Abstract
Epithelial and endothelial tight junctions act as a rate-limiting barrier between an organism and its environment. Continuing studies have highlighted the regulation of the tight junction barrier by cytokines. Elucidation of this interplay is vital for both the understanding of physiological tight junction regulation and the etiology of pathological conditions. This review will focus on recent advances in our understanding of the molecular mechanisms of tight junctions modulation by cytokines.
1. Introduction
Cytokine mediated changes in paracellular permeability contribute to a multitude of pathologic conditions including inflammatory bowel disease (IBD), airway inflammation in asthma [1]and cystic fibrosis [2], and diseases that perturb the blood-brain barrier (BBB) [3]. Cytokines also regulate diverse physiological, developmental, and non-inflammatory processes such as spermatocyte transmigration across the blood testis barrier [4] and mammary epithelial cell differentiation [5]. Epithelial and endothelial barrier function is maintained by intercellular Tight Junctions (TJs), multi-protein complexes that seal the space between adjacent cells. It is therefore easy to envision that cytokine mediated perturbation of TJ function results in enhanced paracellular permeability and increased exposure of tissues to luminal antigens in organ systems such as the gastrointestinal and respiratory tracts. While the molecular mechanisms that regulate these processes are incompletely understood, our knowledge is rapidly expanding through the use of reductionistic model cell culture systems of inflammation and epithelial/endothelial barrier function. This review will focus on recent findings that clarify the signaling processes underling cytokine modulation of epithelial and endothelial barrier function.
As a case in point, chronic recurring inflammation of the intestinal mucosa and loss of the epithelial barrier is observed in IBD. One of the major clinical manifestations of IBD, which encompasses both Crohn's disease (CD) and ulcerative colitis (UC), is chronic relapsing diarrhea. While the pathophysiology of these disorders is complex, an important underlying basis of these diseases is the existence of an abnormal “leaky” epithelial barrier that results in aberrant tissue exposure to luminal antigens and pathogens. Increased epithelial paracellular permeability has been documented in epithelium from acutely inflamed and chronically damaged intestinal mucosa. Furthermore, enhanced epithelial barrier dysfunction has been observed in first-degree relatives of patients with Crohn's disease, which suggests that a genetic component contributes to loss of barrier function and the patholophysiology of this disorder [6, 7]. In animal models of IBD such as the SAMP/Yit model, increased epithelial paracellular permeability precedes chronic intestinal mucosal inflammation [8]. Additionally, in animal models such as the mdr1a-/- mouse, altered epithelial barrier function has been associated with the subsequent development of colitis [9]. These observations further support the critical role of epithelial TJ protein complexes in maintaining mucosal tissue homeostasis. A broad array of cytokines perturb epithelial and endothelial barrier function by influencing the structure and function of the TJ. Table 1 contains a list of cytokines that influence epithelial/endothelial permeability to ions (*), and/or small molecules (#), and highlights postulated cytokine mechanisms of action. Experimentally, TJ barrier function is assessed by measurement of transepithelial (or endothelial) electrical resistance (TER), and the ability of TJs to restrict the passage of small molecules such as inulin, mannitol, or dextran through the paracellular space. Elucidating the molecular mechanisms behind the interplay between cytokines and epithelial permeability is vital for understanding the causes and complications of inflammatory disorders such as IBD.
Table 1.
Paracellular permeability changes due to cytokine treatment. IFN-γ, interferon gamma; TNF-α, tumor necrosis factor alpha; LIGHT, lymphotoxin-like inducible protein that competes with glycoprotein D for herpes virus entry on T cells; *, transepithelial resistance; #, small molecule flux; HUVECs, human umbilical vascular endothelial cells; BPAEC, bovine pulmonary artery endothelial cell; Caco-2, human colonic adenocarcinoma; UEC, uterine epithelial cells; T84, human colonic epithelial cells; Calu-3, human lung epithelial cells; MVEC, microvascular endothelial cells; PAC, primary airway cells; LLC-PK1, porcine renal epithelial cells; ↔, change in localization; ↓, decrease protein or mRNA levels; ↑, increased protein or mRNA levels; Unkn, unknown; JAM-A, junctional adhesion molecule A; NF-κB, nuclear factor-kappa B; MLCK, myosin light chain kinase; ZO-1, zonula occludins 1; ROCK, Rho associated kinase.
| CYTOKINE | PERMEABILITY | CELLS | MECHANISM |
|---|---|---|---|
| IFN-γ | Increased*# | T84 | Actin reorganization, ↓ ZO-1[21] |
| Decrease* # | Calu-3 | Unkn[14] | |
| - | T84 | myosin II-dependent vacuolarization, MLC/Rho/ROCK[25] | |
| Increase* # | T84 | ↔JAM-A, occludin, claudins1/4[22] | |
| Increase* # | T84 | Unkn[17, 86] | |
| Increase* | MVECs | Actin structure [20] | |
| Increase*# | Cholangiocytes | Unkn[60] | |
| Increase* | HUVECs | ↓Occludin, ↓E-Cadherin [27] | |
| TNFα | Increase# | HUVECs | ↓Occludin, ↔claudin 5 and JAM-A [40] |
| Increase# | BPAEC | Actin restructuring[107] | |
| Increase* | Caco-2 | Unkn[108] | |
| Increase* | Caco-2 | NF-κB, MLCK[38] | |
| Increase* # | Caco-2 | NF-κB, ↓ZO-1[36] | |
| Decrease* | UEC | Unkn[32] | |
| Decrease* | LLC-PK1 | Unkn[31] | |
| Increase* # | HT29/B6 | Lowered TJ complexity[99] | |
| Increase* # | MVEC | Actin restructuring [20] | |
| Increase* # | LLC-PK1 | Apoptosis [34] | |
| Increase*# | LLC-PK1 | Unkn[33] | |
| Increase*# | cholangiocytes | Unkn[60] | |
| IFN-γ+TNF-α | - | HEC | Unkn[6], ↔JAM-A[40] |
| Increase* | PAC | Unkn[2] | |
| Increase* # | T84 | Unkn[43], ↓Claudin 2,3 ↔Claudin 4[41] | |
| Increase* | T84 | Altered lipid composition[46] | |
| Increase* # | T84/Caco-2 | MLC/MLCK[42] | |
| Increase* # | Caco-2 | MLCK[44] | |
| Increased# | MVECs | ↔Claudin 5 [45] | |
| IFN-γ +LIGHT | Increased* | Caco-2 | MLCK, caveolar endocytosis[47] |
2. IFN-γ
Interferon gamma (IFN-γ) is a Th1 pro-inflammatory cytokine found at elevated levels in the intestinal mucosa of IBD patients [10-12]. In addition to its immunomodulatory role during inflammation, IFN-γ acts to modify epithelial and endothelial barrier function. In model cell culture systems of inflammation, direct treatment with IFN- γ increases the paracellular permeability of endothelial and epithelial monolayers (see Table 1). However, in airway epithelial cells, IFN-γ exposure has anti-inflammatory properties and promotes epithelial barrier function; indicative of pleiotropic effects [13, 14]. The reason for this discrepancy is unclear, although it should be noted that airway inflammatory episodes are considered primarily a Th2 mediated response [15]. The mechanisms through which IFN-γ influences epithelial/endothelial permeability are beginning to be understood at the molecular level. First observed in endothelial cell cultures by Stophen et.al., treatment with recombinant IFN-γ causes actin rearrangement into stress fibers [16]. More subtle changes in actin structure were observed in T84 epithelial cells which, with IFN-γ treatment, show contraction of cortical actin co-incident with epithelial barrier dysfunction [17]. TJ transmembrane proteins are linked by scaffolding proteins to the actin-myosin cytoskeleton, and disruption of acto-myosin structures has long been understood to modulate paracellular permeability [18, 19]. These observations are consistent with the hypothesis that actin-myosin restructuring plays a central role in cytokine mediated permeability changes.
