Abstract
Effects of optical correction on best-corrected grating acuity (vertical (V), horizontal (H), oblique (O)), vernier acuity (V, H, O), contrast sensitivity (1.5, 6.0, and 18.0 cy/deg spatial frequency, V and H), and stereoacuity were evaluated prospectively in 4- to 13-year-old astigmats and a non-astigmatic age-matched control group. Measurements made at baseline (eyeglasses dispensed for astigmats), 6 weeks, and 1 year showed greater improvement in astigmatic than non-astigmatic children for all measures. Treatment effects occurred by 6 weeks, and did not differ by cohort (< 8 vs. 8 years), but astigmatic children did not attain normal levels of visual function.
Keywords: astigmatism, amblyopia, children, visual performance, treatment
1. Introduction
Degraded visual input during early development can result in neural visual deficits that are clinically termed amblyopia. These deficits are evidenced by reduced visual performance in the absence of any ocular cause. Previous research has shown that patterns of visual deficits in amblyopia can be dependent on the nature of the disruption of visual input present during development (Mitchell, Freeman, Millodot, & Haegerstrom, 1973; Levi & Klein, 1982; Dobson, Miller, Harvey, & Mohan, 2003; McKee, Levi & Movshon, 2003). For example, in astigmatism-related amblyopia, presence and severity of visual deficits can be specific to stimulus orientation. This pattern of amblyopia, termed meridional amblyopia (Mitchell et al., 1973), develops as a result of the orientation-specific defocus characteristic of uncorrected astigmatism, although some studies have found deficits that are independent of stimulus orientation in individuals with some types of astigmatism (Dobson et al., 2003; Harvey, Dobson, Miller, & Clifford-Donaldson, 2007b). Meridional amblyopia has been documented in several types of visual function, including grating acuity (Freeman, Mitchell, & Millidot, 1972; Mitchell et al, 1973; Mitchell & Wilkinson, 1974; Freeman, 1975a; Cobb & MacDonald, 1978; Mohindra, Jacobson, & Held, 1983; Gwiazda, Scheiman, & Held, 1984; Atkinson, Braddick, Bobier, Anker, Ehrlich, King, Watson, & Moore, 1996; Dobson et al., 2003; Harvey et al., 2007b), vernier acuity (Gwiazda, Bauer, Thorn, & Held, 1986; Mitchell et al., 1973), contrast sensitivity (Mitchell & Wilkinson, 1974; Freeman, 1975b; Freeman & Thibos, 1975), and stereoacuity (Mitchell et al., 1973). Previous studies of astigmatic individuals have also documented reduced best-corrected recognition acuity (Kershner & Brick, 1984; Atkinson et al, 1996; Dobson, Tyszko, Miller, & Harvey, 1996; Harvey 2002; Dobson et al 2003; Harvey, Dobson, Clifford-Donaldson, & Miller, 2007a; Harvey et al., 2007b), reduced stereoacuity for complex stimuli (Harvey et al., 2007b), and reduced best-corrected vision across stimulus orientations in grating acuity, vernier acuity, and contrast sensitivity (Harvey et al., 2007b).
Previous research has demonstrated that optical correction of astigmatism, i.e., restoration of normal visual input, can be an effective treatment of astigmatism-related amblyopia during early childhood (Mitchell et al., 1973; Cobb & MacDonald, 1978; Mohindra et al., 1983). However, some evidence suggests that this form of plasticity may be limited to a sensitive period. Retrospective studies of a small number of astigmatic adults have found that meridional amblyopia occurs rarely in those who received eyeglasses prior to age seven years, but frequently in those who received eyeglasses after age seven (Mitchell et al., 1973; Cobb & MacDonald, 1978). Two prospective studies (Harvey, Dobson, Miller, & Sherrill, 2004; Harvey et al., 2007a) have examined the effect of optical correction on astigmatism-related amblyopia in children who are members of a Native American tribe with a high prevalence of astigmatism. The first study, which included subjects three to five years of age, found no significant improvement in best-corrected recognition acuity or grating acuity, and no reduction in meridional amblyopia, in astigmatic children after an average optical treatment duration of four months, relative to a non-astigmatic control group(Harvey et al., 2004). In contrast, the second study, which included subjects four to 13 years of age, showed significantly greater improvement in best-corrected recognition acuity in astigmatic children compared to the improvement over time shown by a normal (non-astigmatic) age-matched control group after an average treatment duration of six weeks (Harvey et al., 2007a). Furthermore, the significant improvement in best-corrected recognition acuity was found both in children age seven years or younger, the age previously believed to mark the end of the sensitive period for successful treatment (Mitchell et al., 1973; Cobb & MacDonald, 1978), and in children older than age seven years (Harvey et al., 2007a). However, the results also indicated that after one year of optical treatment, astigmatic children still had significantly poorer best-corrected visual acuity than did non-astigmatic children. It was not clear whether the persistence of reduced acuity after one year of treatment was due to reduced plasticity in this age range, or to poor treatment compliance in some subjects.
In the present study, we examine prospectively changes in grating acuity for vertical (V), horizontal (H), and oblique (O) stimuli, vernier acuity for V, H, and O stimuli, contrast sensitivity for V and H stimuli, and stereoacuity for complex stimuli that occur following optical treatment of astigmatism-related amblyopia. Because previous retrospective and prospective studies suggest that meridional amblyopia may not be responsive to optical correction beyond age seven years (Mitchell et al., 1973; Cobb & MacDonald, 1978), or perhaps even earlier (Harvey et al., 2004), the present report groups subjects into a younger cohort (YC, < 8 years of age) and an older cohort (OC, 8 years of age). Outcome of best-corrected recognition acuity for these subjects has been reported previously (Harvey et al., 2007a).
2. Methods
2.1. Subjects
Subjects were children in grades K-2 (recruited during the 2003/04 school year) and children in grades 4–6 (recruited during the 2001/02 school year) who attended one of five elementary schools located on the Tohono O’odham Reservation in southern Arizona, and children at a sixth elementary school on the reservation who participated in a preliminary study during the 2000/01 and 2001/02 school years. Recruitment years for different grades were selected in order to minimize the possibility of recruiting children who participated in a previous eyeglass treatment study of Tohono O’odham preschool children (1997–2001, Miller, Dobson, Harvey, & Sherrill, 2000, 2001; Dobson et al. 2003; Harvey et al., 2004). This population was chosen for the study because there is a high prevalence of astigmatism (Dobson, Miller, & Harvey, 1999; Dobson, Miller, Harvey, & Sherrill, 1999; Harvey, Dobson, & Miller, 2006) and astigmatism-related amblyopia (Dobson et al., 1996; Dobson et al., 2003; Harvey et al., 2004) among the Tohono O’odham.
The Institutional Review Board of the University of Arizona approved this study. Prior to each child’s participation, written informed consent was obtained from a parent or guardian, and written assent was obtained from children in grades 4, 5, and 6.
2.2. Procedures
Each child was scheduled to participate in an initial eye examination, a baseline best-corrected vision testing session, a six-week follow-up best-corrected vision testing session, a one-year follow-up eye examination, and a one-year follow-up best-corrected vision testing session. Refractive error correction for the baseline and six-week follow-up vision testing sessions was determined at the initial eye examination, and refractive error correction for the one-year follow-up vision testing session was determined at the one-year follow-up eye examination.
At the eye examinations, each child underwent a complete eye examination including cycloplegic refraction, conducted by a pediatric ophthalmologist (JMM) at least 40 min after instillation of one drop of proparacaine (0.5%) and two drops of cyclopentolate (1%) separated by an interval of 5 min. Eyeglasses were prescribed for (a) children who had ≥ 2.00 diopters (D) of astigmatism in either eye, and (b) children who had uncorrected recognition acuity worse than 20/20 and significant refractive error (myopia ≥ 0.75 D in either meridian, hyperopia ≥ 2.50 D in either meridian, astigmatism ≥ 1.00 D in either eye, anisometropia ≥ 1.50 D spherical equivalent). Eyeglass prescriptions were determined by cycloplegic autorefraction (Nikon Retinomax K+, Nikon Inc, Tokyo, now manufactured by Righton Manufacturing Co., Tokyo), confirmed by retinoscopy and by subjective refinement (when possible). Correction of hyperopic refractive error was reduced by one-third or by 1.00 D, whichever was greater (Guyton, Miller, & West, 2003).
The baseline vision testing session was conducted on a separate day approximately two to three weeks after the initial eye exam. The first follow-up vision testing session was conducted approximately six weeks after the baseline session, and the one-year follow-up vision testing session was conducted approximately two to three weeks after the one-year follow-up eye examination (approximately one year after the baseline vision testing session). Eyeglasses were prescribed only for children who met the above criteria, and these children were given their eyeglasses at the beginning of the baseline vision testing session. However, all children wore eyeglasses containing their refractive correction (with hyperopic refractive error reduced by one-third or by 1.00 D, whichever was greater) during the vision testing sessions. Thus, each child was tested with his/her best-correction, and testers were masked as to which children had been prescribed eyeglasses. Children who did not meet the prescribing criteria wore a pair of eyeglasses selected from a set of “stock” eyeglasses in which the right and left lens corrections were no more than 0.50 vector dioptric difference (Long, 1976; Harris, 1990; Harvey, Miller, Dobson, Tyszko, & Davis, 2000) from the child’s refractive error.
