Abstract
Context
Puberty is associated with increased testicular testosterone (TT) synthesis, which is required to trigger spermatogenesis and to repress anti-Mullerian hormone (AMH) production. However, testicular gonadotropin stimulation during fetal and newborn life neither initiates spermatogenesis nor represses AMH.
Objective
We postulated that a lack of androgen receptor (AR) expression in Sertoli cells (SC) might explain why these processes do not occur during early human development.
Methods and patients
Using immunohistochemistry and quantitative PCR we examined the relationship between AR, AMH and FSH receptor (FSHR) expression in fetal, newborn and adult human testis. The ability of T to repress AMH secretion was evaluated in male newborns, neonates and two adults with androgen insensitivity syndrome (AIS) and also in vitro using SMAT1 Sertoli cells.
Results
FSHR was present in SC at all developmental stages. In fetal and newborn testis, AR was expressed in peritubular and Leydig cells but not in SC. This coincided with the absence of spermatogenesis and with strong SC AMH expression. In adult testis, spermatogenesis was associated with AR expression and with a decrease in SC AMH content. Accordingly, AR mRNA expression was lower and AMH mRNA expression higher in fetal testes than in adult testes. In AIS patients combined gonadotropin stimulation induced an increase in circulating T and AMH, a finding consistent with a failure of TT to repress AMH in the absence of AR signalling. Finally, direct androgen repression of AMH only occurred in AR-expressing SMAT1 cells.
Conclusion
Functional androgen receptors are essential for TT-mediated AMH repression in Sertoli cells.
Keywords: Human testis, Fetal, Newborn, Anti-Mullerian Hormone, Androgen receptor, Sertoli cell, Follicle-stimulating-hormone receptor, congenital hypogonadotropic hypogonadism.
INTRODUCTION
In the human adult testis, spermatogenesis is under the control of two gonadotropins, namely follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH acts directly on Sertoli cells (SC), while LH induces testosterone (T) production after Leydig cell stimulation. Intratesticular testosterone (TT) acts via a paracrine mechanism on androgen receptors (AR) expressed by target cells situated in the seminiferous tubules. In adulthood, the action of androgens on the seminiferous tubules is essential for full, quantitatively normal spermatogenesis and fertility. Most evidence suggests that this effect is mediated through an effect on SC, although the precise mechanisms are unclear. Recently, spermatogenesis was found to be arrested in mice specifically lacking AR in their SC (1–3).
Anti-Mullerian hormone (AMH) is produced by SC from fetal life until puberty and is responsible for Mullerian duct regression in male fetuses. AMH and T serum levels correlate negatively during puberty and adulthood, indicating that TT is responsible for inhibiting SC AMH production. This was confirmed in adult congenital hypogonadotropic hypogonadism (CHH) patients, whose testicular AMH secretion is inhibited by both hCG and androgen administration (4, 5).
AMH and T levels are both high during fetal and neonatal life (6, 7), indicating that testicular T is unable to repress AMH during these periods.
One important physiological issue is why the human testis is unable to produce sperm and to repress AMH secretion during fetal and neonatal life, despite stimulation by chorionic and pituitary gonadotropins, respectively. The aim of this study was to test the hypothesis that the absence of spermatogenesis and the AMH repression observed during human fetal and newborn life are related to the absence of AR in SC, despite FSH receptor (FSHR) expression. For this purpose, we compared the expression profiles of AR, FSHR and AMH proteins and their messenger RNAs in human fetal, newborn and adult testes by means of immunohistochemistry and real-time quantitative PCR (qPCR). In addition, we compared T and AMH levels in umbilical arterial cord blood of male newborns, 20- to 30-day-old neonates, and adults with normal or altered androgen sensitivity, in order to evaluate the ability of T to repress AMH secretion. Our results clearly demonstrate that in humans, AR expression in Sertoli cells is required both to induce spermatogenesis and to repress AMH.
MATERIALS AND METHODS
Tissues collection and quality
Archival paraffin-embedded human testicular samples were collected at different stages of development. Archival fetal testis samples (n=46), collected at 14 to 35 weeks of gestation, were selected from the organ bank of the Pathology Institute of Bari University with local ethics committee approval. Newborn (n=10) and adult (n=10) human testis samples were collected between 2 days and 6 months and between 20–40 years of age, respectively. These samples were obtained from the licensed collections of three pathology departments (Bicêtre, Créteil Intercommunal and Necker-Enfants Malades Hospitals, Paris). Snap-frozen testis specimens from 9 human fetuses of 16 to 38 weeks and from adult men were also obtained from the same licensed tissue collection (French Bioethics law n° 2004–800).
The fetal samples originated from fetuses that had died in utero and had been rapidly delivered and autopsied within 24–36 h postmortem. None of the terminations were performed for fetal abnormalities. Tissue integrity was demonstrated as described in (8). Neonatal testis samples were obtained from cases of sudden infant death, and adult samples were obtained from men undergoing surgical investigation for obstructive azoospermia. The selected samples had a normal karyotype (46 XY) and normal macroscopic and histological features.
Immunohistochemistry
Briefly, 5-μm-thick tissue sections were deparaffinized and rehydrated in successive baths of toluene and graded alcohol solutions. Slides were then subjected to microwave antigen retrieval for 15 min in pH 6 citrate buffer. For FSHR immunostaining the sections were preincubated with ready-to-use proteinase K (DakoCytomation, Carpinteria, CA) for 10 min at room temperature prior to 15 min of microwaving at full power in Tris–EDTA buffer pH 9. Slides were incubated overnight at 4°C with the following primary antibodies: anti-3-β-hydroxysteroid-dehydrogenase (3 βHSD) (kind gift from Prof. Van-Luu The, Laval University, Canada), anti-AMH (9), anti-FSH receptor (FSHR 323) (10) and anti-androgen receptor (sc-816, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at 1:2000, 1:1000 and 1:50 dilution, respectively. Bound immunoglobulins were revealed with a streptavidin-biotin-peroxidase-aminoethylcarbazole kit (LSAB+, DakoCytomation).
