependent phosphatase, calcineurin, and the activity of serine/threonine kinases(12). NFAT cannot normally enter the nucleus until it is dephosphorylated, but can be activated by calcineurin. Loss-of-function mutants have shown that NFAT signaling is crucial for normal heart valve and vascular development during embryogenesis(12;24). Postnatally, this pathway contributes to the regulation of cell growth, differentiation, and cell cycle progression in various cell types, and there is increasing data supporting a critical role of NFAT in mediating angiogenic responses(25;26). Importantly, NFAT activation was identified as a critical component of VEGF-induced angiogenesis and linked to the induction of cyclooxygenase-2, which is also a critical player in angiogenesis. NFAT is a crossing point for the endothelial cell survival pathways initiated by proangiogenic VEGF and bFGF(26). Based on these observations we hypothesized that SFRP2 activates NFAT in endothelial cells.
We found that NFATc3 protein was increased in the nucleus after treatment of endothelial cells in vitro with SFRF2, and was present in human angiosarcomas that also expressed SFRP2. Angiosarcoma is a biologically aggressive vascular malignancy with a high metastatic potential and subsequent mortality. The 2- and 5-year overall survival for all patients with angiosarcoma is 50 and 30%, respectively, with a median overall survival of 24 months(27). There is a desperate need for novel therapies to improve survival in patients with this highly lethal disease, and our findings suggest that targeting NFAT may be therapeutic strategy for angiosarcoma.
Tacrolimus is an immunosuppressive drug that binds to the immunophlin FKBP12. The FK506-FKBP12 complex associates with calcineurin and inhibits its phosphatase activity, which inhibits nuclear translocation of NFAT(13). We found that tacrolimus inhibited SFRP2 induced tube formation in vitro and the growth of angiosarcoma xenografts in vivo, suggesting that SFRP2 induces tube formation via a calcineurin-dependent NFAT pathway. Tacrolimus has previously been shown to inhibit growth of a leukemia cell line in mice(28), and our report is the first to our knowledge to demonstrate the tacrolimus inhibits angiogenesis in vitro and solid tumor growth in vivo. The mechanism of tacrolimus needs to be distinguished from other drugs used to prevent transplant rejection that are being studied in clinical trials for cancer, such as sirolimus (rapomycin) and everolimus (RAD001, a deriviated of rapomycin). These drugs have the same intracellular target as tacrolimus, FKBP12, but unlike tacrolimus these drugs inhibit mTOR and have no effect on NFAT(29).
In summary, SFRP2, a secreted protein overexpressed in the vasculature of a large variety of human tumors, is a novel angiogenesis stimulator. Our data suggests that SFRP2 mediates angiogenesis via a calcineurin-dependent NFAT pathway, and that an inhibitor of the calcineurin-NFAT pathway inhibit tumor growth. Based on its expression pattern and function, SFRP2 and NFAT may be favorable targets for the inhibition of angiogenesis in solid tumors, and direct tumor targets in angiosarcoma.
Acknowledgments
Financial support: Department of Defense Physician Scientist Training Award for Breast Cancer Research (W23RYX-3340-N609), National Cancer Institute (1 K08 CA098034-01A2), University Cancer Research Fund to NK-D, and the NIH National Cancer Institute Breast Cancer Specialized Program of Research Excellence grants P50-CA58223 to NK-D and CMP. J.L. Arbiser was supported by a Veterans Administration Merit Award, NIH grant R01 AR02030, and grants from the Jamie Rabinowitch-Davis Foundation and the Minsk Foundation.
Footnotes
Potential Conflict of Interest: “Discovery of Novel Targets for Angiogenesis Inhibition” Report of Invention Ref. # OTD07-008, patent pending 3/08 (NK-D and CP).