Endothelial and epithelial cells respond to IFN-γ by restructuring actin and by decreasing protein levels or subcellular localization of the scaffolding protein ZO-1 [20, 21]. In T84 epithelial cells, the TJ transmembrane proteins, claudin, occludin, and junction adhesion molecule A (JAM-A), are internalized away from cell-cell contact regions [22, 23]. When visualized by immunoflouescence microscopy, these TJ components become disorganized and discontinuous at the lateral membrane after IFN-γ treatment [21, 22]. This may indicate an enhancement of constitutive TJ remodeling as opposed to gross TJ dissolution. Indeed, internalization of TJ proteins in response to IFN-γ proceeds by macropinocytosis into early recycling endosomes and requires acto-myosin based contraction [24, 25]. It is therefore feasible that IFN-γ increases acto-myosin contractility, promoting endocytosis of TJ structures. Endothelial cells treated with IFN-beta 1a and 1b blocked IFN- γ induced endocytosis of cadherin based junctions and maintained barrier integrity, suggesting a conserved mechanism between endothelial and epithelial responses[26]. Interestingly, mucosal biopsies from patients with actively inflamed UC show internalized sub-apical vesicles, similar to those found in T84 cells treated with IFN-γ, that contain TJ transmembrane proteins. This suggests that vesicle mediated internalization of TJ proteins is an in-vivo mechanism involved in permeability changes [24].
Recent studies have explored in greater depth the molecular mechanisms behind actin contractility, TJ protein endocytosis, and barrier function. IFN-γ exposure was found to activate the small GTPase RhoA and increase the expression of Rho associated kinase (ROCK), which in turn phosphorylates and activates myosin light chain (MLC) [25]. ROCK can also regulate MLC through inactivation of MLC phosphatase [27]. RhoA is a powerful regulator of actin remodeling associated with the formation of stress fibers (reviewed in [28]). Importantly, these data help to explain two consistently observed cellular responses to IFN-γ treatment: actin restructuring and acto-myosin contractility. However, the signaling pathways by which IFN-γ activates Rho/ROCK and the mechanisms of acto-myosin induced macropinocytosis remain unclear.
3. TNF-α
The pro-inflammatory cytokine tumor necrosis factor alpha (TNF-α), like IFN-γ, is implicated in IBD pathogenesis and is found in increased levels in the pulmonary sputum of cystic fibrosis patients [29, 30]. Using in-vitro model systems, TNF-α has been shown to directly impair TJ function in a number of epithelial and endothelial cell lines (see Table 1). Yet conflicting reports exist in several systems that complicate mechanistic interpretations of TNF-α responses. These may reflect cell type specific variation, as well as differences in the length and dose of cytokine treatment [31, 32]. TNF-α mediated increases in permeability were first described using the porcine renal cell line LLC-PK1, which shows an early (<3hrs) transient increase in permeability that quickly returns to normal levels (∼5hrs) [33]. A more recent study by this group implicated cell apoptosis, although the authors speculated that barrier dysfunction was due to actin and TJ rearrangement as apoptotic cells were extruded from the monolayer [34]. Through pharmacological inhibition and the overexpression of dominant negative I kappa B alpha (IκBα) mutants, the subsequent barrier recovery was shown to be mediated by nuclear factor-kappa B(NF-κB) [35]. TNF-α-stimulated NF-κB signal transduction has garnered much attention in recent studies. In the intestinal epithelial cell line Caco-2, TNF-α exerts a delayed effect on cell permeability. This results in an increase in small molecule flux within 24hrs of treatment, and alters TER by 48hrs post-treatment [36]. The authors point to a decrease in ZO-1 protein levels at both these time points, indicating an additional mechanism may be involved. The role of NF-κB was investigated by pharmacological inhibition, which ameliorated barrier defects, and stabilized ZO-1 subcellular localization and protein levels. A thorough investigation over several studies revealed that NF-κB acts to increase myosin light chain kinase (MLCK) transcription during TNF-α treatment [36-38]. Moreover, this correlated with increases in MLCK protein levels, MLC hyperphosphorylation, and increased paracellular permeability. In endothelial cells, MLC phosphorylation and RhoA activation are early events after TNF-α treatment and correlate with increased permeability [39]. Together, these findings are suggestive of a mechanism involving enhanced actin contractility similar to IFN-γ. However, in endothelial cells, pharmacological inhibition of ROCK or MLCK altered the early morphological changes observed but failed to improve barrier function [39]. Permeability changes in endothelial cells, which occur after several days of treatment, do not correlate with RhoA or MLC activation, but rather with decreased occludin levels and mislocalization of TJ transmembrane components claudin 5 and JAM-A. The authors conclude that long-term barrier dysfunction caused by TNF-α treatment is therefore due to TJ remodeling rather than acto-myosin contractility. The reason for the discrepancy between epithelial and endothelial systems is unclear, but may be indicative of cell-specific mechanisms for TJ remodeling during acute or chronic exposure.