RT- PCR
Total RNA was isolated from frozen samples with the TRIZOL reagent (Invitrogen, Cergy Pontoise, France) as recommended by the manufacturer. RNA integrity was checked on agarose gel before processing for RT-PCR as previously described (8)
Real-time RT-PCR
Specific AMH and AR gene expression was quantified by real-time PCR (see Supplemental Table 1 for primers). Total RNA, extracted as described above, was retrotranscribed and amplified on an ABI 7300 Sequence Detector (Applied Biosystems, Foster City, CA) as previously described (8). Ribosomal 18S RNA was used as the internal control for data normalization. Results are means ± SEM of at least two independent analyses of at least two different reverse-transcribed samples. The relative expression level of each gene is expressed relative to 18S RNA (attomoles of specific gene per femtomole of 18S).
T and AMH measurements in umbilical cords of male newborns, neonates and adults
Neonatal blood samples were obtained from umbilical cords arteries of 17 full- term eutrophic male newborns immediately after delivery. Written informed consent was obtained from the mothers. The study was conducted in accordance with the Declaration of Helsinki and after approval from the local ethics committee. Blood samples were also obtained between day 20 and 30 of life from 21 normal boys with intrascrotal testes, and from 20 normal fertile males aged 17–37 years being evaluated in the context of female partner infertility. This part of the study was also approved by the local ethics committee, and we only used surplus serum taken for diagnostic purposes. Plasma testosterone (T) was measured with a commercial RIA method with a detection limit of 0.06 ng/ml (0.19 nmol/l) and intra- and interassay variation coefficients of 5.8% and 8.0%, respectively. Serum AMH levels were measured with an ELISA method (AMH/Mullerian-inhibiting substance ELISA, Immunotech-Beckman, Marseille, France), as previously described (4). The detection limit was 0.7 pmol/L (0.1 ng/ml), and the intra- and interassay coefficients of variation were 5.3% and 8.7%, respectively, for a serum AMH concentration of 35 pmol/l and 4.9% and 7.8% for a serum AMH concentration of 1100 pmol/l.
Clinical investigation of men with moderate androgen insensitivity syndrome (MAIS) or congenital hypogonadotropic hypogonadism (CHH)
We analyzed testicular responses to combined human gonadotropins in two azoospermic MAIS patients by comparison with 10 CHH patients receiving gonadotropin combination therapy with hCG (Organon, Puteaux, France) and rhFSH (recombinant human FSH) (Gonal-F®, Serono, Aubonne, Switzeland) at respective doses of 1500 IU and 150 IU, three times a week, for infertility.
MAIS patient #1, who was hemizygous for the M780I mutation of the AR gene, has been described in detail elsewhere (11). MAIS patient #2, a Caucasian boy, was first seen at 19 years of age with bilateral gynecomastia and impaired virilization but no hypospadias. Testicular volume was 10 ml bilaterally (normal range 15–30 ml), the penis was less than 4 cm long and his pubic hair was at Tanner stage III. His total plasma T level was 13.4 ng/mL (normal, 3.2–9.7 ng/mL, × 3.467 = nmol/L), and his LH was 14.8 IU/L (normal, 4–7 IU/L). Analysis of two semen samples showed azoospermia. The previously reported A to G transition (12) was found in exon 8 at nucleotide position 2973 causing an unconserved arginine residue to be replaced by a glycine (R871G).
Ten previously untreated men, aged 18–31 years, with idiopathic CHH (n=5) or Kallmann syndrome (n=5), were selected for diagnosis and choice of therapy. The diagnostic criteria for CHH were as reported elsewhere (4, 5, 13, 14). None of the patients had a history of cryptorchidism. All had low circulating T and gonadotropin levels. None had previously received gonadotropin or androgen replacement therapy. The patients were offered treatment consisting of FSH combined with hCG in order to increase testicular size, virilization and fertility as previously reported (4, 5, 13).
All the subjects gave their informed written consent to participate in the study, which was approved by the local ethics committee. Treatment was always well tolerated and compliance, evaluated by measuring circulating FSH and T levels, was excellent (see Results). AMH was assayed in blood to evaluate the testicular response. In MAIS and CHH patients, blood samples were drawn before treatment and every month during combined treatment; hormone measurements were performed in a single assay.
Cells culture and transfection
SMAT1 cells were cultured as described in (15). Cells were seeded at a density of 2 ×105/well in six-well plates and transiently transfected with 1 μg of human AR expression vector (hAR-pcDNA3) using the Lipofectamine reagent (Invitrogen, Carlsbad, CA92008). In brief, one day after initial plating, DMEM with 10% fetal calf serum was replaced by DMEM with Dextran-coated charcoal treated serum., Transfections were performed 24 h later. Fresh medium containing 10−5M forskolin alone or combined with 10−7M dihydrotestosterone was added to the cells for 24 h. The cells were then rinsed twice with PBS and processed for RNA extraction and real-time PCR as described above.
Western blot analysis and immunoprecipitation assay
Total protein extracts were prepared from SMAT1 cells transiently transfected or not with hAR-pcDNA3. Immunoprecipitation was performed by incubating 0.5 mg of total proteins with 1 μg of anti-AR antibody (sc-816, Santa-Cruz) overnight at 4°C. Immunoprecipitates were purified with protein A Sepharose CL-4B beads, and submitted to Western blot analysis using the same antibody at 1:200 dilution, followed by incubation with a peroxidase-conjugated goat anti-rabbit antibody (1:15000, Vector Laboratories, Burlingame, CA). The blots were visualized with the ECL+ detection kit (GE Healthcare).