Vision testing was conducted by a team of trained testers who were masked to each child’s refractive error and to results obtained at previous testing sessions. Each vision testing session included five tests: (1) monocular (right eye (RE) and left eye (LE)) distance (4 m) logMAR recognition acuity using 62- by 65-cm Early Treatment Diabetic Retinopathy Study (ETDRS) charts (Ferris, Kassoff, Bresnick, & Bailey, 1982) mounted in an illuminator cabinet (Precision Vision, Inc., LaSalle, IL); (2) monocular (RE) grating acuity for V, H, and O gratings tested at 1.5 m using stimuli constructed from unmounted Teller acuity cards (Vistech Consultants, Inc., Dayton, OH) (Teller, McDonald, Preston, Sebris, & Dobson, 1986); (3) monocular (RE) vernier acuity for V, H, and O stimuli tested at 1.75 m using stimuli that were generated using a computer program (Miller, Harvey, & Dobson, 2002); (4) monocular (RE) contrast sensitivity tested at 3 m for low (1.5 cy/deg), middle (6 cy/deg), and high (18 cy/deg) spatial frequency V and H sinewave grating stimuli using stimuli constructed from unmounted VCTS6500 Contrast Sensitivity Charts (Vistech Consultants, Inc., Dayton, OH); and (5) stereoacuity tested at 40 cm using the Randot Preschool Stereoacuity Test (Stereo Optical Co., Chicago, IL) (Birch, Williams, Hunter, & Lapa, 1997). Test order was counterbalanced across subjects but remained constant for each child across all testing sessions (baseline, six weeks, and one year). During monocular testing, the fellow eye was occluded with 5-cm wide adhesive paper tape (3M micropore, Minneapolis, MN). A detailed description of the tests and testing procedures is provided in another report (Harvey et al., 2007b). Results of longitudinal recognition acuity testing are reported elsewhere (Harvey et al., 2007a).
2.3. Resolution (Grating) Acuity
Grating acuity was assessed using a 3-alternative forced-choice (3AFC) procedure in which the subject’s task was to identify which one of three circles (number 1, 2, or 3) contained a grating. The remaining two circles on each trial contained gray stimuli constructed from the same Teller Acuity Card as the grating. Stimuli were organized into a test book that included grating spatial frequencies ranging from 0.86 to 38 cy/cm (2.3 to 99.5 cy/deg), ordered from lowest to highest spatial frequency, with V, H, and O gratings interleaved. Order of presentation of orientations within each spatial frequency was always the same for an individual child (at baseline, six weeks, and one year), but was counterbalanced across the five schools at which testing was conducted. Testing began with the 6.5 cy/cm (17 cy/deg) grating and continued until the subject could no longer identify the location of the grating on three of three or on three of four trials for an orientation. Grating acuity for each orientation was scored as the highest spatial frequency at which a subject could correctly locate the grating on at least three out of a maximum of four trials. For subjects who were judged unable to resolve the largest grating available (0.86 cy/cm, 2.3 cy/deg), a grating acuity corresponding to the next lower spatial frequency in the Teller Acuity Card set (0.64 cy/cm, 1.7 cy/deg) was assigned.
2.4. Vernier Acuity
Vernier acuity for V, H, and O lines was tested using a 3AFC procedure similar to that used to assess grating acuity. The subject’s task was to identify which one of three circles (number 1, 2, or 3) contained the “wiggly” line. The remaining two circles on each trial contained a straight line of the same width and length as the vernier stimulus. Stimuli were organized into a test book that included stimuli with offsets ranging from 80 to 5 arc sec, ordered from largest to smallest offset, with V, H, and O gratings interleaved. Order of presentation of orientations within each offset size was always the same for an individual child (at baseline, six weeks, and one year), but was counterbalanced across the five schools at which testing was conducted. Testing began with the 80 arc sec offset and continued until the subject could no longer identify the location of the vernier stimulus on three of three or on three of four trials for an orientation. Vernier acuity for each stimulus orientation was scored as the smallest vernier offset at which the child could correctly identify the vernier stimulus on three out of a maximum of four trials. Subjects who were judged unable to resolve the largest offset (80 arc sec) were assigned a vernier acuity 100 arc sec, i.e., 0.1 log unit larger than the largest level included in the test book.
2.5. Contrast Sensitivity
Assessment of contrast sensitivity was conducted using a test design similar to that used to test grating and vernier acuity: each trial was a 3AFC task (V, tilted clockwise, or tilted counter-clockwise; or H, tilted clockwise, or tilted counter-clockwise), and the subject had to correctly identify the grating orientation by holding up a pen, and matching the orientation of the pen to the orientation of the grating. For each of the three spatial frequencies tested, the test included eight levels of contrast (max-min/max+min) ranging from 0.33 to 0.006 for 1.5 cy/deg, 0.20 to 0.004 for 6 cy/deg, and 0.25 to 0.011 for 18 cy/deg, with V and H stimuli interleaved within the test book. Order (V or H first) was constant across sessions for each child, but was counterbalanced across schools. Contrast sensitivity for each grating orientation for each spatial frequency was scored as the lowest contrast level on which the child was able to correctly identify the orientation of the grating on at least three out of a maximum of four trials. Order of testing across the three spatial frequencies was always the same for an individual child, was randomly selected by the tester prior to the child’s first test session, and was counterbalanced across subjects.
A contrast sensitivity threshold one step larger than the highest contrast level included in the test book (average step size was 0.2 log unit) was assigned for subjects who were judged unable to resolve the highest contrast stimulus (contrast threshold values of 0.52, 0.32, and 0.39 were assigned for 1.5, 6.0, and 18.0 cy/deg stimuli, respectively).
2.6. Stereoacuity
The Randot Preschool Stereoacuity Test includes six levels of retinal disparity that range from 800 to 40 arc sec. Subjects wore test-specific polarized glasses over their eyeglasses. Stereoacuity was recorded as the smallest disparity at which the subject could correctly identify two of three shapes in the random dot display. For subjects who were judged unable to resolve the largest disparity level, a stereoacuity of 1600 arc sec was assigned.
2.7. Encouraging and Monitoring Treatment Compliance
Eyeglasses were initially dispensed at the baseline vision testing session. At the end of the testing session, children who required eyeglasses were given one pair and were instructed to wear them all the time. Each child’s teacher was given a spare pair of eyeglasses to keep in the classroom. Classroom eyeglasses were for use on days when children did not bring their glasses, or their glasses were lost or broken. Teachers were asked to provide the children with their spare pair when needed, and to try to collect them at the end of the day so that the child would always have a pair at school. Children were given the classroom spare to take home over the summer vacation, and were instructed to use them if their other pair became lost or broken. A study staff member made periodic visits to each classroom to check on glasses and to encourage eyeglass wear. This staff member carried a spare pair for each child so that they could be dispensed as soon possible whenever a child’s glasses became lost, broken, or badly scratched. As soon as this spare was dispensed, a replacement spare was ordered. Daily log books were given to each teacher to record whether children were or were not wearing their eyeglasses. The log books also served as a reminder to teachers to dispense the classroom pair if the child did not have his/her eyeglasses.
2.8. Data Analysis
Threshold values for each measure were transformed to log values for data analyses. Subjects were assigned to astigmatism groups based on the cycloplegic refraction results of the initial eye examination. The non-astigmatic control (NonA) group included children with little or no astigmatism (< 0.75 D in the RE and the LE), and the astigmatic group included children with ≥ 1.00 D RE with-the-rule (plus cylinder axis 90±15 deg) astigmatism. Subjects in the astigmatic group were further divided into two subgroups: (a) children with hyperopic astigmatism (HA), sphere (plus cyl) ≥ 0, and (b) children with myopic or mixed astigmatism (M/MA), sphere (plus cyl) < 0. Subjects were also categorized by age cohort. The younger cohort (YC) included children < 8 years of age, and the older cohort (OC) included children ≥ 8 years of age on the day of baseline best-corrected vision testing (and glasses dispensing).
Data from subjects who did not meet the criteria for any of the three astigmatism groups (NonA, HA, M/MA), subjects with anisometropia (≥1.50 D difference in spherical equivalent between eyes), subjects with ocular abnormalities other than refractive error, astigmatic subjects whose uncorrected RE recognition acuity was 20/20 or better, and subjects who did not participate in best-corrected vision testing at baseline, six weeks, and one year were excluded from analyses.
Amblyopia is a clinical term, and while it is generally defined as reduced best-corrected vision in the absence of ocular causes, the specific deficits that indicate the presence of amblyopia, both in clinical and research settings, can vary. In order to avoid any confusion with this term, for the purpose of the present report we define astigmatism-related amblyopia as significantly reduced vision in astigmats, relative to vision in an age-matched non-astigmatic control group. Similarly, we define meridional amblyopia as a significant difference in vision across stimulus orientation in astigmats, relative to meridional differences observed in a non-astigmatic age-matched control group. In the present study amblyopia is examined in astigmats as a group (e.g., mean acuity in astigmats vs. non-astigmats), rather than for individuals (i.e., data on deficits in individual astigmatic subjects are not reported).
The first set of analyses was aimed at determining if there were significant deficits at baseline for the HA and M/MA groups relative to the NonA group (significantly reduced best-corrected vision and/or significant meridional amblyopia). We have previously published a detailed report of baseline measurements of visual performance in the three astigmatism groups (Harvey et al., 2007b). However, the present report includes only those subjects followed for a full year after baseline, i.e., a subset of the 805 subjects for whom we reported results of baseline testing. Therefore, we present analyses here to document whether or not baseline deficits were significant in this smaller sample. Separate analyses of variance (ANOVAs) compared baseline measures of grating acuity for V, H, and O stimuli, vernier acuity for V, H, and O stimuli, contrast sensitivity for V and H stimuli (with separate analyses for low, middle, and high spatial frequency stimuli), and stereoacuity across astigmatism group (HA, M/MA, NonA) and age cohort (YC vs. OC). In order to evaluate meridional amblyopia, analyses (ANOVAs) also compared vertical-horizontal (V-H) grating acuity, vernier acuity, and contrast sensitivity across astigmatism group and age cohort.
The second set of analyses focused on determining if there was a significant effect of treatment, i.e., whether the amount of change in best-corrected visual performance over time in the HA and M/MA groups was greater than that observed in the NonA group. Separate repeated measures analyses of variance (RM-ANOVAs) compared mean change over time in resolution (grating) acuity for V, H, and O stimuli, in vernier acuity for V, H, and O stimuli, in contrast sensitivity for V and H stimuli (with separate analyses for low, middle, and high spatial frequency stimuli), and in stereoacuity across age cohort (YC vs. OC) from baseline to six weeks to one year. Analyses (RM-ANOVAs) also evaluated change over time in meridional amblyopia (the difference between performance for V and H stimuli) for grating acuity, vernier acuity, and contrast sensitivity for the astigmatic groups, relative to the NonA group. All significant main effects and interactions are reported. Main effects of age cohort are reported, and when significant, always reflect better vision in the older cohort unless otherwise noted. Planned post hoc comparisons (t-tests with Bonferroni correction for multiple comparisons) were conducted only on significant interactions that included both the “time” and the “astigmatism group” variables, as these effects were pertinent to the primary aims of the study: To determine if there was significantly greater improvement in the astigmatic groups over time, relative to the non-astigmatic group. Because a previous detailed report of baseline data showed no evidence that visual deficits were associated with presence of astigmatic anisometropia or with previous eyeglass wear in this sample (Harvey et al., 2007b), these variables were not entered into the present analyses.