4. Combined effects of TNF-α and IFN-γ
Under inflammatory conditions, target cells are exposed to a variety of cytokines. While model cell culture systems exposed to multiple cytokines may complicate mechanistic interpretation, it likely reflects many endogenous cellular environments. Both epithelial and endothelial cell culture systems exposed to TNF-α and IFN-γ simultaneously show increased paracellular permeability (see Table 1). Investigations into the molecular pathways involved report findings that are largely consistent with studies using either cytokine alone. These include altered actin structure, displacement or down regulation of TJ proteins, and activation of acto-myosin contractility pathways [16, 40-42]. Interestingly, cotreatment reveals a synergy between TNF-α and IFN-γ, with relative increases in barrier dysfunction and sensitization of airway epithelial cells to IFN-γ [2, 43, 44]. Combined treatment with TNF-α and IFN-γ results in mislocalization into the cytoplasm of tight junction proteins such as JAM-A, claudin 4 and claudin 5 [40, 41, 45]. Indeed, TJ proteins such as occludin and JAM have decreased membrane raft association in model intestinal epithelial cells exposed to a combination of TNF-α and IFN-γ [46]. The lymphotoxin-like inducible protein LIGHT, a TNF family member, also synergizes with IFN-γ to increase paracellular permeability [47]. MLC phosphorylation increases with combined TNF-α and IFN-γ treatment in Caco-2 epithelial cells [42]. The engagement of the myosin motor is essential for IFN-γ/TNF-α induced permeability changes in T84 cells, as myosin inhibition with pharmacological inhibitors reduces endocytosis in cytokine treated cells [42]. Although not required for protein internalization in T84 cells, MLCK is upregulated in Caco-2 cells treated with IFN-γ and TNF-α [25, 42]. Consistent with these observations, MLCK transcript was found to be upregulated by priming cultured cells with IFN-γ prior to TNF-α exposure [48]. This response, as with TNF-α alone, proceeds in a NF-κB dependent manner [44]. These data suggest that IFN-γ and TNF-α signal through independent pathways that converge at MLC phosphorylation (see figure 1.2). The mechanism of IFN-γ and TNF-α synergy is unclear, yet it is interesting to note that IFN-γ is believed to prime cultures for TNF-α treatment by upregulating the TNF-α cell surface receptor [49].
Figure 1. Mechanisms of TJ remodeling by cytokines.
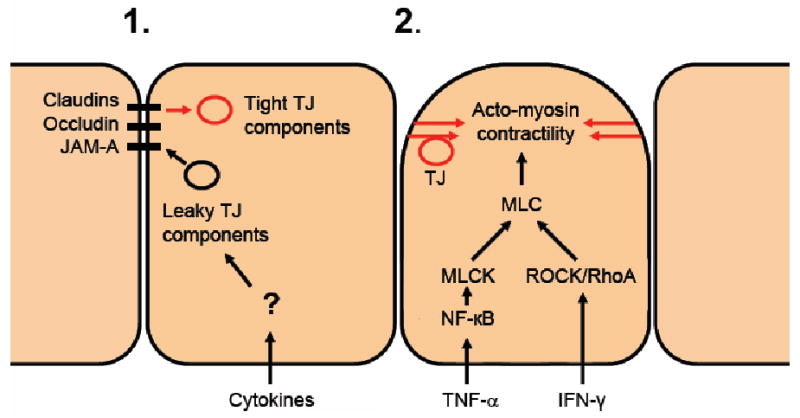
Mechanisms of paracellular permeability modulation by cytokines. The figure depicts cells in a monolayer undergoing stimulation and tight junction remodeling that results in barrier dysfunction. 1) In response to cytokine activity, tight junction structure is maintained but tight junction protein composition is altered. Tight junction proteins that confer “tight” barrier properties are replaced with those with “leaky” properties. 2) Internalization of TJ structures is due acto-myosin contractility. The pathways are cytokine specific yet converge on MLC.
5. Interleukins
Interleukins (IL) are a large family of cytokines, and several have been studied for effects on paracellular permeability in-vitro. These include IL-1, 2, 4, 6, 8, 10, and 13, all of which have been found to have a variety of effects on epithelial and endothelial paracellular permeability (Table 2). Interleukin-1 (IL-1) is type 1 pro-inflammatory cytokine that is elevated in the intestinal mucosa of patients with IBD and in the bronchoalveolar lavage fluid from asthma patients [50, 51]. In both epithelial and endothelial in-vitro cell culture systems, IL-1 addition to growth media directly increases paracellular permeability to ions and small molecules [52, 53]. In the model intestinal epithelial cell line Caco-2, IL-1 treatment decreases occludin protein levels due, at least in part, to the reduction of occludin mRNA levels [53]. This is consistent with previous findings in astrocytes, where IL-1β treatment suppressed occludin protein levels [54]. While occludin knockout mice have normal intestinal permeability (as measured by both trans-epithelial resistance (TER) and mannitol flux) they develop chronic inflammation, gastritis, and bone defects [55-57]. Indeed, exogenous occludin expression in MDCK cells increased TJ strand complexity and number, as well as decreasing cell permeability [58]. While the role of occludin in regulation of TJs is still incompletely understood, these studies clearly highlight a relationship between aberrant occludin expression and TJ permeability. IL-2 knockout mice exhibit spontaneous UC-like symptoms, yet specific effects on TJ protein composition and morphology have not shown direct effects by IL-2 [59, 60]. IL-4 treatment increases permeability in model intestinal epithelial T84 cells as well as in Calu-3 airway epithelial cells, which after 24hrs of treatment demonstrate decreased TER [14, 61]. Small molecule flux across the epithelium increases with extended IL-4 treatment (48hrs), which correspondes with a decrease in the protein levels of ZO-1 and occludin [14]. Interestingly, an IL-4-induced increase in intestinal epithelial permeability has also been associated with increased protein expression of claudin-2 [41, 62]. Increases in claudin 2 alter cell permeability, as exemplified by claudin 2 overexpression in epithelial cells, which lowers transepithelial resistance (TER) and confers increased Na+ conductance [63-65]. In IL-6 knockout mice, increased intestinal permeability to small molecules has been linked to stability of ZO-1 in TJs [66]. Consistent with these findings, IL-6 treatment in-vitro also increases permeability across endothelial cells and produces ZO-1 misslocalization, actin structure remodeling, and increased actin contractility [67]. In many circumstances IL-10 opposes the influence of pro-inflammatory cytokines such as IFN- γ on the barrier properties of the epithelium or endothelium [68, 69]. Mazzon et. al. have observed that IL-10 knockout mice, a model of spontaneous colitis, have increased levels of pro-inflammatory cytokines TNF-α, IL-1, and IL-6 [70]. IL-10 ablation also correlates with misslocalization of ZO-1 and claudin-1 away from TJs and may reflect the action of increased pro-inflammatory cytokines [70]. Direct treatment of airway epithelial cells with IL-13 causes decreased TER and enhanced manatol flux and lower ZO-1 protein levels [14]. IL-13 mediated barrier dysfunction in T84 cells also correlates with increased claudin 2 protein levels [41]. IL-13 and IL-4 act synergistically to stimulate the classical STAT6 pathway, although the involvement of this pathway in TJ structure and function has not been directly evaluated [71].