RESULTS
3βHSD expression in human testis
We first examined the immunoexpression pattern of the key enzyme 3βHSD in order to evaluate the steroidogenic activity of Leydig cells. As expected, the protein was located in the cytoplasm of Leydig cells in all fetal (Fig. 1A), newborn (Fig. 1B) and adult (Fig. 1C) testicular samples.
Figure 1. Immunoexpression of 3βHSD, AR and AMH in human testis.
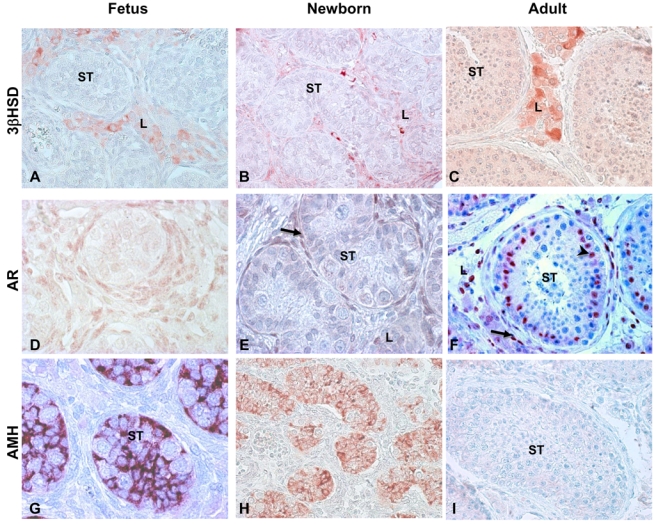
Representative illustrations of immunoreactivity in fetal, neonatal and adult testis.
A, B, C: Immunoexpression of 3βHSD in fetal (A), neonatal (B) and adult (C) testis: Enzyme expression is localized to the cytoplasm of Leydig cells (L) in fetal, newborn and adult testis. Sertoli cells and germ cells are negative.
D, E, F: Immunoexpression of AR in fetal (D), newborn (E) and adult (F) testis. In fetal (D) and newborn testis (E), nuclear AR expression is restricted to Leydig cells (L) and myoid peritubular cells (arrows). Both Sertoli cells and germ cells are negative. In adult testis (F), AR expression is present in the nucleus of Leydig cells (L), myoid peritubular cells (arrows) and also Sertoli cells (arrowheads).
G, H, I: Immunoexpression of AMH in fetal (G), newborn (H) and adult (I) testis AMH is strongly expressed in Sertoli cell cytoplasm in fetal (G) and newborn testis (H).
AMH is undetectable in adult human testis.
ST: seminiferous tubule
Original magnification: x20.
Immunohistochemical detection of AR and AMH in human testis
To determine the time course of AR and AMH expression during human testicular development, which might account for the relative testosterone resistance of SC, we compared AR and AMH expression in fetal, newborn and adult testes. In fetal and newborn testes, AR protein was detected in Leydig and myoid peritubular cells, but not in germ cells or SC (Fig. 1D and 1E). The absence of AR in SC coincided with very strong AMH expression in the same cells of all fetal and neonatal testis samples (Fig. 1G and 1H). In contrast, in all adult human testis samples examined, AR was detected not only in Leydig cells and myoid peritubular cells but also in SC (Fig. 1F), whereas AMH was not expressed (Fig. 1I). Semi-quantitative results for AR and AMH expression during human testis development are summarized in Table 1.
Table 1.
Summary of AR and AMH immunoexpression in human testis
| Fœtal N=46 | Newborn n =10 | Adult n =10 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sertoli | Leydig | Peritubular | Sertoli | Leydig | Peritubular | Sertoli | Leydig | Peritubular | |
| AR | 0 | ++ | ++ | 0 | ++ | ++ | + | ++ | ++ |
| AMH | ++ | 0 | 0 | ++ | 0 | 0 | 0 | 0 | 0 |
Immunoexpression was classified as: 0: absent or not detectable; Moderate: + and Strong: ++ as described in (23).
AR and AMH mRNA levels in human fetal testis
To examine quantitative changes in AMH and AR expression during testis development, mRNA levels of these genes were determined by real-time quantitative PCR in 10 fetal testes obtained at 16 (n= 2), 20 (n=1), 21 (n=1), 22 (n=1), 24 (n=2), 25 (n=2) and 38 (n=1) weeks of gestation, by comparison with adult testes (Fig. 2). The relative expression level of AR mRNA in fetal testis was 30% or lower than that in adult testis (Fig. 2A). This difference was probably due to the absence of AR expression in fetal testis SC. We then examined AMH mRNA expression and found that levels were 2- to 30-fold higher in fetal than in adult testes (Fig. 2B). Thus, the AR/AMH mRNA ratio, an index of AR expression in SC, was 151 ± 8 in adult testis, compared to 1–20 in fetal testis, in keeping with the lack of AR immunoexpression in fetal SC.
Figure 2. AMH and AR messenger levels in fetal human testis.
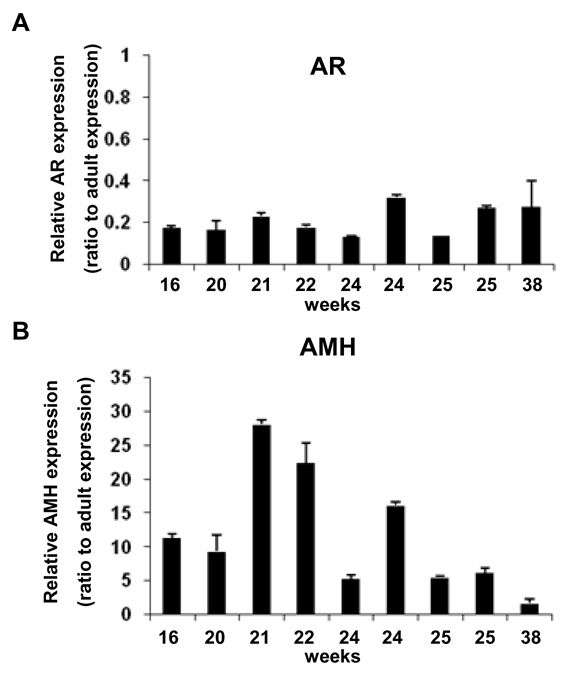
A, B: Relative expression of AR (A) and AMH (B) mRNA in 10 fetal human testis obtained at 16 (n=2), 20 (n=1), 21 (n=1), 22 (n=1), 24 (n=2), 25 (n=2) and 38 (n=1) weeks of gestation. (See Supplemental Table 1 for primers). Results were normalized to 18S ribosomal RNA. Relative expression in a given sample was calculated as attomoles per femtomol of 18S RNA. Results are means ± SEM of at least two RT samples run in duplicate, and represent the relative expression compared with control adult testes (arbitrarily 1).