Finally, the last set of analyses were aimed at determining if significant baseline deficits for the HA and M/MA groups relative to the NonA group (significantly reduced best-corrected vision and/or significant meridional amblyopia) remained significant after one year of treatment. The same analyses conducted on baseline data (see summary of first set of analyses above) were conducted on one-year data for measures that yielded significantly reduced performance for astigmatic children at baseline.
3. Results
Of 1,048 K-2nd and 4th–6th grade children enrolled in the study, 243 were excluded from analyses for the following reasons: undilated at initial exam (2: 1 refused, 1 poorly dilated), history of patching (1), anisometropia (18), strabismus (11), ocular abnormality other than strabismus (11), anisometropia and strabismus (2), anisometropia and ocular abnormality (1), strabismus and ocular abnormality (1), lost to follow-up after baseline eye exam (no baseline visual acuity data collected, 39), did not meet the criteria for any astigmatism group (NonA, HA, M/MA, 157).
A total of 805 children provided baseline data and met the inclusion criteria. Of these children, 95.3% (767/805) were tested at 6 weeks (38 (5%) lost to follow-up), and 67.9% (547/805) were tested at both 6 weeks and 1 year (113 (14%) lost to follow-up at 6 weeks and/or 1 year, 145 (18%) entered the study during the second year of testing for their cohort and contributed data only at baseline and 6 weeks). Final analyses include the 547 children who met the inclusion criteria and were followed up at both six weeks and one year. Sample sizes were 324 (136 YC, 188 OC) for the NonA group, 109 (74 YC, 35 OC) for the HA group, and 114 for the M/MA group (44 YC, 70 OC). Some children did not complete all measurements at each follow-up session. Sample sizes for each measure are provided in the results section. The average age for the final sample of 547 children was 8.66 years (range: 4.75 to 13.53 (SD=2.30)).
Follow-up vision testing occurred an average of 44.7 days (SD 16.1) (six-week follow-up) and 375.9 days (SD 35.6) (one-year follow-up) after the baseline vision testing session. ANOVA on duration of baseline to six-week follow-up interval yielded a significant main effect of age cohort (F(1, 541)=57.28, p < 0.001), with follow-up interval shorter for the OC (39.7, SD 13.6) than the YC (50.5, SD 16.9) and a main effect of astigmatism group (F(2,541)=4.65, p < 0.02), with the NonA group having a significantly shorter follow-up interval than the HA group (42.9 (SD 15.3) vs. 48.4 (SD 16.2), p < 0.004). There was also a significant interaction between astigmatism group and age cohort (F(2,541)=4.27, p < 0.02). For the YC, follow-up interval for the NonA group was significantly shorter than for the M/MA group (48.2 (SD 16.2) vs. 57.3 (SD 18.9), p < 0.006), whereas there were no significant differences across astigmatism group for the OC. There were no significant differences or interactions between age cohorts and astigmatism groups for the baseline to one year interval. Age cohort effects are likely due to differences in scheduling limitations between grades/schools (primary vs. intermediate schools), and astigmatism group effects are likely due to occasional delays in receiving prescription glasses (i.e., glasses for the astigmatic children) from the optical laboratory.
Detailed results of data analyses for each measure of vision are provided below. In each section, analyses address the following: (1) Performance at Baseline: Was vision significantly reduced and was meridional amblyopia present in the HA and M/MA groups relative to the NonA group at baseline? (2) Treatment Effects: Was there significantly greater improvement in best-corrected vision and/or a significant reduction in meridional amblyopia over time in the HA or M/MA groups, relative to the NonA group, and was there any evidence of reduced treatment effects in OC children, compared to the YC? and (3) Performance at One Year: Did vision remain significantly reduced and did meridional amblyopia persist in the HA and M/MA groups relative to the NonA group after 1 year of treatment? A summary of results for all measures is provided in Table 1.
Table 1.
| Measure | Stimulus |
* Visual Deficits: Baseline |
* Visual Deficits: 1 Year |
† Improvement: Baseline to 6 Weeks |
† Improvement: 6 Weeks to 1 Year |
||||
|---|---|---|---|---|---|---|---|---|---|
| HA | M/MA | HA | M/MA | HA | M/MA | HA | M/MA | ||
| Grating Acuity | V | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ns | ns |
| H | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| O | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| V-H | ns | <0.001 | --- | <0.001 | ns | ns | ns | ns | |
| Vernier Acuity | V | <0.001 | <0.001 | <0.004 | ns | <0.003 | < 0.001 | ns | ns |
| H | <0.001 | <0.001 | <0.02 | <0.001 | |||||
| O | <0.001 | <0.001 | <0.009 | <0.02 | |||||
| V-H | ns | ns | -- | -- | ns | ns | ns | ns | |
| 1.5 cy/deg Contrast | V | ns | ns | -- | -- | <0.008 | <0.02 | ns | ns |
| H | <0.001 | <0.04 | ns | ns | |||||
| V-H | <0.04 | ns | ns | ns | ns | ns | ns | ns | |
| 6.0 cy/deg Contrast | V | <0.001 | <0.001 | <0.03 | <0.001 | YC: ns OC:<0.009 | YC: ns OC:<0.02 | ns | ns |
| H | <0.001 | <0.001 | ns | <0.001 | YC: ns OC:<0.001 | YC: ns OC:<0.002 | YC:<0.03 OC:ns | ||
| V-H | ns | ns | -- | -- | ns | ns | ns | ||
| 18.0 cy/deg Contrast | V | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ns | ns | ns |
| H | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| V-H | <0.02 | ns | <0.04 | -- | ns | ns | ns | ns | |
| Stereo Acuity | YC:<0.001 OC:<0.001 | YC:<0.001 OC:<0.005 | YC:<0.001OC:<0.001 | YC:<0.001 OC:<0.001 | <0.001 | ns | ns | ns | |
Numbers indicate p values, when significant deficits relative to the NonA group were present, “ns” indicates vision not significantly reduced (relative to NonA group), and “—“ indicates that test was not conducted (in cases in which deficits were not significant at baseline). Results by cohort presented only in cases in which cohort interacted significantly with astigmatism group.
Numbers indicate p values, when there is significant improvement relative to improvement in the NonA group, and “ns” indicates no significant improvement (relative to NonA group). Results by cohort presented only in cases in which cohort interacted significantly with both “time” and “astigmatism group” variables (i.e., only when improvement differed significantly across astigmatism group and cohort). Cells are merged across orientation in cases in which stimulus orientation did not interact with both “time” and “astigmatism group” variables (i.e., improvement was not dependent upon stimulus orientation).
3.1. Grating Acuity (Figure 1)
Figure 1.
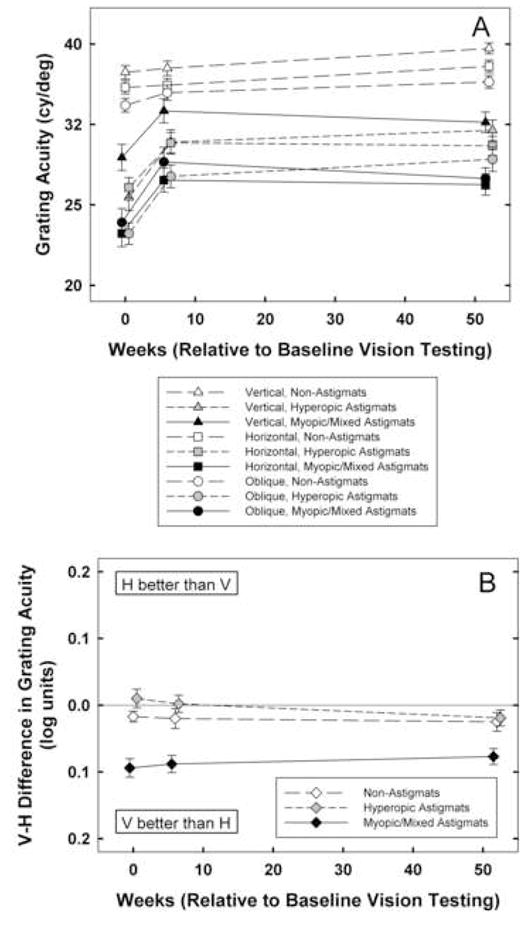
A: Mean best-corrected grating acuity at baseline, 6 weeks, and 1 year by astigmatism group (NonA, HA, M/MA) for vertical (triangle symbols), horizontal (square symbols), and oblique (circle symbols) stimuli. Y axis is scaled logarithmically, with 0.1 log unit between tick marks. B: Mean of V-H best-corrected grating acuity at baseline, 6 weeks, and 1 year for by astigmatism group (NonA, HA, M/MA). Bars indicate ± 1 sem.
3.1.1. Performance at Baseline
Analyses of grating acuity (V, H, O) measurements at baseline (n=537) yielded main effects of astigmatism group (F(2,531)=44.79 for V, F(2,531)=72.03 for H, and F(2,531)=57.19 for O, ps < 0.001) and age cohort (F1,531)=11.22, p < 0.002 for V, F(2,531)=5.91, p < 0.02 for H, F(2,531)=12.61, p < 0.001 for O), but no significant interactions between astigmatism group and age cohort. Post hoc analyses indicated that both HA and M/MA groups had significantly reduced acuity for V, H, and O stimuli compared to the NonA group (all ps < 0.001, after Bonferroni correction). Analyses of meridional amblyopia (V-H grating acuity) at baseline yielded a significant main effect of astigmatism group (F(1,531)=15.72, p < 0.001), but no effect of age cohort or interaction between astigmatism group and age cohort. Post hoc analyses indicated that the M/MA group had significant meridional amblyopia compared to the NonA group (p < 0.001), but the HA group did not show evidence of meridional amblyopia at baseline.