Reference List
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971 Nov 18;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007 Dec 27;357(26):2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004 Jun 3;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006 Dec 14;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 5.Kane RC, Farrell AT, Saber H, Tang S, Williams G, Jee JM, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006 Dec 15;12(24):7271–8. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 6.Bhati R, Patterson C, Livasy CA, Fan C, Ketelsen D, Hu Z, et al. Molecular characterization of human breast tumor vascular cells. Am J Pathol. 2008 May;172(5):1381–90. doi: 10.2353/ajpath.2008.070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, et al. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002 Jan;22(1):108–14. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- 8.Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL-18 as a novel angiogenic mediator. J Immunol. 2001 Aug 1;167(3):1644–53. doi: 10.4049/jimmunol.167.3.1644. [DOI] [PubMed] [Google Scholar]
- 9.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. Angiogenesis assays: a critical overview. Clin Chem. 2003 Jan;49(1):32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 10.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997 Feb 4;94(3):861–6. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003 Jul 1;116(Pt 13):2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson LM, Sun ZW, Nilsson J, Nordstrom I, Chen YW, Molkentin JD, et al. Novel blocker of NFAT activation inhibits IL-6 production in human myometrial arteries and reduces vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2007 Mar;292(3):C1167–C1178. doi: 10.1152/ajpcell.00590.2005. [DOI] [PubMed] [Google Scholar]
- 13.Martinez-Martinez S, Redondo JM. Inhibitors of the calcineurin/NFAT pathway. Curr Med Chem. 2004 Apr;11(8):997–1007. doi: 10.2174/0929867043455576. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki H, Toyota M, Carraway H, Gabrielson E, Ohmura T, Fujikane T, et al. Frequent epigenetic inactivation of Wnt antagonist genes in breast cancer. Br J Cancer. 2008 Mar 25;98(6):1147–56. doi: 10.1038/sj.bjc.6604259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sogabe Y, Suzuki H, Toyota M, Ogi K, Imai T, Nojima M, et al. Epigenetic inactivation of SFRP genes in oral squamous cell carcinoma. Int J Oncol. 2008 Jun;32(6):1253–61. doi: 10.3892/ijo_32_6_1253. [DOI] [PubMed] [Google Scholar]
- 16.Takagi H, Sasaki S, Suzuki H, Toyota M, Maruyama R, Nojima M, et al. Frequent epigenetic inactivation of SFRP genes in hepatocellular carcinoma. J Gastroenterol. 2008;43(5):378–89. doi: 10.1007/s00535-008-2170-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee JL, Chang CJ, Wu SY, Sargan DR, Lin CT. Secreted frizzled-related protein 2 (SFRP2) is highly expressed in canine mammary gland tumors but not in normal mammary glands. Breast Cancer Res Treat. 2004 Mar;84(2):139–49. doi: 10.1023/B:BREA.0000018412.83348.ff. [DOI] [PubMed] [Google Scholar]
- 18.Lee JL, Chang CJ, Chueh LL, Lin CT. Secreted Frizzled Related Protein 2 (sFRP2) Decreases Susceptibility to UV-Induced Apoptosis in Primary Culture of Canine Mammary Gland Tumors by NF-kappaB Activation or JNK Suppression. Breast Cancer Res Treat. 2006 Nov;100(1):49–58. doi: 10.1007/s10549-006-9233-9. [DOI] [PubMed] [Google Scholar]
- 19.Roth W, Wild-Bode C, Platten M, Grimmel C, Melkonyan HS, Dichgans J, et al. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene. 2000 Aug 31;19(37):4210–20. doi: 10.1038/sj.onc.1203783. [DOI] [PubMed] [Google Scholar]
- 20.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007 Jan 30;104(5):1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melkonyan HS, Chang WC, Shapiro JP, Mahadevappa M, Fitzpatrick PA, Kiefer MC, et al. SARPs: a family of secreted apoptosis-related proteins. Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):13636–41. doi: 10.1073/pnas.94.25.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gehmert S, Sadat S, Song YH, Yan Y, Alt E. The anti-apoptotic effect of IGF-1 on tissue resident stem cells is mediated via PI3-kinase dependent secreted frizzled related protein 2 (Sfrp2) release. Biochem Biophys Res Commun. 2008 Jul 11;371(4):752–5. doi: 10.1016/j.bbrc.2008.04.151. [DOI] [PubMed] [Google Scholar]
- 23.Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000 Apr 28;275(17):12701–11. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- 24.Schulz RA, Yutzey KE. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev Biol. 2004 Feb 1;266(1):1–16. doi: 10.1016/j.ydbio.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Minami T, Horiuchi K, Miura M, Abid MR, Takabe W, Noguchi N, et al. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J Biol Chem. 2004 Nov 26;279(48):50537–54. doi: 10.1074/jbc.M406454200. [DOI] [PubMed] [Google Scholar]
- 26.Zaichuk TA, Shroff EH, Emmanuel R, Filleur S, Nelius T, Volpert OV. Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J Exp Med. 2004 Jun 7;199(11):1513–22. doi: 10.1084/jem.20040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espat NJ, Lewis JJ, Woodruff JM, Antonescu C, Xia J, Leung D, et al. Confirmed angiosarcoma: prognostic factors and outcome in 50 prospectively followed patients. Sarcoma. 2000;4(4):173–7. doi: 10.1155/2000/575781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medyouf H, Alcalde H, Berthier C, Guillemin MC, dos Santos NR, Janin A, et al. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med. 2007 Jun;13(6):736–41. doi: 10.1038/nm1588. [DOI] [PubMed] [Google Scholar]
- 29.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006 Feb 10;124(3):471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]