FSHR expression in human testis
To determine whether the lack of spermatogenesis in the fetal and newborn testes is related to the lack of FSHR during these periods of life, we studied FSHR expression by immunohistochemistry and RT-PCR. As shown in Fig. 3A–C, FSHR immunoreactivity was localized to the SC membrane in fetal, newborn and adult testis. Furthermore, we confirmed that, as in adult testis, FSHR mRNA is also detected in human fetal testis (Fig. 3D). Using FSHR as another SC marker, we confirmed that the AR/FSHR mRNA ratio is higher in adult testis (406 ± 25) than in fetal testis (7.5 ± 1.5), providing additional support for the lack of AR expression in SC during human fetal development.
Figure 3. FSHR expression in human testis.
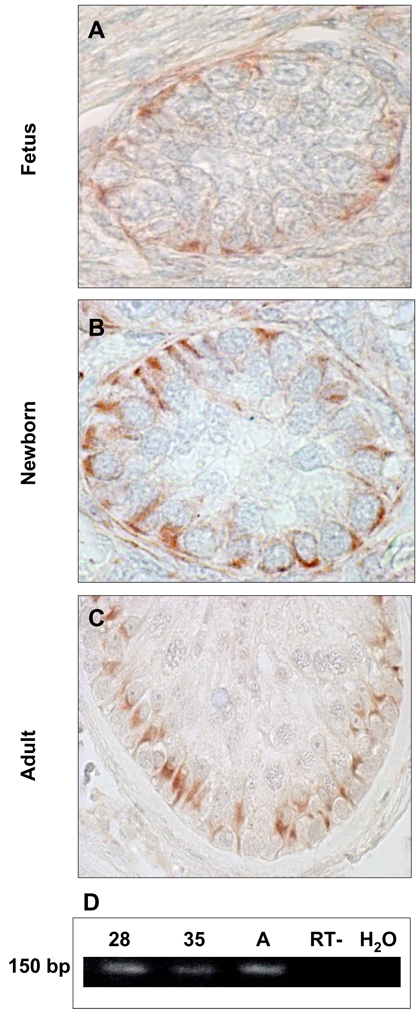
A-C: FSHR protein expression in human fetal (A), newborn (B) and adult testis (C). The receptor is expressed on the Sertoli cell membrane (arrows). Germ cells are negative.
D: Representative FSHR mRNA expression in human adult and fetal testis at 28 and 35 weeks. RNA was extracted and reverse transcribed as described in Material and Methods. (See Supplemental Table 1 for primers). A: Adult. RT- (omission of the reverse transcriptase) and H2O are negative controls.
Inability of combined gonadotropin stimulation to repress AMH in adult patients with an AR signaling defect
We then compared the testicular responses to combined hCG and rhFSH in two adult MAIS patients and 10 CHH patients used as positive controls of preserved AR signaling in post-pubertal testes (Fig. 4A left panel). As expected (4, 5) gonadotropin therapy increased the serum levels of FSH (from 0.6 ± 0.3 to 11.2 ± 2.2 IU/L) and T (from 0.46 ± 0.2 to 5.8 ± 2.2 ng/ml) in CHH patients leading to a concomitant collapse of AMH levels (from 406 ± 274 to 37 ± 18 pmol/L; P<0.001) which reached normal adult levels (Fig. 4A, right panel). In contrast, in MAIS patients, despite a further increase in their plasma T levels induced by gonadotropin administration (from 11–13.4 to 15–19.4 ng/ml), the serum AMH level also rose, clearly demonstrating that, in these adult patients, testicular T is unable to repress AMH secretion in the absence of functional AR and therefore to prevent the AMH stimulation induced by rhFSH. This response pattern was similar to that observed during the prenatal and postnatal period in which, despite relatively high circulating T levels driven by chorionic and pituitary gonadotrophin stimulation, mean AMH levels remain significantly higher than in post-pubertal men (6) (Fig. 4A, right panel). Interestingly, AMH levels were significantly higher in 20- to 30-day-old boys than in newborns, a finding consistent with our demonstration of FSHR expression in SC and its stimulation by FSH during the early post-natal period (16). In addition, strong AMH immunostaining was observed in SC of both adult patients with AIS and untreated CHH patients (Fig. 4B), contrasting with undetectable AMH expression in adults with normal testicular T and androgen sensitivity (see Fig. 1 panel I). Collectively, our results clearly indicate that repression of AMH secretion requires both an appropriate intratesticular T levels and functional AR signalling in SC.
Figure 4. Relationship between AMH and testosterone levels in normal and androgen-deficient human males.