3.1.2. Treatment Effects
RM-ANOVA on grating acuity measurements at baseline, six weeks, and one year yielded significant main effects of time (F(2,530)=30.82, p < 0.001), astigmatism group (F(2,531)=86.78, p < 0.001), age cohort (F(1,531)=20.08, p < 0.001), and stimulus orientation (F(2,530)=74.17, p < 0.001). There were significant interactions between time and astigmatism group (F(4,1062)=5.79, p < 0.001) and between orientation and astigmatism group (F(4,1062)=14.83, p < 0.001). Post hoc analyses indicated that there was significantly greater improvement from baseline to six weeks in the HA and M/MA groups than in the NonA group (ps < 0.001). The astigmatism groups did not differ significantly with regard to the amount of change observed from six weeks to one year. RM-ANOVA on V-H grating acuity yielded a significant main effect of astigmatism group (F(2, 531)=29.84, p < 0.001), but no significant effects time, astigmatism group, or age cohort.
3.1.3. Performance at One Year
Analyses of grating acuity (V, H, O) measurements at one-year follow-up yielded main effects of astigmatism group (F(2,531)=37.07 for V, F(2,531)=54.90 for H, F(2,531)=35.84 for O, ps, < 0.001) and age cohort (F(1,531)=4.03, p < 0.05 for V, F(1,531)=7.61, p < 0.007 for H, F(1,531)=3.94, p < 0.05 for O), but no significant interactions between astigmatism group and age cohort. Post hoc analyses indicated that at one year, both HA and M/MA groups still had significantly reduced acuity for V, H, and O stimuli compared to the NonA group (all ps < 0.001, after Bonferroni correction). Analyses of meridional amblyopia (V-H grating acuity) at one-year follow-up yielded a significant main effect of astigmatism group (F(2,531)=7.33, p < 0.002), but not age cohort. Post hoc analyses indicated that the M/MA group still had significant meridional amblyopia compared to the NonA group after one year (p < 0.001).
3.2. Vernier Acuity (Figure 2)
Figure 2.
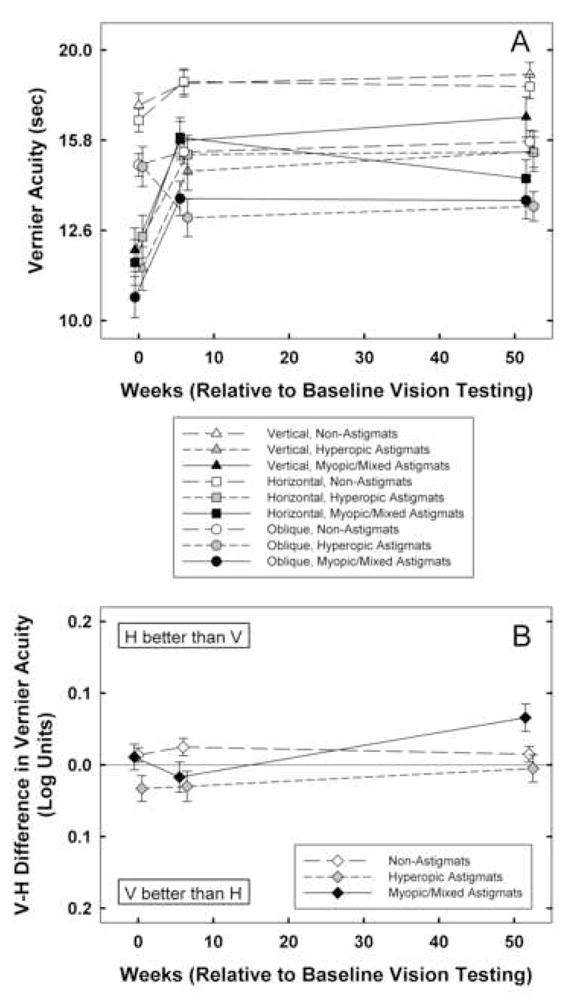
A: Mean best-corrected vernier acuity at baseline, 6 weeks, and 1 year by astigmatism group (NonA, HA, M/MA) for vertical (triangle symbols), horizontal (square symbols), and oblique (circle symbols) stimuli. Y axis is scaled logarithmically, with 0.1 log unit between tick marks. B: Mean of V-H best-corrected vernier acuity at baseline, 6 weeks, and 1 year for by astigmatism group (NonA, HA, M/MA) Bars indicate ± 1 sem.
3.2.1. Performance at Baseline
Analyses of vernier acuity (V, H, O) measurements at baseline (n=527) yielded main effects of astigmatism group (F(2,521)=26.32 for V, F(2,521)=20.30 for H, F(2,521)=23.17 for O, ps < 0.001) and main effects of age cohort (F(1,521)=33.92 for V, F(1,521)=23.46 for H, F(1,521)=18.07 for O, ps, < 0.001). There were no significant interactions between astigmatism group and age cohort. Post hoc analyses indicated that both the HA and the M/MA groups had significantly reduced acuity for V, H, and O stimuli at baseline compared to the NonA group (ps < 0.001, after Bonferroni correction). Analyses of meridional amblyopia (V-H vernier acuity) yielded no significant effects of astigmatism group or age cohort at baseline.
3.2.2. Treatment Effects
RM-ANOVA on vernier acuity measurements at baseline, six weeks, and one year (n=527) yielded significant main effects of time (F(2,520)=31.83, p < 0.001), astigmatism group (F(2,521)=20.93, p < 0.001), age cohort (F(1,521)=22.13, p < 0.001), and stimulus orientation (F(2,520)=73.19, p < 0.001). There were significant interactions between time and astigmatism group (F(4,1042)=5.17, p < 0.001), between time and age cohort (F(2,520)=5.38, p<0.006), and between orientation and astigmatism group (F(4,1042)=2.58, p < 0.04). Post hoc analyses indicated that there was significantly greater improvement in the HA and M/MA groups than in the NonA group from baseline to six weeks (p< 0.003 and p < 0.001, respectively). There were no differences among astigmatism groups with regard to the amount of change observed from six weeks to one year. RM-ANOVA on V-H vernier acuity yielded a significant main effect of time (F(2,520)= 3.05, p < 0.05) and astigmatism group (F(2, 521)=4.51, p < 0.02). The effect of age cohort was not significant, and no interactions were significant.
3.2.3. Performance at One Year
Analyses of vernier acuity (V, H, O) measurements at one year follow-up yielded main effects of astigmatism group (F(2,521)=5.74, p < 0.004, F(2,521)=9.67, p < 0.001for V, F(2,521)=5.80 for H, p < 0.004 for O) and main effects of age cohort for H stimuli (F(1,521)=5.87, p < 0.02). There were no significant interactions between astigmatism group and age cohort. Post hoc analyses indicated that after one year, the HA group still had reduced vernier acuity for V, H, and O stimuli (p < 0.004, p < 0.02, and p < 0.009, respectively) and the M/MA group still had significantly reduced acuity for H and O stimuli (p < 0.001 and p < 0.02, respectively), but acuity for V stimuli in M/MA group was no longer significantly reduced relative to the NonA group.
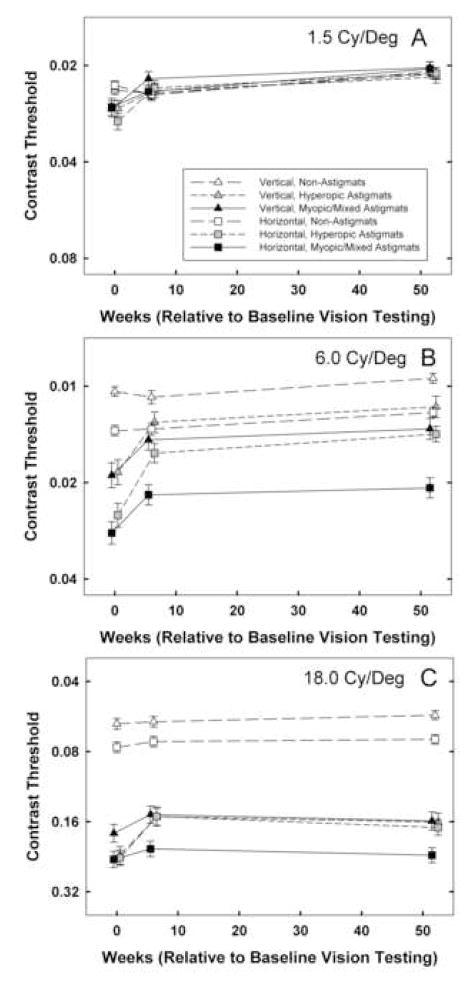
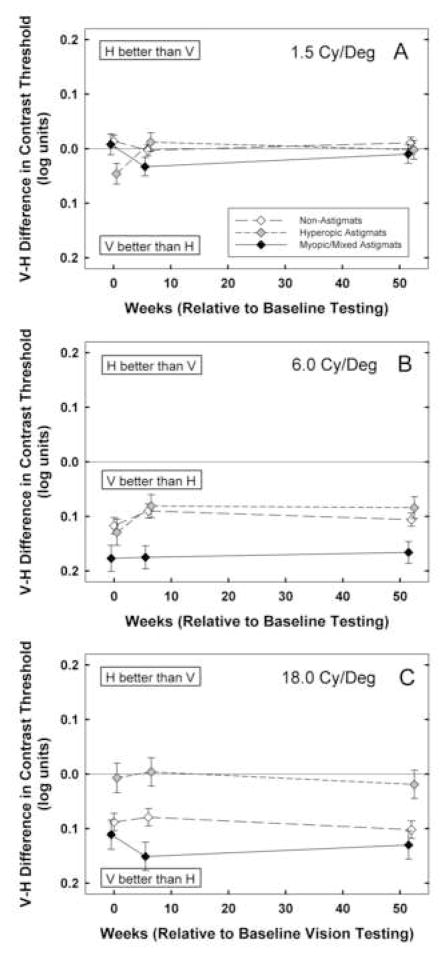
3.3. Contrast Sensitivity: Low Spatial Frequency Stimuli (Figure 3)
Figure 3.
Mean best-corrected contrast sensitivity at baseline, 6 weeks, and 1 year by astigmatism group (NonA, HA, M/MA) for vertical (triangle symbols) and horizontal (square symbols) stimuli for A: low spatial frequency (1.5 cy/deg) stimuli. Y axis is scaled logarithmically, with 0.3 log unit between tick marks. B: middle spatial frequency (6.0 cy/deg) stimuli, and C: high spatial frequency (18.0 cy/deg) stimuli. Bars indicate ± 1 sem.