Table 2.
Permeability modification by interleukins and growth factors. IL-1, interleukin 1; TGF-β, transforming growth factor beta; HGF/SF, hapatocyte growth factor/scatter factor; HB-EGF, heparin-binding-epidermal growth factor; PDGF, platelet derived growth factor; *, transepithelial resistance; #, small molecule flux; HUVECs, human umbilical vascular endothelial cells; Caco-2, human colonic adenocarcinoma; UEC, uterine epithelial cells; T84, human colonic epithelial cells; Calu-3, human lung epithelial cells; PAC, primary airway cells; MDCK, Madine-Darby canine kidney cells; ERK, extracellular signal-regulated kinases; MAPK, mitogen-activated protein kinase; SMAD, small mothers against decapentaplegic; PKC, protein kinase C; TJ, tight junction; ZO-1, zonula occludins 1; NF-κB, nuclear factor-kappa B.
| CYTOKINE | PERMEABILITY | CELLS | MECHANISM |
|---|---|---|---|
| IL-1 | Increase* # | HUVECs | Unkn [52] |
| Increase* # | Caco-2 | NF-κB, ↓Occludin [53] | |
| - | Astrocytes | ↑Claudin 1, ↓Occludin[54] | |
| Increase* | PAE | Unkn[2] | |
| IL-4 | Increase* # | Calu-3 | ↓ZO-1, ↔Occludin[14] |
| Increase* # | T84 | Unknown[61]↑Claudin 2[62] | |
| IL-6 | Increase# | Intestine | ↓ZO-1[66] |
| Increase* | HUVECs | Actin restructuring, ↔ZO-1, PKC (α or β)[67] | |
| IL-10 | Decrease# | Liver | ↑ZO-1, ↔Claudin 1[70] |
| Decrease* # | T84 | Antagonizes IFN-γ[68] | |
| Decrease* # | HUVECs | Antagonizes IFN-γ, ↑Occludin[69] | |
| IL-13 | Increase* # | T84 | ↑Claudin 2[41] |
| Increase* # | Calu-3 | ↓ZO-1, ↔Occludin[14] | |
| TGFβ | Increase* | UEC | Unkn[32] |
| - | hepatocytes | ↓Claudin 1, ↑Claudin 2, SMAD[79] | |
| Decrease* | T84 | ↑Claudin 2, ERK, MAPK, SMAD [72, 73] | |
| HGF/SF | Increase# | cerebrovascula | ↓Occludin, ↓ZO-1 [81] |
| Decrease* | UEC | Unkn[32] | |
| HB-EGF | Decrease* | MDCK | ↓Claudin 2[83] |
| PDGF | Increased# | MDCK | TJ structure, ↔Occludin[84] |
Many interleukins act as modulators of TJ components, controlling occludin, claudin, and ZO-1 protein levels. These in turn form TJs with altered permeability characteristics. Alternatively, interleukins 2 and 10 act to antagonize the action of pro-inflammatory cytokines on TJ permeability, although the mechanisms involved are poorly understood.
6. Growth Factors
Growth factors have a variety of effects on paracellular permeability, either increasing or decreasing permeability, depending on the cell environment (Table 2). Transforming growth factor beta (TGF-β) is a multifunctional cytokine that has been shown to enhance epithelial barrier properties in-vitro [72, 73]. TGF-β binding to its cell surface receptor TGFRβI/II promotes SMAD mediated signaling to the nucleus. Recently, a SMAD independent pathway for TGF-β function was linked to partitioning defective protein 6 (Par6) through SMURF1 ubiquitination and degradation of RhoA [74]. Par6, an evolutionarily conserved regulator of cell polarity, is believed to act as a negative regulator of TJ establishment, yet may support TJ integrity through RhoA degradation [74, 75]. Par6 is located at TJs and is in a constitutive complex with atypical protein kinase C (aPKC), which together forms a complex with Par3 and Cdc42 [76]. Cdc42, a small Rho family GTPase, is thought to act together with Par6 as a GTP dependent molecular switch for the activation of aPKC [77]. Interestingly, aPKC activity is required for SMURF1 degradation of RhoA [78], presenting the possibility of a counter-acting mechanism to proinflammatory cytokines. Like many cytokines, TGF-β exhibits pleiotropic effects, as it increases permeability in uterine epithelial cells [32]. This is consistent with studies in rat hepatocytes, which show decreases in claudin 1 and increases in claudin 2 protein expression following exposure of cells to TGF-β [79]. TGF-β regulation of claudin 1 was found to proceed by a SMAD-dependent mechanism [73]. This may reflect multiple functions for TGF-β that are dependent on the biological setting. Indeed, TGF-β treatment is a model for the study of cell-cell contact disruption during epithelial-mesenchymal transition (EMT). The factors that dictate which of these pathways predominate after TGF treatment are unknown. Variable effects in cell barrier function are also seen with Hepatocyte Growth Factor/Scatter Factor (HGF/SF) treatment. An HGF-induced increased permeability is observed in epithelial and endothelial cells, and correlates with a decrease in occludin and ZO-1 protein levels [80, 81]. In contrast, HGF/SF exposure decreases uterine epithelial cell monolayer permeability [32]. HGF/SF is also used to study EMT and acts to internalize junction proteins in a clathrin dependent mechanism in Madin-Darby canine kidney cells (MDCK) [82]. Heparin binding epidermal growth factor (HB-EGF) decreases permeability in MDCK cells, which is correlated with suppression of claudin 2 protein levels [83]. Platelet-derived growth factor (PDGF) increases small molecule flux in MDCK cells through modulation of TJ structure and displacement of occludin [84]. Although varied in their effects on barrier properties, growth factors and cytokines act by similar mechanisms, including displacement or down regulation of TJ protein components and regulation of RhoA. Further studies will be needed to determine the signaling pathways that mediate these effects.