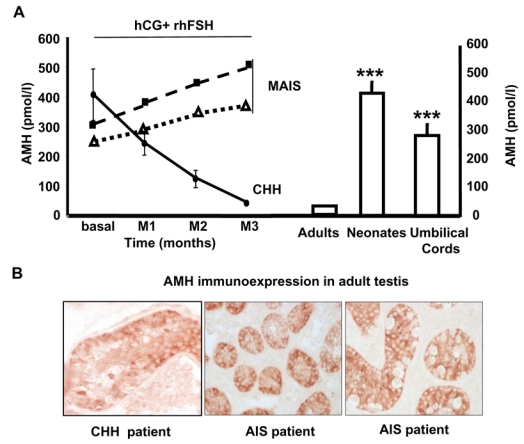
A: Left Panel: Time course of individual serum AMH levels (pmol/L) in two adult MAIS patients and mean (±SD) serum AMH in 10 adult CHH patients during combined gonadotropins stimulation (recombinant human FSH (rhFSH) and human chorionic gonadodotropin (hCG)).
Right panel: Serum AMH levels (pmol/L) were measured in adult, neonatal (20 and 30 post-natal days) and umbilical cord arterial bloods. Results are means ± SEM of at least 19 values. ***, P <0.001 vs adult values, Mann-Whitney test..
B: High AMH immunoexpression in testis of an untreated adult patient with congenital hypogonadic hypogonadotropism (CHH) and two adults with mild androgen insensitivity syndrome (AIS).
AMH repression by androgens requires functional androgen receptors in SMAT1 cells
To investigate direct androgen regulation of AMH in a Sertoli cell model, we examined endogenous AMH expression by means of real-time PCR in SMAT1 cells, an immortalized immature Sertoli cell line which does not express AR (Fig. 5A). Forskolin induced a 2.5-fold increase in AMH expression (Fig. 5B) through a PKA-dependent mechanism, as already reported (15). However, coadministration of dihydrotestosterone did not affect forskolin-induced AMH expression. In contrast, when SMAT1 cells were transiently transfected with a human AR expression vector (Fig. 5A and C), forskolin-stimulated AMH mRNA levels returned to basal values after dihydrotestosterone exposure, providing the first evidence that functional AR are crucial for full AMH repression by androgens in Sertoli cells.
Figure 5. Androgens repress endogenous AMH expression in SMAT1 Sertoli cells in the presence of AR.
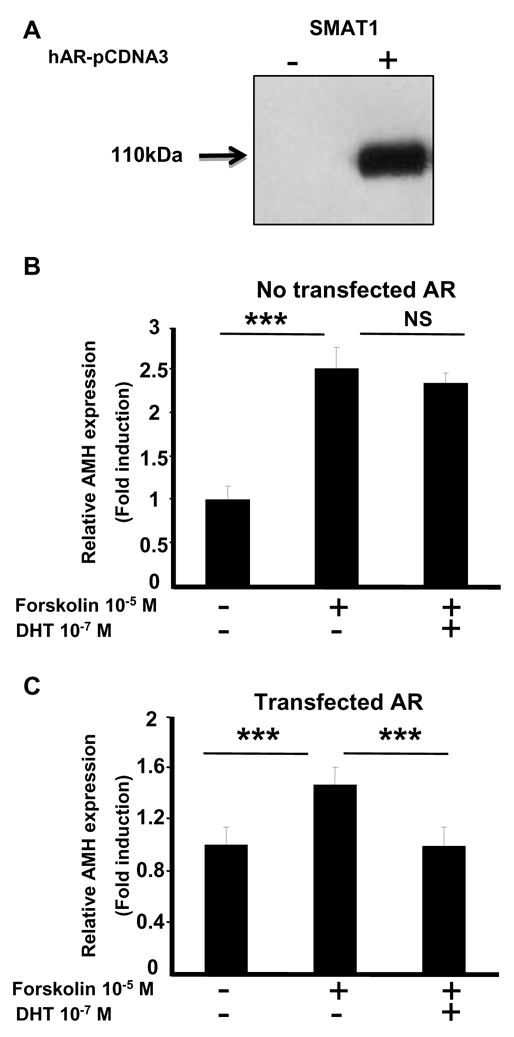
A: AR was expressed in SMAT1 cells transfected with pcDNA3-hAR plasmid. Proteins (0.5 mg) were immunoprecipitated (IP) with an anti-AR antibody, followed by Western blotting (WB) with the same antibody. AR protein was only expressed in SMAT1 cells transiently transfected with pcDNA3-hAR plasmid.
B, C: Relative AMH expression was analyzed by using real-time PCR in control SMAT1 cells (B) and in SMAT1 cells transiently transfected with pcDNA3-hAR plasmid (C). Cells were treated with 10−5M forskolin alone or combined with 10−7M dihydrotestosterone (DHT) for 24 h. Results are means ± SEM of at least six independent determinations and represent the relative expression compared with basal levels in untreated cells (arbitrarily set at 1). ***, P < 0.001 non parametric Mann-Whitney test. NS: not significant
DISCUSSION
The role of androgen receptor (AR) in spermatogenesis has been the subject of intense interest for many years. Multiple rodent studies of androgen withdrawal and disruption of AR activity by surgical, chemical or genetic means have produced convergent results: spermatogenesis rarely occurs in the absence of androgens and functional AR. It has been assumed that the main cellular mediator of this regulatory function is the Sertoli cell (SC), given its intimate contact with germ cells devoid of AR (17–19). Our findings endorse this assumption. Other rodent studies have revealed the major role played by SC AR in the completion of spermatogenesis (20). Several groups (1–3) recently generated mice with SC-selective AR knockout (SCARKO mice), opening up new possibilities to elucidate the respective contributions to spermatogenesis of AR expression in different testicular cell types. Thus, despite normal testicular descent, all SCARKO mice generated to date display arrested spermatogenesis. These results prove that the initiation and maintenance of spermatogenesis are both crucially dependent on AR activation in SC. Several authors, while studying the profile of AR expression in SC during post-natal development of normal rodents and non human primates (21–24), failed to detect immunochemical AR expression in the immediate post-natal period. This could explain why the elevated T levels present in rodent and marmoset testis are unable to induce morphological maturation of SC, or to trigger and maintain spermatogenesis, despite high levels of androgens and gonadotropins in the fetus and neonate.