3.3.1. Performance at Baseline
Analyses of 1.5 cy/deg contrast sensitivity measurements (V and H) at baseline (n=500) yielded main effects of astigmatism group only for H stimuli (F(2,494)=4.49, p < 0.02), significant main effects of age cohort for V and H stimuli (F(1,494)=52.56 for V, F(1,494)=36.69 for H, ps < 0.001), and no interactions between astigmatism group and age cohort. Post hoc analyses indicated that HA and M/MA groups had reduced contrast sensitivity only for H stimuli (p < 0.001 and p < 0.04, respectively) relative to the NonA group. Analyses of meridional amblyopia (V-H contrast sensitivity) at baseline yielded a main effect of astigmatism group (F(2,494)=3.40, p < 0.04). Post hoc analyses indicated that at baseline, only the HA group had significant meridional amblyopia compared to the NonA group (p < 0.04).
3.3.2. Treatment Effects
RM-ANOVA on low spatial frequency contrast sensitivity at baseline, six weeks, and one year (n=500) yielded significant main effects of time (F(2,493)=31.71, p < 0.001) and age cohort (F(1,494)=64.55, p < 0.001). There was a significant interaction 21 between time and astigmatism group (F(4,988)=3.11, p < 0.02). Post hoc analyses indicated that there was significantly greater improvement from baseline to 6 weeks in the HA and M/MA groups than in the NonA group (p < 0.008 and p < 0.02, respectively). There were no significant differences among astigmatism groups in amount of change observed from six weeks to one year. RM-ANOVA on V-H low spatial frequency contrast sensitivity yielded no significant effects of time, astigmatism group, or age cohort.
3.3.3. Performance at One Year
Analyses of 1.5 cy/deg contrast sensitivity measurements (V and H) at one-year follow-up yielded significant effects of age cohort (F(1,494)=45.78 for V, F(1,494)=30.00 for H, ps, < 0.001), but no significant effects of astigmatism group and no significant interactions between astigmatism group and age cohort. Analyses of meridional amblyopia (V-H contrast sensitivity) at one-year follow-up yielded no significant main effects, but there was a significant interaction between astigmatism group and age cohort (F(2,494)=4.13, p<0.02). When the YC and OC data were analyzed separately, there was no evidence of significant meridional amblyopia at baseline or one year for either the HA or M/MA groups, relative to the NonA group. The significant interaction between astigmatism group and age cohort reflected the fact that in the YC, there was a significant difference between the HA group and the M/MA group on V-H contrast sensitivity.
3.4. Contrast Sensitivity: Middle Spatial Frequency Stimuli (Figure 4)
Figure 4.
Mean of V-H best-corrected contrast sensitivity at baseline, 6 weeks, and 1 year by astigmatism group (NonA, HA, M/MA) for A: low spatial frequency (1.5 cy/deg) stimuli, B: middle spatial frequency (6.0 cy/deg) stimuli, and C: high spatial frequency (18.0 cy/deg) stimuli. Bars indicate ± 1 sem.
3.4.1. Performance at Baseline
Analyses of 6.0 cy/deg contrast sensitivity measurements (V and H) at baseline (n=501) yielded significant main effects of astigmatism group (F(2,495)=35.30 for V, F(2,495)=47.26 for H, ps < 0.001), and significant main effects of age cohort ( F(1,495)= 13.73, p < 0.001 for V, F(1,495)=11.81p < 0.002 for H). In addition, there was a significant interaction between astigmatism group and age cohort for V contrast sensitivity (F(2,495)=3.38, p < 0.04). Post hoc analyses indicated that both HA and M/MA groups had reduced contrast sensitivity for V and H stimuli compared to the NonA group (ps < 0.001). The interaction between astigmatism group and age cohort for V stimuli reflected the fact that the NonA OC had significantly better contrast sensitivity than the YC, but there was no difference between OC and YC for the HA and M/MA groups. Analyses of meridional amblyopia (V-H contrast sensitivity) at baseline yielded no significant effects.
3.4.2. Treatment Effects
RM-ANOVA on middle spatial frequency contrast sensitivity measurements at baseline, six weeks, and one year (n=501) yielded significant main effects of time (F(2,494)=41.52, p < 0.001), astigmatism group (F(2,495)=40.04, p < 0.001), age cohort (F(1,495)=16.89, p < 0.001), and orientation (F(1,495)=251.84, p < 0.001). There were significant interactions between time and astigmatism group (F(4,990)=7.11, p < 0.001), orientation and astigmatism group (F(2,495) = 8.73, p < 0.001), among orientation, astigmatism group, and age cohort (F(2,495)=3.27, p < 0.04), and among time, orientation, astigmatism group and age cohort (F(4,990)=3.90, p<0.005). For the YC, there were no significant differences between astigmatism groups with regard to the amount of change observed from baseline to six weeks or from six weeks to one year.
For the OC, there was significantly greater change observed from baseline to six weeks in the HA and the M/MA groups than in the NonA groups for both V (p< 0.009 and p < 0.02, respectively) and H (p < 0.001 and p < 0.002, respectively) stimuli. RM-ANOVA on V-H middle spatial frequency contrast sensitivity yielded a significant main effect of astigmatism group (F(2, 495)=8.73, p < 0.001), and significant interactions between astigmatism group and age cohort (F(2,495)=3.27, p < 0.04), and between time, astigmatism group, and age cohort (F(4,990)=4.07, p < 0.004). The interaction between time, astigmatism group and age cohort reflects the fact that when analyzed separately by age cohort, there was a significant interaction between time and astigmatism group for the YC but not the OC. For the YC, although mean contrast sensitivity was always better for V than for H stimuli, the V-H difference increased from 6 weeks to 1 year for the NonA group, and decreased for the HA and the M/MA groups (although only the NonA vs. M/MA comparison was significant (p < 0.03)).
3.4.3. Performance at One Year
Analyses of 6.0 cy/deg contrast sensitivity measurements (V and H) at one-year follow-up yielded significant main effects of astigmatism group ( F(2,495)=15.04 for V, F(2,495)=24.55 for H, ps < 0.001), and a significant main effect of age cohort for V stimuli (F(1,495)=10.01, p < 0.003). Post hoc analyses indicated that after one year, the HA group had significantly reduced contrast sensitivity only for V stimuli (p < 0.03), and the M/MA group had significantly reduced contrast sensitivity for both V and H stimuli (ps < 0.001).
3.5. Contrast Sensitivity: High Spatial Frequency Stimuli (Figure 5)
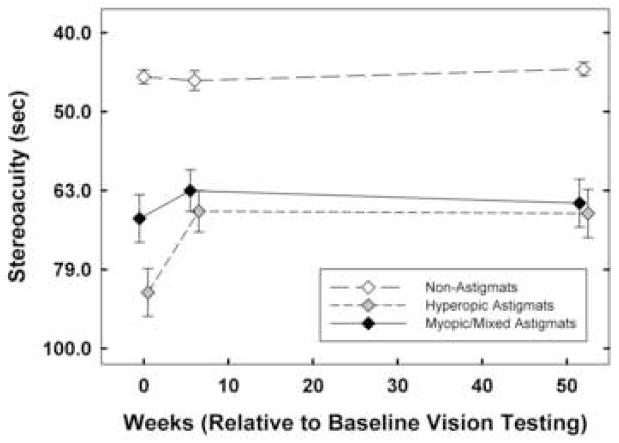
Figure 5.
Mean best-corrected stereoacuity at baseline, 6 weeks, and 1 year by astigmatism group (NonA, HA, M/MA). Y axis is scaled logarithmically, with 0.1 log unit between tick marks. Bars indicate ± 1 sem.
3.5.1. Performance at Baseline
Analyses of 18.0 cy/deg contrast sensitivity measurements (V and H) at baseline (n=501) yielded significant main effects of astigmatism group ( F(2,495)=93.88 for V, F(2,495)=85.95 for H, ps < 0.001), and significant main effects of age cohort ( F(1,495)=11.44, p < 0.002 for V and F(1,495)=7.36, p < 0.007 for H). In addition, there was a significant interaction between astigmatism group and age cohort for V stimuli (F(2,495)=3.63, p < 0.03, reflecting significantly better contrast sensitivity in the OC than the YC only in the NonA group). Post hoc analyses indicated that both HA and M/MA groups had reduced contrast sensitivity for V and H stimuli compared to the NonA group (ps < 0.001). Analyses of meridional amblyopia (V-H contrast sensitivity) yielded a significant main effect of astigmatism group at baseline (F(2,495)=4.01, p < 0.02), and no effects of age cohort and no interaction. Post hoc analyses indicated that the HA group showed evidence of meridional amblyopia (p < 0.02), but the M/MA group did not.
3.5.2. Treatment Effects
RM-ANOVA on high spatial frequency contrast sensitivity measurements at baseline, six weeks, and one year (n=501) yielded significant main effects of time (F(2,494)=12.91, p < 0.001), astigmatism group (F(2,495)=136.30, p < 0.001), age cohort (F(1,495)=11.80, p<0.002), and orientation (F(1,495)=60.47, p < 0.001). There were significant interactions between time and astigmatism group (F(4,990)=3.15, p < 0.02), and orientation and astigmatism group (F(2,495) = 10.81, p < 0.001). Post hoc analyses indicated that only the HA group showed significantly greater improvement than the NonA group from baseline to six weeks (p < 0.001). The astigmatism groups did not differ significantly with regard to the amount of change observed from six weeks to one year. RM-ANOVA on V-H high spatial frequency contrast sensitivity yielded a significant main effect of astigmatism group (F(2, 495)=10.81, p < 0.001). No other effects or interactions among time, astigmatism group and age cohort were significant.
3.5.3. Performance at One Year
Analyses of 18.0 cy/deg contrast sensitivity measurements (V and H) at one-year follow-up yielded significant main effects of astigmatism group (F(2,495)=97.43 for V, F(2,495)=92.45 for H, ps < 0.001), significant main effects of age cohort (F(1,495)= 11.14, p < 0.002 for V, F(1,495)=6.17, p < 0.02 for H), and no significant interactions. Post hoc analyses indicated that at one year, both HA and M/MA groups still had reduced contrast sensitivity for V and H stimuli compared to the NonA group (ps < 0.001). Analyses of meridional amblyopia (V-H contrast sensitivity) indicated that there was still a significant main effects of astigmatism group (F(2,495)=4.61, p < 0.02), with no significant effect of age cohort or interaction. Post hoc analyses indicated that the HA group still showed evidence of meridional amblyopia (p < 0.04).