7. Mechanisms of Tight Junction regulation by cytokines
Although cellular responses to cytokines show cell type specific, pleiotropic, and time and dose-dependent effects, common mechanisms for TJ modulation emerge. These include cytokine induced actin remodeling and changes in TJ structure. By and large, in-vitro cell culture systems have shown that cytokines alter TJs independent of apoptosis. Epithelial and endothelial barrier continuity, when disrupted by cell apoptosis, leads to increases in tissue permeability. Cytotoxic effects due to cytokines have been described in a variety of cell lines and are dependent on the dose and duration of TNF-α and IFN-γ exposure [85]. Yet under experimental conditions that increase cell permeability, apoptosis was excluded as a mechanism in T84 cells treated with TNF-α and IFN-γ [17, 22, 36, 86]. For example, pharmacological inhibition of apoptosis fails to attenuate increases in monolayer permeability [46]. Therefore, cytokines are capable of directly modifying TJ composition and structure through signaling pathways independent of cell death.
7.1 Claudin/Occludin turnover at the tight junction
A recent study by Zeissig et. al. found aberrant expression of claudin 2, 5 and 8 in Crohn's disease patients. Surprisingly, these claudins were found to be increased in non-inflamed segments of the intestine [87]. Similar changes in claudin expression were not found in the same patients during periods of remission, suggesting that cytokines induce TJ remodeling and changes in claudin expression [88]. Indeed, recent studies in our laboratory demonstrated that increased susceptibility to experimentally induced colitis in JAM-A knockout animals correlated with increased expression of pore forming claudins (10 and 15) prior to experimental injury [89]. Changes in the claudin compliment within TJs effects the size and charge selectivity of the paracellular pathway as well as the structure of the TJ stands. A review of recent data groups claudins into two types, pore-forming claudins (2, 7, 10, 15, and 16) and sealing claudins (1, 4, 5, 8, 11, 14 and 19) [90]. Most of these claudins remain to be investigated and it is unclear by what mechanism claudin exchange takes place after cytokine exposure. It is believed that claudins are constitutively internalized and recycled in epithelial cells during normal junction maintenance and homeostasis [91]. Changes in TJ components could be due to increases in turnover rate at the expense of integration. Paracellular pore characteristic may therefore be altered through the exchange of transmembrane components, or by increasing/decreasing the number of existing pores (Figure 1.1). Indeed, cell line specific paracellular permeability has recently been attributed to changes in the density and number of pores between cells [92]. Therefore, both the number and the character of claudins expressed in a cell after cytokine treatment have the potential to alter paracellular barrier function.
Occludin and claudin protein function and stability can be regulated by post-transcriptional modification of C-terminal residues [93-95]. Phosphorylation in these domains has been shown to increase protein internalization. For example, EphA2 phosphorylation of the claudin 4 C-terminus disrupts the interaction of ZO-1 and claudin 4 localization in the membrane [96]. Claudin C-terminal cytoplasmic domains have PKC and Protein kinase A (PKA) phosphorylation sites within the C-terminal cytoplasmic domain [90, 97]. PKA-dependent phosphorylation of the serine residue in position -2 of the K+ channel Kir 2.3 disrupts interaction with PSD-95 [98]. These modifications may therefore increase protein turnover or disrupt interactions with scaffolding proteins. Both are thought to disrupt TJ structure and function. Interestingly, TNF-α induced permeability changes can be ameliorated by the PKA inhibitor H-8, and cytokine effects can be attenuated by PKC inhibitors [2][99]. Cytokine-induced changes in TJ permeability have previously been linked to alterations in tyrosine kinases and PKC [67, 99]. Indeed, IL-6 induced permeability increases can be attenuated by pharmacological inhibition of PKC and with a PKC pseudosubstrate [67].
Cytokine regulation of transmembrane protein transcription would be a powerful mechanism for TJ remodeling. Recent studies indicate that occludin downregulation by IL-1 is dependent on NFκB signaling [53]. NFκB is a co-factor for transcription and a classic mediator of inflammatory signals to the nucleus. NFκB activation within the first hour of IL-1β treatment is vital for TER disruption [53]. Therefore, NFκB activation occurs prior to permeability increases and mediates the observed changes in occludin and claudin protein levels. SMAD4, a mediator of TGF-β signaling, suppresses claudin 1 levels in SW480 cells, although the link to cytokine signaling and claudin 1 expression is unclear [100].
7.2 Actin cytoskeletal contraction and endocytosis of tight junction proteins
Wholesale disassembly of TJs is studied experimentally by incubation of cells in low or calcium-free media (calcium switch), which internalizes both adherens junctions and TJs. In low calcium conditions, endothelial cell monolayers lose barrier function through actin mediated internalization of E-cadherin[101]. Epithelial cultures lose barrier properties after calcium reduction and this has been shown to proceed via clathrin mediated membrane vesicle endocytosis [102]. Interestingly, endocytosis in both calcium depletion experiments and cytokine treatments lead to actin reorganization and activation of myosin dependent contractility [103]. In this model, peripheral actin ring structures contract in a non-muscle myosin dependent manner, which coincides with internalization of TJ structural proteins [103]. Cytochalasin D treatment increases permeability in MDCK cells and inhibits TJ restructuring after calcium depletion [104]. The same is true for Cytochalasin B, indicating actin related processes are required for endocytosis as well as junction reassembly [105]. Blebbistatin treatment, which inhibits the myosin II motor, also attenuates loss of junction complexes in the calcium switch model of junction disassembly [103]. Enteropathogenic bacteria induce increases in epithelial permeability in a manner similar to cytokine exposure, and requires MLC phosphorylation [42, 106]. These experimental systems are models of junction disassembly and barrier dysfunction, and share several features seen in cytokine treated cells. These include vesicle endocytosis and contraction of acto-myosin based cytoskeletal networks.