The main purpose of the present study was to extend to humans this concept of a physiological state of transient androgen insensitivity of SC during fetal and neonatal life. Our results clearly show that, as reported for rodents and marmoset, AR protein is present in Leydig and peritubular cells of fetal and neonatal human testis, but not in SC. This expression pattern is in keeping with the absence of spermatogenesis, as expected (25–27), in our samples. During these two periods of human testicular development, hCG- and LH-induced biosynthesis of T is activated, as indicated by the strong 3βHSD expression in Leydig cells. This lack of AR expression in SC during early testicular development contrasted with the expected (17) strong AR expression we observed in these cells in sperm-producing adult human testis.
The role of FSH in the initiation of human spermatogenesis remains controversial (15, 28–32). In order to confirm that the lack of FSH receptor expression is not responsible for the lack of spermatogenic development in the fetal and early post-natal testis, we examined the FSHR expression pattern in SC in the same testicular samples. Immunochemical studies clearly showed FSHR expression in fetal and neonatal SC, as well as in adult SC. These findings, which concord with the FSHR transcript expression we also observed in human fetal testis, are also in line with the presence of FSH binding sites in fetal primate testis (33). It is generally accepted that FSH stimulates SC inhibin B (IB) secretion (4, 31, 34). Indeed, the presence of functional FSHR in human neonatal testis is suggested by two recent reports showing an increase in circulating IB in response to the neonatal rise in FSH (16, 35). Collectively, these results rule out the possibility that the absence of spermatogenesis in fetal and neonatal life is due to a lack of FSHR.
AMH, which is responsible for the repression of Mullerian ducts in male fetuses, is measurable in human male umbilical cord serum, as well as in the neonatal period and during infancy (36, 37). The T elevation during puberty correlates with a fall in circulating AMH (6). This negative correlation is also observed in boys with central or gonadotropin-independent early puberty, suggesting that T is the main player in AMH down-regulation.
Our combined T and AMH assays in umbilical cord, neonatal infants and adult blood provide additional in vivo support for the lack of functional AR expression in SC during the perinatal period. Indeed, the elevated T levels in fetal and neonatal males, in keeping with previous studies (16), are consistent with an increase in TT. However, AMH levels remained elevated during these two developmental stages, in keeping with the absence of AR in SC, as demonstrated here and in another very recent publication (25). Furthermore, circulating AMH levels were markedly increased in neonates compared to cord blood, owing to the reported FSH surge between these two periods (16, 38). This AMH increase is probably related to FSH-induced SC proliferation (which increases the AMH-producing cell population), and also to activation of AMH gene transcription through a pathway mediated by cyclic AMP (15, 21).
The negative correlation between testicular T and AMH levels is not found in neonatal rodents (21), human fetuses (6), or normal or cryptorchid human male newborns (16, 37), indicating that testicular T is unable to repress AMH in the fetal and neonatal periods. In newborn rodents (21, 24) and in adult SCARKO mice (1), the absence of AR expression in SC is also associated with a lack of AMH repression, suggesting that a similar mechanism might exist in human male newborns. Our immunohistochemical findings demonstrating strong AMH expression in SC lacking AR, and the marked fall observed in post-pubertal SC (which express AR) support this assumption. These results also argue against a mechanism in which a change in directional testicular AMH secretion (39) causes the fall in serum AMH after puberty.
Patients with androgen insensitivity syndrome or CHH, a pathological condition associated with low testicular T, have abnormally elevated serum AMH levels. As we have previously shown (4, 5) the hCG-driven increase in intratesticular T in the 10 CHH patients studied here, was associated with a marked reduction in the serum AMH level. In contrast, AMH levels were not reduced in the two adult MAIS patients, who received the same combined gonadotropin treatment; on the contrary, they were markedly enhanced, in keeping with an absence of androgen action owing to the lack of AR signaling in SC. Thus, this AMH response pattern in MAIS is similar to that recently reported in two CHH neonates (35) receiving a similar treatment, indicating that in both cases the failure to repress AMH is related to the absence of functional AR expression in SC.
In accordance to these in vivo findings, we obtained the first direct evidence that AMH repression by androgens only occurs when AR is expressed in Sertoli SMAT1 cells. The molecular mechanisms underlying the repressive effect of AR on AMH expression remain to be elucidated. AR might directly repress AMH transcription even though no androgen response elements are present in the regulatory regions of the AMH gene. Alternatively, AR might compromise FSHR-mediated AMH stimulation by altering AP2 and NFκB signaling (15). We are currently investigating the functional interaction between AR and FSHR activation cascade on AMH expression using a Sertoli cell model.
In conclusion, the absence of androgen receptor expression in Sertoli cells of fetal and neonatal human testis contributes to the lack of germ cell maturation and of anti-Mullerian hormone repression despite strong testicular testosterone biosynthesis.
Acknowledgments
The authors are indebted to Dr Anne-Lise Delezoïde (APHP, Hôpital Robert Debré, Paris, France), Dr Sophie Ferlicot (APHP, Hôpital Bicêtre, Paris, France), Dr Francis Joubert (APHP, Hôpital Necker, Paris, France) and Dr Martine Sinico (CHIC, Creteil, France) for their permission to use testis samples. We thank Annick Ganieux (IFR 93 Bicêtre) for help with the plasmid preparation. This work was supported by funds from Inserm and University Paris-Sud 11 (BQR). KB was the recipient of a fellowship from the Ministère de l′Enseignement Supérieur et de la Recherche, France and from la Fondation pour la Recherche Médicale (FRM).