3.6. Stereoacuity (Figure 6)
3.6.1. Performance at Baseline
Analyses of stereoacuity measurements at baseline (n=537) yielded a significant main effect of astigmatism group (F(2,531)=69.50, p < 0.001) and age cohort (F(1,531)=9.17, p < 0.004), and a significant interaction between astigmatism group and age cohort (F(2,531)=11.57, p < 0.001, reflecting no significant difference between age cohorts for the NonA and HA groups, but significantly better stereoacuity for the YC than in the OC M/MA group). Post hoc analyses indicated that for the YC and for the OC, stereoacuity was significantly reduced at baseline (for the YC, ps < 0.001 for the HA and M/MA groups; for the OC, ps < 0.001 for the HA group and p < 0.005 for the M/MA group).
3.6.2. Treatment Effects
RM-ANOVA on stereoacuity measurements at baseline, six weeks, and one year yielded significant main effects of time (F(2,530)=10.71, p < 0.001), astigmatism group (F(2,531)=71.14, p < 0.001), and age cohort (F(1,531)=5.05, p < 0.03). There were significant interactions between astigmatism group and age cohort (F(2,531)=13.87, p < 0.001), between time and astigmatism group (F(4,1062)=4.60, p<0.002), and time and age cohort (F(2,530)=3.35, p < 0.04). Post hoc analyses indicated that only the HA group showed significant improvement compared to the in the NonA group from baseline to six weeks (p < 0.001). None of the astigmatism groups differed significantly with regard to amount of change observed from six weeks to one year.
3.6.3. Performance at One Year
Analyses of stereoacuity measurements at one-year follow-up yielded a significant main effect of astigmatism group (F(2,531)=45.62, p < 0.001), no significant effect of age cohort, and a significant interaction between astigmatism group and age cohort (F(2,531)=6.76, p < 0.002, reflecting no age cohort difference in the NonA group or M/MA group, and significantly better stereoacuity in the OC than the YC HA group). Post hoc analyses indicated that for both the YC and for the OC, stereoacuity was still significantly reduced for the HA and M/MA groups, relative to the NonA group (ps < 0.001).
4. Discussion
In a previous report, we provided evidence that (a) optical correction of astigmatism results in significant improvement in best-corrected recognition acuity in astigmatic children four to 13 years of age, relative to an age-matched control group of non-astigmatic children, (b) most of the improvement was observed within the first six weeks of treatment, and (c) improvement in best-corrected recognition acuity in four- to 13-year-old astigmatic children was not related to age, suggesting that the sensitive period for treatment of astigmatism-related amblyopia with eyeglass correction alone extends beyond age seven years (Harvey et al., 2007a). In the present report, we extend our previous findings to indicate that optical treatment of astigmatism-related amblyopia also results in significant improvement in other aspects of vision: grating acuity, vernier acuity, contrast sensitivity, and stereoacuity. Furthermore, as in the results for recognition acuity (Harvey et al., 2007a), treatment effects occurred early in treatment, and there was no evidence that improvement was limited to a sensitive period that ended prior to age 7 years. These findings, which indicate that the introduction of clear visual input (through eyeglass correction of astigmatic refractive error) is sufficient to result in an increase in best-corrected visual performance in children with astigmatism-related amblyopia, are consistent with the results of recent studies of patients with anisometropic, strabismic, and isoametropic amblyopia that have also demonstrated a significant treatment response as a result of optical correction alone (Moseley, Neufeld, McCarry, Charnock, McNamara, Rice, & Fielder, 2002; Stewart, Moseley, Fielder, Stephens, & the MOTAS Cooperative, 2004; PEDIG, 2005b; PEDIG, 2006; Chen, Chen, Tai, Fu, Chang,& Lu, 2007; Cotter, Edwards, Arnold, Astle, Barnhardt, Beck, Birch, Donahue, Everett, Felius, Holmes, Kraker, Melia, Repka, Wallace, Weise, & the Pediatric Eye Disease Investigator Group, 2007; Wallace, Chandler, Beck, Arnold, Bacal, Birch, Felius, Frazier, Holmes, Hoover, Klimek, Lorenzana, Quinn, Repka, Suh, & Tamkins, on behalf of the Pediatric Eye Disease Investigator Group, 2007).
4.1. Patterns of Treatment Effects
4.1.1. Grating Acuity and Vernier Acuity
Similar patterns of treatment effects were obtained for measurements of best-corrected grating acuity and vernier acuity. At baseline, both the HA and the M/MA groups had significantly reduced grating acuity and vernier acuity for V, H, and O stimuli. There was significantly greater improvement from baseline to six weeks in the HA and M/MA groups than in the NonA group, but no difference among astigmatism groups in the amount of improvement between six weeks and one year, indicating that there was a significant treatment effect that was concentrated in the first six weeks of treatment. Comparison of results from children four to seven years of age with results from children from eight to 13 years of age showed no difference in treatment effect, thus providing no evidence to support the idea that children less than eight years of age may be more amenable to treatment (Mitchell et al., 1973; Cobb & McDonald, 1978).
4.1.2. Contrast Sensitivity for Low (1.5 cy/deg), Middle (6.0 cy/deg), and High (18.0 cy/deg) Spatial Frequency Stimuli
There was little evidence of significantly reduced contrast sensitivity for low spatial frequency stimuli in the astigmatic groups at baseline (the only significant reduction was in the sensitivity of the HA group for H stimuli). Despite this, there was 29 evidence of a significant treatment effect in the HA and M/MA groups, i.e., greater improvement in sensitivity, relative to the NonA group, from baseline to six weeks. The presence of a significant treatment effect, despite little evidence of significantly reduced contrast sensitivity at baseline, is due to the fact that contrast sensitivity tended to be poorer in the astigmatism groups than in the NonA group at baseline, and from baseline to six weeks, there was a slight reduction in contrast sensitivity for the NonA group and an improvement in contrast sensitivity for the astigmatism groups (see Figure 3).
For middle and high spatial frequency stimuli, the astigmatism groups had significantly reduced contrast sensitivity for V and H stimuli at baseline, and there was evidence of significant treatment effects from baseline to six weeks for both middle and high spatial frequency stimuli. However, treatment effects for middle spatial frequency stimuli differed by age cohort: there was a significant effect of treatment in the OC, but not in the YC. This finding is contrary to the prediction, based on studies suggesting no treatment effect of optical correction of astigmatic amblyopia after age seven years (Mitchell et al., 1973; Cobb & MacDonald, 1978), that there would be greater treatment effects in younger (< 8 years), rather than older (≥ 8 years) children. For high spatial frequency stimuli, there was a significant treatment effect in the HA group but not the M/MA group.
In summary, the results of contrast sensitivity outcome measures indicate that optical correction had a significant treatment effect on contrast sensitivity for low, middle, and high spatial frequency stimuli. Thus, the benefits of optical correction of astigmatism extend beyond the improvements in processing of high spatial frequency stimuli.
4.1.3. Stereoacuity
Both the HA and the M/MA groups had significantly reduced stereoacuity at baseline, relative to the NonA group. There was a significant treatment effect from baseline to six weeks in the HA group, but not the M/MA group, perhaps because initial baseline deficits were greater in the HA group. These findings of significant improvement in stereoacuity are consistent with the findings in a study of three- to nine-year-old children with high bilateral hyperopia and/or astigmatism, most of whom were treated with optical correction alone, that found significant improvement in stereoacuity measured with the Randot Preschool Stereoacuity test over a treatment duration of one year (Wallace et al., 2007).
4.1.4 Meridional Amblyopia
At baseline, significant meridional amblyopia was present only in measurements of grating acuity for the M/MA group and in measurements of low and high spatial frequency contrast sensitivity for the HA group. Results indicated that there was no significant treatment effect, i.e., no significant reduction in these meridional amblyopia effects over time compared to the NonA group. At one year, while there was still significant meridional amblyopia in measurements of grating acuity for the M/MA and high spatial frequency contrast sensitivity for the HA group, meridional amblyopia in measurements of low spatial frequency contrast sensitivity for the HA was no longer significant, most likely related to the fact that these effects were significant, but weak, at baseline (p < 0.04).
Our failure to find a significant reduction in meridional amblyopia for grating acuity in the M/MA group and meridional amblyopia for high spatial frequency contrast sensitivity for the HA group after a treatment duration of one year is consistent with the results of a previous study of optical treatment of astigmatism-related amblyopia in preschool Tohono O’odham children, which showed no significant reduction in meridional amblyopia for grating acuity after four months of optical correction in children in the M/MA group (Harvey et al., 2004). However, our data are not consistent with previous retrospective studies that found evidence of meridional amblyopia in astigmatic adults who received eyeglasses after age seven years but not in those who received glasses prior to age seven years (Mitchell et al., 1973; Cobb & MacDonald, 1978). The apparent discrepancy between the results of the retrospective studies of adults and the results of the previous (Harvey et al., 2004) and present studies of Tohono O’odham children could be explained if elimination of meridional amblyopia occurs only following treatment duration longer than one year (the longest treatment duration provided in the two studies of children).