Cytokine mediated restructuring of TJs proceeds through a variety of different signaling pathways to effect paracellular permeability. A synthesis of current data with the aim of distilling common mechanisms, is complicated by pleiotropic cytokine actions as well as temporal and dose dependent variation in cell culture systems. For example, endothelial cells in-vivo may be required to maintain barrier function during exposure to chronic low-levels of systemic cytokines during an inflammatory response, and therefore respond differentially to low cytokine levels in model cell culture systems. Yet modulation of TJ properties by cytokines appears to proceed through two distinct processes, the remodeling of TJs by selectively removing or introducing TJ components (Figure 1.1) or the wholesale restructuring of TJ and actin networks (Figure 1.2). Several lines of evidence indicate that these processes may function simultaneously or sequentially. Although it should be recognized that during in-vivo inflammatory events the cytokine environment experienced by the cell is quite complex and the processes of cytokine modulation of TJs unclear, our understanding of the molecular mechanisms involved is expanding rapidly through the use of reductionisic cell culture systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–44. doi: 10.1016/j.jaci.2007.10.025. quiz 1245-6. [DOI] [PubMed] [Google Scholar]
- 2.Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218–34. doi: 10.1091/mbc.E02-03-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–36. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 4.Lui WY, Cheng CY. Regulation of cell junction dynamics by cytokines in the testis: a molecular and biochemical perspective. Cytokine Growth Factor Rev. 2007;18:299–311. doi: 10.1016/j.cytogfr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khaled WT, Read EK, Nicholson SE, Baxter FO, Brennan AJ, Came PJ, Sprigg N, McKenzie AN, Watson CJ. The IL-4/IL-13/Stat6 signalling pathway promotes luminal mammary epithelial cell development. Development. 2007;134:2739–50. doi: 10.1242/dev.003194. [DOI] [PubMed] [Google Scholar]
- 6.Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, Dadufalza VD, Krugliak P, Rotter JI. Intestinal permeability in patients with Crohn's disease and their healthy relatives. Gastroenterology. 1989;97:927–31. doi: 10.1016/0016-5085(89)91499-6. [DOI] [PubMed] [Google Scholar]
- 7.Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, Baert F, Vermeire S, Vlietinck R, Rutgeerts P. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology. 1997;113:802–7. doi: 10.1016/s0016-5085(97)70174-4. [DOI] [PubMed] [Google Scholar]
- 8.Olson TS, Reuter BK, Scott KG, Morris MA, Wang XM, Hancock LN, Burcin TL, Cohn SM, Ernst PB, Cominelli F, Meddings JB, Ley K, Pizarro TT. The primary defect in experimental ileitis originates from a nonhematopoietic source. J Exp Med. 2006;203:541–52. doi: 10.1084/jem.20050407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resta-Lenert S, Smitham J, Barrett KE. Epithelial dysfunction associated with the development of colitis in conventionally housed mdr1a-/- mice. Am J Physiol Gastrointest Liver Physiol. 2005;289:G153–62. doi: 10.1152/ajpgi.00395.2004. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura M, Saito H, Kasanuki J, Tamura Y, Yoshida S. Cytokine production in patients with inflammatory bowel disease. Gut. 1992;33:933–7. doi: 10.1136/gut.33.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niessner M. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR) Clin Exp Immunol. 1995;101:428–35. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 13.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 14.Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol. 2001;281:C2029–38. doi: 10.1152/ajpcell.2001.281.6.C2029. [DOI] [PubMed] [Google Scholar]
- 15.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–20. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stolpen AH, Guinan EC, Fiers W, Pober JS. Recombinant tumor necrosis factor and immune interferon act singly and in combination to reorganize human vascular endothelial cell monolayers. Am J Pathol. 1986;123:16–24. [PMC free article] [PubMed] [Google Scholar]
- 17.Madara JL, Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989;83:724–7. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–36. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht G, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82:1516–24. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blum MS, Toninelli E, Anderson JM, Balda MS, Zhou J, O'Donnell L, Pardi R, Bender JR. Cytoskeletal rearrangement mediates human microvascular endothelial tight junction modulation by cytokines. Am J Physiol. 1997;273:H286–94. doi: 10.1152/ajpheart.1997.273.1.H286. [DOI] [PubMed] [Google Scholar]
- 21.Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–88. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- 22.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–72. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 23.Utech M, Bruwer M, Nusrat A. Tight junctions and cell-cell interactions. Methods Mol Biol. 2006;341:185–95. doi: 10.1385/1-59745-113-4:185. [DOI] [PubMed] [Google Scholar]
- 24.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. Faseb J. 2005;19:923–33. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 25.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–52. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minagar A, Long A, Ma T, Jackson TH, Kelley RE, Ostanin DV, Sasaki M, Warren AC, Jawahar A, Cappell B, Alexander JS. Interferon (IFN)-beta 1a and IFN-beta 1b block IFN-gamma-induced disintegration of endothelial junction integrity and barrier. Endothelium. 2003;10:299–307. doi: 10.1080/10623320390272299. [DOI] [PubMed] [Google Scholar]
- 27.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–90. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 28.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 29.van Deventer SJ. Anti-TNF antibody treatment of Crohn's disease. Ann Rheum Dis. 1999;58 1:I114–20. doi: 10.1136/ard.58.2008.i114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salva PS, Doyle NA, Graham L, Eigen H, Doerschuk CM. TNF-alpha, IL-8, soluble ICAM-1, and neutrophils in sputum of cystic fibrosis patients. Pediatr Pulmonol. 1996;21:11–9. doi: 10.1002/(SICI)1099-0496(199601)21:1<11::AID-PPUL2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 31.Marano CW, Laughlin KV, Russo LM, Peralta A, Mullin JM. Long-term effects of tumor necrosis factor on LLC-PK1 transepithelial resistance. J Cell Physiol. 1993;157:519–27. doi: 10.1002/jcp.1041570311. [DOI] [PubMed] [Google Scholar]
- 32.Grant-Tschudy KS, Wira CR. Paracrine mediators of mouse uterine epithelial cell transepithelial resistance in culture. J Reprod Immunol. 2005;67:1–12. doi: 10.1016/j.jri.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Mullin JM, Snock KV. Effect of tumor necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990;50:2172–6. [PubMed] [Google Scholar]