Footnotes
Disclosure Statement: The authors have nothing to disclose
References
- 1.Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci U S A. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci U S A. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 4.Young J, Chanson P, Salenave S, Noel M, Brailly S, O’Flaherty M, Schaison G, Rey R. Testicular anti-mullerian hormone secretion is stimulated by recombinant human FSH in patients with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2005;90:724–728. doi: 10.1210/jc.2004-0542. [DOI] [PubMed] [Google Scholar]
- 5.Young J, Rey R, Couzinet B, Chanson P, Josso N, Schaison G. Antimullerian hormone in patients with hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1999;84:2696–2699. doi: 10.1210/jcem.84.8.5972. [DOI] [PubMed] [Google Scholar]
- 6.Rey R, Lordereau-Richard I, Carel JC, Barbet P, Cate RL, Roger M, Chaussain JL, Josso N. Anti-mullerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development. J Clin Endocrinol Metab. 1993;77:1220–1226. doi: 10.1210/jcem.77.5.8077315. [DOI] [PubMed] [Google Scholar]
- 7.Rajpert-De Meyts E, Jorgensen N, Graem N, Muller J, Cate RL, Skakkebaek NE. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84:3836–3844. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 8.Boukari K, Ciampi ML, Guiochon-Mantel A, Young J, Lombes M, Meduri G. Human fetal testis: source of estrogen and target of estrogen action. Hum Reprod. 2007;22:1885–1892. doi: 10.1093/humrep/dem091. [DOI] [PubMed] [Google Scholar]
- 9.Rey R, al-Attar L, Louis F, Jaubert F, Barbet P, Nihoul-Fekete C, Chaussain JL, Josso N. Testicular dysgenesis does not affect expression of anti-mullerian hormone by Sertoli cells in premeiotic seminiferous tubules. Am J Pathol. 1996;148:1689–1698. [PMC free article] [PubMed] [Google Scholar]
- 10.Vannier B, Loosfelt H, Meduri G, Pichon C, Milgrom E. Anti-human FSH receptor monoclonal antibodies: immunochemical and immunocytochemical characterization of the receptor. Biochemistry. 1996;35:1358–1366. doi: 10.1021/bi952290f. [DOI] [PubMed] [Google Scholar]
- 11.Rodien P, Mebarki F, Mowszowicz I, Chaussain JL, Young J, Morel Y, Schaison G. Different phenotypes in a family with androgen insensitivity caused by the same M780I point mutation in the androgen receptor gene. J Clin Endocrinol Metab. 1996;81:2994–2998. doi: 10.1210/jcem.81.8.8768864. [DOI] [PubMed] [Google Scholar]
- 12.Shkolny DL, Beitel LK, Ginsberg J, Pekeles G, Arbour L, Pinsky L, Trifiro MA. Discordant measures of androgen-binding kinetics in two mutant androgen receptors causing mild or partial androgen insensitivity, respectively. J Clin Endocrinol Metab. 1999;84:805–810. doi: 10.1210/jcem.84.2.5453. [DOI] [PubMed] [Google Scholar]
- 13.Nistal M, Abaurrea MA, Paniagua R. Morphological and histometric study on the human Sertoli cell from birth to the onset of puberty. J Anat. 1982;134:351–363. [PMC free article] [PubMed] [Google Scholar]
- 14.Salenave S, Chanson P, Bry H, Pugeat M, Cabrol S, Carel JC, Murat A, Lecomte P, Brailly S, Hardelin JP, Dode C, Young J. Kallmann’s syndrome: a comparison of the reproductive phenotypes in men carrying KAL1 and FGFR1/KAL2 mutations. J Clin Endocrinol Metab. 2008;93:758–763. doi: 10.1210/jc.2007-1168. [DOI] [PubMed] [Google Scholar]
- 15.Lukas-Croisier C, Lasala C, Nicaud J, Bedecarras P, Kumar TR, Dutertre M, Matzuk MM, Picard JY, Josso N, Rey R. Follicle-stimulating hormone increases testicular Anti-Mullerian hormone (AMH) production through sertoli cell proliferation and a nonclassical cyclic adenosine 5′-monophosphate-mediated activation of the AMH Gene. Mol Endocrinol. 2003;17:550–561. doi: 10.1210/me.2002-0186. [DOI] [PubMed] [Google Scholar]
- 16.Bergada I, Milani C, Bedecarras P, Andreone L, Ropelato MG, Gottlieb S, Bergada C, Campo S, Rey RA. Time course of the serum gonadotropin surge, inhibins, and anti-Mullerian hormone in normal newborn males during the first month of life. J Clin Endocrinol Metab. 2006;91:4092–4098. doi: 10.1210/jc.2006-1079. [DOI] [PubMed] [Google Scholar]
- 17.Suarez-Quian CA, Martinez-Garcia F, Nistal M, Regadera J. Androgen receptor distribution in adult human testis. J Clin Endocrinol Metab. 1999;84:350–358. doi: 10.1210/jcem.84.1.5410. [DOI] [PubMed] [Google Scholar]
- 18.Bremner WJ, Millar MR, Sharpe RM, Saunders PT. Immunohistochemical localization of androgen receptors in the rat testis: evidence for stage-dependent expression and regulation by androgens. Endocrinology. 1994;135:1227–1234. doi: 10.1210/endo.135.3.8070367. [DOI] [PubMed] [Google Scholar]
- 19.Berensztein EB, Baquedano MS, Gonzalez CR, Saraco NI, Rodriguez J, Ponzio R, Rivarola MA, Belgorosky A. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr Res. 2006;60:740–744. doi: 10.1203/01.pdr.0000246072.04663.bb. [DOI] [PubMed] [Google Scholar]
- 20.Johnston DS, Russell LD, Friel PJ, Griswold MD. Murine germ cells do not require functional androgen receptors to complete spermatogenesis following spermatogonial stem cell transplantation. Endocrinology. 2001;142:2405–2408. doi: 10.1210/endo.142.6.8317. [DOI] [PubMed] [Google Scholar]