4.2. Timing of Treatment Effects and Performance at One Year: Implications for Effective Treatment Duration
In general, across measures, treatment effects occurred primarily early in treatment (within the first six weeks). Specifically, for all measures, significant treatment effects occurred from baseline to six weeks, but not between six weeks and one year. These results are consistent with recognition acuity outcome in this sample (Harvey et al., 2007a). They are also consistent with results of studies of children with anisometropic, strabismic, or bilateral refractive amblyopia, which show that most improvement resulting from optical correction occurs within the first few weeks after initiation of optical treatment (Stewart et al., 2004; PEDIG, 2006; Wallace et al., 2007; Chen et al., 2007). A study of 65 newly-diagnosed anisometropic and/or strabismic amblyopes, with a mean age of five years, found an average improvement in acuity in amblyopic eyes of 1.1 logMAR lines following six weeks of optical correction, which doubled to 2.2 logMAR lines by 12 weeks, with little improvement (0.02 logMAR line) between the 12-week and the final 18-week test session (Stewart et al., 2004). In a study of 60 children with previously untreated anisometropic amblyopia who were compliant with optical treatment, Chen et al. (2007) found that most of the improvement with optical correction occurred within the first 12 weeks of treatment. Mean improvement from baseline to acuity stabilization (defined as 6 weeks with ≤ 0.1 logMAR improvement) was 3.8 logMAR lines, with 2.5 logMAR lines improvement observed from baseline to 12 weeks. Two other studies, one that included 84 newly-diagnosed anisometropic amblyopes (PEDIG, 2006) and one that included 113 bilateral refractive (hyperopic and/or astigmatic) amblyopes (Wallace et al., 2007), with a mean age of five years in both studies, showed that much of the improvement observed occurred within the first 5 weeks of treatment. Wallace et al. (2007) reported a mean improvement of 2.4 logMAR lines over the first 5 weeks, and 1.6 logMAR lines from 5 weeks to 1 year. In the PEDIG (2006) study, children were treated with optical correction only until they no longer showed improvement over a 5 week follow-up interval, or until their acuity in the amblyopic eye was no longer worse than the fellow eye, at which time they were randomized to either a control group (optical treatment alone) or to a group prescribed patching along with optical treatment. The results showed that much of the observed improvement occurred early in treatment: there was an average of 1.8 logMAR lines improvement in the first 5 weeks of optical treatment, and a maximum improvement averaging 2.9 logMAR lines (with the longest duration of improvement of 30 weeks in one patient). Although much of the reduction in amblyopia occurred within the first few weeks, children who were randomized to the control group and continued to receive optical treatment alone showed an additional reduction in amblyopia (average of 1.2 logMAR lines). Overall, these studies suggest that much of the reduction in amblyopia due to optical correction alone occurs within the first 12 weeks of optical treatment. However, in contrast to the results of the present study, these previous studies also showed evidence of additional improvement beyond this early treatment period.
Despite significant effects of treatment, analyses of visual functions after one year of eyeglass treatment indicated that, with just a few exceptions (low spatial frequency contrast sensitivity (which was reduced only for H stimuli in the HA group at baseline), vertical vernier acuity for the M/MA group, and 6.0 cy/deg contrast sensitivity for the HA group), the astigmatic groups still did not reach normal levels, i.e., levels of visual function seen in the NonA control group, even after one year of optical treatment. Furthermore, after one year, there was little evidence of elimination of the meridional amblyopia that was present at baseline. The failure of astigmatic subjects to reach normal levels of visual function with optical correction, even after one year of treatment, is consistent with findings for one-year recognition acuity outcome in this sample (Harvey et al., 2007a). Similarly, previous results from other groups indicate that 55–80% of children fail to achieve resolution of anisometropic, strabismic, or bilateral refractive amblyopia with eyeglass correction alone (Stewart et al., 2004; PEDIG, 2006; Cotter et al., 2007, Chen et al., 2007). In contrast, results from a study of three- to nine-year-old children with bilateral refractive error, most of whom were treated with optical correction alone, found mean monocular best-corrected acuity improved from 20/80 at baseline to 20/32 after one year of treatment (Wallace et al., 2007). However, this study did not include a normal age-matched comparison group, so while it is clear that there were significant improvements with treatment, it is not clear if acuity, on average, reached “normal” levels after one year of treatment.
It is not clear if persistent below-normal acuity in the astigmatic children is the result of limited plasticity in this age range or less-than-optimal treatment compliance. However, secondary analyses of recognition acuity data for compliant astigmatic subjects did not provide evidence of greater treatment effects in compliant subjects, nor did compliant subjects reach, on average, the acuity level of the NonA group after one year of treatment (Harvey et al., 2007a). As suggested previously (Harvey et al, 2007a), it is possible that other factors, such as uncorrected spherical aberration or coma, may in part be responsible for persistent below-normal acuity in the astigmatic children. A study currently in progress will allow us to determine if spherical aberration and coma are more prevalent among astigmatic than non-astigmatic Tohono O’odham children, and allow us to determine if spherical aberration and coma contribute to poor best-corrected vision in this population.
4.4. Effects of Age Cohort: Implications Regarding Plasticity
The results of the present study indicated that, although the older cohort tended to have significantly better visual performance than the younger cohort on almost all measures, treatment effects did not differ by age cohort. Furthermore, we did not find differences in treatment effectiveness across age cohort despite the fact that, on average, the older cohort, in which we expected to see reduced treatment effect relative to the younger cohort, had a shorter “six week” treatment interval in than the younger cohort (40 days vs. 50 days). These findings contradict evidence in the literature that suggests that there is a sensitive period for successful treatment of astigmatism-related amblyopia that ends by age seven years (Mitchell et al., 1973; Cobb & MacDonald, 1978; Gwiazda et al.,1986) or earlier (Harvey et al., 2004). However, the finding of treatment effects in the older cohort in the present study and in a previous report on recognition acuity in this sample (Harvey et al., 2007a) is consistent with the results of a study that reported that eyeglass correction alone in anisometropic and/or strabismic amblyopes resulted in significant improvement in 25% of 203 seven- to twelve-year-old children and 23% of 48 thirteen- to seventeen-year-old children (PEDIG, 2005b).
Overall, the available studies that have addressed the issue of a sensitive period in treatment of astigmatism-related amblyopia do not provide a clear indication of the timing of the end of the sensitive period for successful treatment, or allow us to determine if in fact there is a sensitive period for successful treatment. It is possible that the unexpected pattern of results across our studies (i.e., significant effects of optical treatment in school-age children (Harvey et al., 2007a) but not preschool children (Harvey et al., 2004), who were believed to be within the sensitive period for successful treatment) may reflect a complex interaction of factors. For example, treatment of astigmatism-related amblyopia may be influenced by the visual experiences of the child and the types of tasks that the child engages in. A recent pilot study of three- to six-year-old children with anisometropic and/or strabismic amblyopia, conducted by the PEDIG group (2005a), reported a suggestion of greater improvement in best-corrected visual acuity in children assigned to perform near activities during patching compared to children assigned to non-near activities during patching. Furthermore, several recent studies have shown that practice on fine perceptual tasks (“perceptual learning”) can result in improvement in recognition visual acuity and contrast sensitivity, as well as in perceptual task performance, in amblyopic adults (Levi and Polat, 1996; Levi, Polat, & Hu, 1997, Polat, Ma-Naim, Belkin, & Sagi, 2004; Li and Levi, 2004), as well as in amblyopic children (Li, Young, Hoenig, & Levi, 2005). Based on these results, Levi (2005) has suggested that perceptual learning may be responsible for some of the improvement seen in the clinical treatment of amblyopia. It is possible that the lack of effectiveness of eyeglass treatment in reducing astigmatism-related amblyopia in three-to fiv-year-old children (Harvey et al., 2004) may have been related to the types of visual tasks that young children typically perform, i.e., tasks that do not require fine perceptual discrimination, while treatment effects may have been enhanced in older children, who spend more time reading and doing fine perceptual tasks.
4.5. Summary and Conclusions
Previous research has shown that astigmatism in childhood is associated with a broad range of visual deficits, including recognition acuity, grating acuity, vernier acuity, contrast sensitivity and stereoacuity (Freeman et al., 1972; Mitchell et al, 1973; Mitchell & Wilkinson, 1974; Freeman, 1975a,b; Freeman & Thibos, 1975; Cobb & MacDonald, 1978; Mohindra et al., 1983; Gwiazda et al, 1984, 1986; Kershner & Brick, 1984; Atkinson et al., 1996; Dobson et al., 1996; Harvey 2002; Dobson et al., 2003; Harvey et al., 2007b). In a previous report, we observed significant improvements in best-corrected recognition acuity over time with optical correction of astigmatism (Harvey et al., 2007a). In the present report, we extend those findings, and report significant improvement in best-corrected grating acuity, vernier acuity, contrast sensitivity, and stereoacuity over time as a result of optical correction. In addition, the present report provides further evidence that treatment effects can persist beyond what was previously hypothesized to be the end of the sensitive period for successful treatment of astigmatism-related amblyopia.
While it is clear that optical treatment provides a significant benefit, this research also demonstrates that there may be limits to plasticity related to optical correction of astigmatism-related amblyopia in this age range: after one year of treatment, subjects did not attain normal levels of visual function. Future research might focus on use of near activities or perceptual learning as a means of further improving visual function in astigmatic children who have been unable to achieve normal levels of visual function as a result of optical correction alone. Perceptual learning may prove to be an ideal method for targeting the deficits specific to astigmatism-related amblyopia, i.e., meridional amblyopia, as perceptual learning tasks could be tailored to target the child’s specific deficits, e.g., resolution acuity for H stimuli in M/MA astigmats.
Acknowledgments
The authors thank the National Institutes of Health National Eye Institute’s Data Monitoring and Oversight Committee for their guidance and comments (Maureen Maguire, Ph.D. (former chair), Robert Hardy, Ph.D. (current chair), Donald Everett, M.A., Jonathan Holmes, M.D., Cynthia Norris, and Karla Zadnik, Ph.D., O.D.). The authors thank the following for their help and support in conducting this study: The Tohono O’odham Nation, The Indian Oasis/Baboquivari School District, the Bureau of Indian Affairs Office of Indian Education Programs (BIA OIEP, Papago/Pima Agency), the San Xavier Mission School, and the parents and children who participated in the study.
This work was supported by NIH/NEI (Grants EY11155 (JMM) and EY13153 (EMH)) and Research to Prevent Blindness (unrestricted grant to the Department of Ophthalmology and Vision Science (JMM), Career Development Award (EMH), Walt and Lilly Disney Award for Amblyopia Research (JMM)).