- 34.Peralta Soler A, Mullin JM, Knudsen KA, Marano CW. Tissue remodeling during tumor necrosis factor-induced apoptosis in LLC-PK1 renal epithelial cells. Am J Physiol. 1996;270:F869–79. doi: 10.1152/ajprenal.1996.270.5.F869. [DOI] [PubMed] [Google Scholar]
- 35.Soler AP, Marano CW, Bryans M, Miller RD, Garulacan LA, Mauldin SK, Stamato TD, Mullin JM. Activation of NF-kappaB is necessary for the restoration of the barrier function of an epithelium undergoing TNF-alpha-induced apoptosis. Eur J Cell Biol. 1999;78:56–66. doi: 10.1016/s0171-9335(99)80007-7. [DOI] [PubMed] [Google Scholar]
- 36.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–76. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 37.Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–30. doi: 10.1152/ajpgi.00412.2004. [DOI] [PubMed] [Google Scholar]
- 38.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496–504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 39.McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–8. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- 40.Ozaki H, Ishii K, Horiuchi H, Arai H, Kawamoto T, Okawa K, Iwamatsu A, Kita T. Cutting edge: combined treatment of TNF-alpha and IFN-gamma causes redistribution of junctional adhesion molecule in human endothelial cells. J Immunol. 1999;163:553–7. [PubMed] [Google Scholar]
- 41.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85:1139–62. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 42.Zolotarevsky Y, Hecht G, Koutsouris A, Gonzalez DE, Quan C, Tom J, Mrsny RJ, Turner JR. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–72. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 43.Fish SM, Proujansky R, Reenstra WW. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45:191–8. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–19. doi: 10.1016/s0002-9440(10)62264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong RK, Baldwin AL, Heimark RL. Cadherin-5 redistribution at sites of TNF-alpha and IFN-gamma-induced permeability in mesenteric venules. Am J Physiol. 1999;276:H736–48. doi: 10.1152/ajpheart.1999.276.2.H736. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Interferon-gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol. 2008;126:67–80. doi: 10.1016/j.clim.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–94. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham WV, Wang F, Clayburgh DR, Cheng JX, Yoon B, Wang Y, Lin A, Turner JR. Tumor necrosis factor-induced long myosin light chain kinase transcription is regulated by differentiation-dependent signaling events. Characterization of the human long myosin light chain kinase promoter. J Biol Chem. 2006;281:26205–15. doi: 10.1074/jbc.M602164200. [DOI] [PubMed] [Google Scholar]
- 49.Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. 2006;131:1153–63. doi: 10.1053/j.gastro.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ligumsky M, Simon PL, Karmeli F, Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990;31:686–9. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–67. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 52.Marcus BC, Wyble CW, Hynes KL, Gewertz BL. Cytokine-induced increases in endothelial permeability occur after adhesion molecule expression. Surgery. 1996;120:411–6. doi: 10.1016/s0039-6060(96)80317-5. discussion 416-7. [DOI] [PubMed] [Google Scholar]
- 53.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 2007;178:4641–9. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duffy HS, John GR, Lee SC, Brosnan CF, Spray DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. J Neurosci. 2000;20:RC114. doi: 10.1523/JNEUROSCI.20-23-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta. 2005;1669:34–42. doi: 10.1016/j.bbamem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–42. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109(Pt 9):2287–98. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 59.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 60.Hanada S, Harada M, Koga H, Kawaguchi T, Taniguchi E, Kumashiro R, Ueno T, Ueno Y, Ishii M, Sakisaka S, Sata M. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int. 2003;23:3–11. doi: 10.1034/j.1600-0676.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 61.Colgan SP, Resnick MB, Parkos CA, Delp-Archer C, McGuirk D, Bacarra AE, Weller PF, Madara JL. IL-4 directly modulates function of a model human intestinal epithelium. J Immunol. 1994;153:2122–9. [PubMed] [Google Scholar]
- 62.Wisner DM, Harris LR, 3rd, Green CL, Poritz LS. Opposing regulation of the tight junction protein claudin-2 by interferon-gamma and interleukin-4. J Surg Res. 2008;144:1–7. doi: 10.1016/j.jss.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 63.Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–72. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci. 2002;115:4969–76. doi: 10.1242/jcs.00165. [DOI] [PubMed] [Google Scholar]
- 65.Van Itallie CM, Fanning AS, Anderson JM. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am J Physiol Renal Physiol. 2003;285:F1078–84. doi: 10.1152/ajprenal.00116.2003. [DOI] [PubMed] [Google Scholar]
- 66.Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–9. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 67.Desai TR, Leeper NJ, Hynes KL, Gewertz BL. Interleukin-6 causes endothelial barrier dysfunction via the protein kinase C pathway. J Surg Res. 2002;104:118–23. doi: 10.1006/jsre.2002.6415. [DOI] [PubMed] [Google Scholar]
- 68.Madsen KL, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 1997;113:151–9. doi: 10.1016/s0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 69.Oshima T, Laroux FS, Coe LL, Morise Z, Kawachi S, Bauer P, Grisham MB, Specian RD, Carter P, Jennings S, Granger DN, Joh T, Alexander JS. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc Res. 2001;61:130–43. doi: 10.1006/mvre.2000.2288. [DOI] [PubMed] [Google Scholar]
- 70.Mazzon E, Puzzolo D, Caputi AP, Cuzzocrea S. Role of IL-10 in hepatocyte tight junction alteration in mouse model of experimental colitis. Mol Med. 2002;8:353–66. [PMC free article] [PubMed] [Google Scholar]
- 71.Heller NM, Matsukura S, Georas SN, Boothby MR, Rothman PB, Stellato C, Schleimer RP. Interferon-gamma inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31:573–82. doi: 10.1165/rcmb.2004-0195OC. [DOI] [PubMed] [Google Scholar]
- 72.Howe K, Gauldie J, McKay DM. TGF-beta effects on epithelial ion transport and barrier: reduced Cl- secretion blocked by a p38 MAPK inhibitor. Am J Physiol Cell Physiol. 2002;283:C1667–74. doi: 10.1152/ajpcell.00414.2001. [DOI] [PubMed] [Google Scholar]