- 21.Al-Attar L, Noel K, Dutertre M, Belville C, Forest MG, Burgoyne PS, Josso N, Rey R. Hormonal and cellular regulation of Sertoli cell anti-Mullerian hormone production in the postnatal mouse. J Clin Invest. 1997;100:1335–1343. doi: 10.1172/JCI119653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- 23.McKinnell C, Saunders PT, Fraser HM, Kelnar CJ, Kivlin C, Morris KD, Sharpe RM. Comparison of androgen receptor and oestrogen receptor beta immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reproduction. 2001;122:419–429. doi: 10.1530/rep.0.1220419. [DOI] [PubMed] [Google Scholar]
- 24.Majdic G, Millar MR, Saunders PT. Immunolocalisation of androgen receptor to interstitial cells in fetal rat testes and to mesenchymal and epithelial cells of associated ducts. J Endocrinol. 1995;147:285–293. doi: 10.1677/joe.0.1470285. [DOI] [PubMed] [Google Scholar]
- 25.Chemes HE, Rey RA, Nistal M, Regadera J, Musse M, Gonzalez-Peramato P, Serrano A. Physiologic Androgen Insensitivity of the Fetal, Neonatal, and Early Infantile Testis Is Explained by the Ontogeny of the Androgen Receptor Expression in Sertoli Cells. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2008-0915. [DOI] [PubMed] [Google Scholar]
- 26.Rey R. Regulation of spermatogenesis. In: Karger, editor. The developing testis physiology and pathophysiology. Basel: 2003. pp. 38–55. [Google Scholar]
- 27.Muller J, Skakkebaek NE. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int J Androl. 1983;6:143–156. doi: 10.1111/j.1365-2605.1983.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 28.Lindstedt G, Nystrom E, Matthews C, Ernest I, Janson PO, Chatterjee K. Follitropin (FSH) deficiency in an infertile male due to FSHbeta gene mutation. A syndrome of normal puberty and virilization but underdeveloped testicles with azoospermia, low FSH but high lutropin and normal serum testosterone concentrations. Clin Chem Lab Med. 1998;36:663–665. doi: 10.1515/CCLM.1998.118. [DOI] [PubMed] [Google Scholar]
- 29.Phillip M, Arbelle JE, Segev Y, Parvari R. Male hypogonadism due to a mutation in the gene for the beta-subunit of follicle-stimulating hormone. N Engl J Med. 1998;338:1729–1732. doi: 10.1056/NEJM199806113382404. [DOI] [PubMed] [Google Scholar]
- 30.Tapanainen JS, Aittomaki K, Min J, Vaskivuo T, Huhtaniemi IT. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15:205–206. doi: 10.1038/ng0297-205. [DOI] [PubMed] [Google Scholar]
- 31.Plant TM, Marshall GR. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- 32.Layman LC, Porto AL, Xie J, da Motta LA, da Motta LD, Weiser W, Sluss PM. FSH beta gene mutations in a female with partial breast development and a male sibling with normal puberty and azoospermia. J Clin Endocrinol Metab. 2002;87:3702–3707. doi: 10.1210/jcem.87.8.8724. [DOI] [PubMed] [Google Scholar]
- 33.Huhtaniemi IT, Yamamoto M, Ranta T, Jalkanen J, Jaffe RB. Follicle-stimulating hormone receptors appear earlier in the primate fetal testis than in the ovary. J Clin Endocrinol Metab. 1987;65:1210–1214. doi: 10.1210/jcem-65-6-1210. [DOI] [PubMed] [Google Scholar]
- 34.Young J, Couzinet B, Chanson P, Brailly S, Loumaye E, Schaison G. Effects of human recombinant luteinizing hormone and follicle-stimulating hormone in patients with acquired hypogonadotropic hypogonadism: study of Sertoli and Leydig cell secretions and interactions. J Clin Endocrinol Metab. 2000;85:3239–3244. doi: 10.1210/jcem.85.9.6811. [DOI] [PubMed] [Google Scholar]
- 35.Bougneres P, Francois M, Pantalone L, Rodrigue D, Bouvattier C, Demesteere E, Roger D, Lahlou N. Effects of an early postnatal treatment of hypogonadotropic hypogonadism with a continuous subcutaneous infusion of recombinant follicle-stimulating hormone and luteinizing hormone. J Clin Endocrinol Metab. 2008;93:2202–2205. doi: 10.1210/jc.2008-0121. [DOI] [PubMed] [Google Scholar]
- 36.Josso N, Lamarre I, Picard JY, Berta P, Davies N, Morichon N, Peschanski M, Jeny R. Anti-mullerian hormone in early human development. Early Hum Dev. 1993;33:91–99. doi: 10.1016/0378-3782(93)90204-8. [DOI] [PubMed] [Google Scholar]
- 37.Lee MM, Donahoe PK, Silverman BL, Hasegawa T, Hasegawa Y, Gustafson ML, Chang YC, MacLaughlin DT. Measurements of serum mullerian inhibiting substance in the evaluation of children with nonpalpable gonads. N Engl J Med. 1997;336:1480–1486. doi: 10.1056/NEJM199705223362102. [DOI] [PubMed] [Google Scholar]
- 38.Debieve F, Beerlandt S, Hubinont C, Thomas K. Gonadotropins, prolactin, inhibin A, inhibin B, and activin A in human fetal serum from midpregnancy and term pregnancy. J Clin Endocrinol Metab. 2000;85:270–274. doi: 10.1210/jcem.85.1.6249. [DOI] [PubMed] [Google Scholar]
- 39.Fenichel P, Rey R, Poggioli S, Donzeau M, Chevallier D, Pointis G. Anti-Mullerian hormone as a seminal marker for spermatogenesis in non-obstructive azoospermia. Hum Reprod. 1999;14:2020–2024. doi: 10.1093/humrep/14.8.2020. [DOI] [PubMed] [Google Scholar]