This research was presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting, May 2007, Ft. Lauderdale, Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkinson J, Braddick O, Bobier B, Anker S, Ehrlich D, King J, Watson P, Moore A. Two infant vision screening programmes: Prediction and prevention of strabismus and amblyopia from photo- and videorefractive screening. Eye. 1996;10:189–198. doi: 10.1038/eye.1996.46. [DOI] [PubMed] [Google Scholar]
- Birch E, Williams C, Hunter J, Lapa MC the ALSPAC “Children in Focus” Study Team. Random dot stereoacuity of preschool children. Journal of Pediatric Ophthalmology & Strabismus. 1997;34:217–222. doi: 10.3928/0191-3913-19970701-08. [DOI] [PubMed] [Google Scholar]
- Chen PL, Chen JT, Tai MC, Fu JJ, Chang CC, Lu DW. Anisometropic amblyopia treated with spectacle correction alone: Possible factors predicting success and time to start patching. American Journal of Ophthalmology. 2007;143:54–60. doi: 10.1016/j.ajo.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Cobb SR, MacDonald CF. Resolution acuity in astigmats: Evidence for a critical period in the human visual system. British Journal of Physiological Optics. 1978;32:38–49. [PubMed] [Google Scholar]
- Cotter SA, Edwards AR, Arnold RW, Astle WF, Barnhardt CN, Beck RW, Birch EE, Donahue SP, Everett DF, Felius J, Holmes JM, Kraker RT, Melia BM, Repka MX, Wallace DK, Weise KK the Pediatric Eye Disease Investigator Group. Treatment of strabismic amblyopia with refractive correction. American Journal of Ophthalmology. 2007;143:1060–1063. doi: 10.1016/j.ajo.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson V, Miller JM, Harvey EM. Corneal and refractive astigmatism in a sample of 3- to 5-year-old children with a high prevalence of astigmatism. Optometry and Vision Science. 1999a;76:855–860. doi: 10.1097/00006324-199912000-00022. [DOI] [PubMed] [Google Scholar]
- Dobson V, Miller JM, Harvey EM, Mohan KM. Amblyopia in astigmatic preschool children. Vision Research. 2003;43:1081–1090. doi: 10.1016/s0042-6989(03)00014-2. [DOI] [PubMed] [Google Scholar]
- Dobson V, Miller JM, Harvey EM, Sherrill DL. In Vision science and its applications. Washington, D.C.: Optical Society of America; 1999b. Prevalence of astigmatism, astigmatic anisometropia, and glasses wearing among preschool-and school-age Native American children; pp. 177–180. [Google Scholar]
- Dobson V, Tyszko RM, Miller JM, Harvey EM. Vision science and its applications. Washington, D.C.: Optical Society of America; 1996. Astigmatism, amblyopia, and visual disability among a Native American population; pp. 139–142. [Google Scholar]
- Ferris FL, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. American Journal of Ophthalmology. 1982;94:91–96. [PubMed] [Google Scholar]
- Freeman RD. Asymmetries in human accommodation and visual experience. Vision Research. 1975a;15:483–492. doi: 10.1016/0042-6989(75)90025-5. [DOI] [PubMed] [Google Scholar]
- Freeman RD. Contrast sensitivity in meridional amblyopia. Investigative Ophthalmology. 1975b;14:78–81. [PubMed] [Google Scholar]
- Freeman RD, Mitchell DE, Millodot M. A neural effect of partial visual deprivation in humans. Science. 1972;175:1384–1386. doi: 10.1126/science.175.4028.1384. [DOI] [PubMed] [Google Scholar]
- Freeman RD, Thibos LN. Contrast sensitivity in humans with abnormal visual experience. Journal of Physiology. 1975;247:687–710. doi: 10.1113/jphysiol.1975.sp010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton DL, Miller JM, West CE. Optical pearls and pitfalls: Tricks and traps in strabismus. In: Wright KW, Speigel PH, editors. Pediatric ophthalmology and strabismus. New York: Springer; 2003. pp. 292–296. [Google Scholar]
- Gwiazda J, Bauer J, Thorn F, Held R. Meridional amblyopia does result from astigmatism in early childhood. Clinical Vision Science. 1986;1:145–152. [Google Scholar]
- Gwiazda J, Scheiman M, Held R. Anisotropic resolution in children’s vision. Vision Research. 1984;24:527–531. doi: 10.1016/0042-6989(84)90106-8. [DOI] [PubMed] [Google Scholar]
- Harris WF. The mean and variance of samples of dioptric powers: The basic calculations. Clinical and Experimental Optometry. 1990;73:89–92. [Google Scholar]
- Harvey EM. Visual development and plasticity in children. Dissertation Abstracts International. 2002;63(12B):6115. [Google Scholar]
- Harvey EM, Dobson V, Clifford-Donaldson CE, Miller JM. Optical treatment of amblyopia in astigmatic children: The sensitive period for successful treatment. Ophthalmology. 2007a;114:2293–2301. doi: 10.1016/j.ophtha.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Harvey EM, Dobson V, Miller JM. Prevalence of high astigmatism, eyeglass wear, and poor visual acuity among Native American grade-school children. Optometry and Vision Science. 2006;83:206–212. doi: 10.1097/01.opx.0000214333.84822.71. [DOI] [PubMed] [Google Scholar]
- Harvey EM, Dobson V, Miller JM, Clifford-Donaldson CE. Amblyopia in astigmatic children: Patterns of deficits. Vision Research. 2007b;47:315–326. doi: 10.1016/j.visres.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EM, Dobson V, Miller JM, Sherrill DL. Treatment of astigmatism-related amblyopia in 3- to 5-year-old children. Vision Research. 2004;44:1623–1634. doi: 10.1016/j.visres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Harvey EM, Miller JM, Dobson V, Tyszko R, Davis AL. Measurement of refractive error in Native American preschoolers: Validity and reproducibility of autorefraction. Optometry and Vision Science. 2000;77:140–149. doi: 10.1097/00006324-200003000-00013. [DOI] [PubMed] [Google Scholar]
- Kershner RM, Brick DC. Prevalence of high corneal astigmatism in Papago school children. Investigative Ophthalmology and Visual Science. 1984;25(Suppl):217. (Abstract) [Google Scholar]
- Levi DM. Perceptual learning in adults with amblyopia: a reevaluation of critical periods in human vision. Developmental Psychobiology. 2005;46:222–232. doi: 10.1002/dev.20050. [DOI] [PubMed] [Google Scholar]
- Levi DM, Klein S. Hyperacuity and amblyopia. Nature. 1982;298:268–270. doi: 10.1038/298268a0. [DOI] [PubMed] [Google Scholar]
- Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Polat U, Hu YS. Improvement in vernier acuity in adults with amblyopia: practice makes better. Investigative Ophthalmology and Visual Science. 1997;38:1493–1510. [PubMed] [Google Scholar]
- Li RW, Levi DM. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. Journal of Vision. 2004;4:476–487. doi: 10.1167/4.6.7. [DOI] [PubMed] [Google Scholar]
- Li RW, Young KG, Hoenig P, Levi DM. Perceptual learning improves visual performance in juvenile amblyopia. Investigative Ophthalmology and Visual Science. 2005;46:3161–168. doi: 10.1167/iovs.05-0286. [DOI] [PubMed] [Google Scholar]
- Long WF. A matrix formalism for decentration problems. American Journal of Optometry and Physiological Optics. 1976;53:27–33. doi: 10.1097/00006324-197601000-00005. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. Journal of Vision. 2003;3:380–406. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- Miller JM, Dobson V, Harvey EM, Sherrill DL. Astigmatism and Amblyopia Among Native American Children (AANAC): Design and methods. Ophthalmic Epidemiology. 2000;7:187–207. [PubMed] [Google Scholar]
- Miller JM, Dobson V, Harvey EM, Sherrill DL. Comparison of preschool vision screening methods in a population with a high prevalence of astigmatism. Investigative Ophthalmology and Visual Science. 2001;42:917–924. [PubMed] [Google Scholar]
- Miller JM, Harvey EM, Dobson V. A practical method for testing vernier acuity in children. Investigative Ophthalmology and Visual Science. 2002;43 E-Abstract 2665. [Google Scholar]
- Mitchell DE, Freeman RD, Millodot M, Haegerstrom G. Meridional amblyopia: Evidence for modification of the human visual system by early visual experience. Vision Research. 1973;13:535–558. doi: 10.1016/0042-6989(73)90023-0. [DOI] [PubMed] [Google Scholar]
- Mitchell DE, Wilkinson F. The effect of early astigmatism on the visual resolution of gratings. Journal of Physiology. 1974;243:739–756. doi: 10.1113/jphysiol.1974.sp010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohindra I, Jacobson SG, Held R. Binocular visual form deprivation in human infants. Documenta Ophthalmolgica. 1983;55:237–249. doi: 10.1007/BF00140811. [DOI] [PubMed] [Google Scholar]
- Moseley MJ, Neufeld M, McCarry B, Charnock A, McNamara R, Rice T, Fielder A. Remediation of refractive amblyopia by optical correction alone. Ophthalmic and Physiological Optics. 2002;22:296–299. doi: 10.1046/j.1475-1313.2002.00034.x. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. A randomized pilot study of near activities versus non-near activities during patching therapy for amblyopia. Journal of AAPOS. 2005a;9:129–136. doi: 10.1016/j.jaapos.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Archives of Ophthalmology. 2005b;123:437–447. doi: 10.1001/archopht.123.4.437. [DOI] [PubMed] [Google Scholar]
- Pediatric Eye Disease Investigator Group. Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2006;113:895–903. doi: 10.1016/j.ophtha.2006.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CE, Moseley MJ, Fielder AR, Stephens DA the MOTAS Cooperative. Refractive adaptation in amblyopia: quantification of effect and implications for practice. British Journal of Ophthalmology. 2004;88:1552–1556. doi: 10.1136/bjo.2004.044214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller DY, McDonald MA, Preston K, Sebris SL, Dobson V. Assessment of visual acuity in infants and children: The acuity card procedure. Developmental Medicine & Child Neurology. 1986;28:779–789. doi: 10.1111/j.1469-8749.1986.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Wallace DK, Chandler DL, Beck RW, Arnold RW, Bacal DA, Birch EE, Felius J, Frazier M, Holmes JM, Hoover D, Klimek DA, Lorenzana I, Quinn GE, Repka MX, Suh DW, Tamkins S on behalf of the Pediatric Eye Disease Investigator Group. Treatment of bilateral refractive amblyopia in children three to less than 10 years of age. American Journal of Ophthalmology. 2007;144:487–96. doi: 10.1016/j.ajo.2007.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]