- 73.Howe KL, Reardon C, Wang A, Nazli A, McKay DM. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol. 2005;167:1587–97. doi: 10.1016/s0002-9440(10)61243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–9. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- 75.Gao L, Joberty G, Macara IG. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr Biol. 2002;12:221–5. doi: 10.1016/s0960-9822(01)00663-7. [DOI] [PubMed] [Google Scholar]
- 76.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–9. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 77.Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, Ohno S. PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells. 2001;6:721–31. doi: 10.1046/j.1365-2443.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- 78.Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–9. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- 79.Kojima T, Takano KI, Yamamoto T, Murata M, Son S, Imamura M, Yamaguchi H, Osanai M, Chiba H, Himi T, Sawada N. Transforming growth factor-beta induces epithelial to mesenchymal transition by down-regulation of claudin-1 expression and the fence function in adult rat hepatocytes. Liver Int. 2007 doi: 10.1111/j.1478-3231.2007.01631.x. [DOI] [PubMed] [Google Scholar]
- 80.Nusrat A, Parkos CA, Bacarra AE, Godowski PJ, Delp-Archer C, Rosen EM, Madara JL. Hepatocyte growth factor/scatter factor effects on epithelia. Regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J Clin Invest. 1994;93:2056–65. doi: 10.1172/JCI117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Date I, Takagi N, Takagi K, Tanonaka K, Funakoshi H, Matsumoto K, Nakamura T, Takeo S. Hepatocyte growth factor attenuates cerebral ischemia-induced increase in permeability of the blood-brain barrier and decreases in expression of tight junctional proteins in cerebral vessels. Neurosci Lett. 2006;407:141–5. doi: 10.1016/j.neulet.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 82.Palacios F, Schweitzer JK, Boshans RL, D'Souza-Schorey C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol. 2002;4:929–36. doi: 10.1038/ncb881. [DOI] [PubMed] [Google Scholar]
- 83.Singh AB, Sugimoto K, Dhawan P, Harris RC. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol. 2007;293:C1660–8. doi: 10.1152/ajpcell.00274.2007. [DOI] [PubMed] [Google Scholar]
- 84.Harhaj NS, Barber AJ, Antonetti DA. Platelet-derived growth factor mediates tight junction redistribution and increases permeability in MDCK cells. J Cell Physiol. 2002;193:349–64. doi: 10.1002/jcp.10183. [DOI] [PubMed] [Google Scholar]
- 85.Fransen L, Ruysschaert MR, Van der Heyden J, Fiers W. Recombinant tumor necrosis factor: species specificity for a variety of human and murine transformed cell lines. Cell Immunol. 1986;100:260–7. doi: 10.1016/0008-8749(86)90025-0. [DOI] [PubMed] [Google Scholar]
- 86.Adams RB, Planchon SM, Roche JK. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J Immunol. 1993;150:2356–63. [PubMed] [Google Scholar]
- 87.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, Dermody TS, Nusrat A, Parkos CA. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–76. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE. Structure and function of claudins. Biochim Biophys Acta. 2007;1778:631–645. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 91.Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci. 2004;117:1247–57. doi: 10.1242/jcs.00972. [DOI] [PubMed] [Google Scholar]
- 92.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci. 2008;121:298–305. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 93.Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol. 1999;170:147–56. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- 94.Fujibe M, Chiba H, Kojima T, Soma T, Wada T, Yamashita T, Sawada N. Thr203 of claudin-1, a putative phosphorylation site for MAP kinase, is required to promote the barrier function of tight junctions. Exp Cell Res. 2004;295:36–47. doi: 10.1016/j.yexcr.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 95.Van Itallie CM, Colegio OR, Anderson JM. The cytoplasmic tails of claudins can influence tight junction barrier properties through effects on protein stability. J Membr Biol. 2004;199:29–38. doi: 10.1007/s00232-004-0673-z. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka M, Kamata R, Sakai R. EphA2 phosphorylates the cytoplasmic tail of Claudin-4 and mediates paracellular permeability. J Biol Chem. 2005;280:42375–82. doi: 10.1074/jbc.M503786200. [DOI] [PubMed] [Google Scholar]
- 97.D'Souza T, Agarwal R, Morin PJ. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J Biol Chem. 2005;280:26233–40. doi: 10.1074/jbc.M502003200. [DOI] [PubMed] [Google Scholar]
- 98.Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–67. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 99.Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112(Pt 1):137–46. doi: 10.1242/jcs.112.1.137. [DOI] [PubMed] [Google Scholar]
- 100.Shiou SR, Singh AB, Moorthy K, Datta PK, Washington MK, Beauchamp RD, Dhawan P. Smad4 regulates claudin-1 expression in a transforming growth factor-beta-independent manner in colon cancer cells. Cancer Res. 2007;67:1571–9. doi: 10.1158/0008-5472.CAN-06-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alexander JS, Jackson SA, Chaney E, Kevil CG, Haselton FR. The role of cadherin endocytosis in endothelial barrier regulation: involvement of protein kinase C and actin-cadherin interactions. Inflammation. 1998;22:419–33. doi: 10.1023/a:1022325017013. [DOI] [PubMed] [Google Scholar]
- 102.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays. 2005;27:356–65. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- 103.Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15:2639–51. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stevenson BR, Begg DA. Concentration-dependent effects of cytochalasin D on tight junctions and actin filaments in MDCK epithelial cells. J Cell Sci. 1994;107(Pt 3):367–75. doi: 10.1242/jcs.107.3.367. [DOI] [PubMed] [Google Scholar]
- 105.Ma TY, Hoa NT, Tran DD, Bui V, Pedram A, Mills S, Merryfield M. Cytochalasin B modulation of Caco-2 tight junction barrier: role of myosin light chain kinase. Am J Physiol Gastrointest Liver Physiol. 2000;279:G875–85. doi: 10.1152/ajpgi.2000.279.5.G875. [DOI] [PubMed] [Google Scholar]
- 106.Yuhan R, Koutsouris A, Savkovic SD, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–82. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 107.Goldblum SE, Ding X, Campbell-Washington J. TNF-alpha induces endothelial cell F-actin depolymerization, new actin synthesis, and barrier dysfunction. Am J Physiol. 1993;264:C894–905. doi: 10.1152/ajpcell.1993.264.4.C894. [DOI] [PubMed] [Google Scholar]
- 108.Marano CW, Lewis SA, Garulacan LA, Soler AP, Mullin JM. Tumor necrosis factor-alpha increases sodium and chloride conductance across the tight junction of CACO-2 BBE, a human intestinal epithelial cell line. J Membr Biol. 1998;161:263–74. doi: 10.1007/s002329900333. [DOI] [PubMed] [Google Scholar]


