Abstract
Objective
Malnutrition is a powerful predictor of mortality in chronic kidney disease (CKD); however, its etiology is unclear. We hypothesized that adipocyte-derived proteins leptin and adiponectin, inflammation (C-reactive protein –CRP), and insulin resistance (Homeostasis Model Assessment –HOMA); implicated in the malnutrition-inflammation complex syndrome commonly seen in maintenance dialysis patients, would be associated with the loss of muscle mass in earlier stages of CKD. Arm muscle area was used as an indicator of muscle mass.
Setting
The Modification of Diet in Renal Disease (MDRD) Study cohort of people with CKD stages 3 and 4 was used for analysis.
Main Outcome Measures
Regression models were carried out to examine the relationships of leptin, adiponectin, CRP, and HOMA with arm muscle area (the main study outcome).
Results
Arm muscle area was 39 ± 15 cm2 (mean ± standard deviation, SD) and adiponectin levels were 13 ± 7 μg/mL. Median and (inerquartile range, IQR) concentrations were: 9.0 (13.6) ng/mL for leptin, 2.3 (4.9) mg/L for CRP, and 2.4 (2.0) form HOMA. Higher leptin [beta coefficient and (95% confidence interval): −6.9 (−8.7, −5.1), P<0.001] and higher CRP [−2.7 (−3.9, −1.4), P<0.001] were associated with lower arm muscle area. There was a trend toward lower arm muscle area with higher adiponectin (P=0.07) but no association with HOMA (P=0.80).
Conclusion
Leptin and CRP were associated with lower muscle mass in subjects with CKD stages 3–4. Further studies are needed to understand the mechanisms underlying these associations and to develop targeted interventions for this patient population.
Keywords: Chronic kidney disease, arm muscle area, adipokines, inflammation, insulin resistance
Introduction
Malnutrition is common in the early stages of chronic kidney disease (CKD) and rises steadily with its progression (1). Indicators of nutritional status such as serum albumin and muscle mass are powerful predictors of morbidity and mortality in CKD. Muscle mass, in particular, confers a survival advantage in kidney failure (2, 3). The loss of muscle mass with CKD may be the result of an imbalance between catabolic and anabolic signals (1, 4). Although, the mechanisms underlying muscle wasting are not well understood, adipocyte-derived protein called adipokines, may play an important role given that they mediate insulin resistance and inflammation in kidney disease (5).
Two adipokines of interest are leptin and adiponectin. High levels of leptin are present in kidney failure (6). Leptin suppresses appetite, increases satiety, and is directly correlated with body fat (7). Hyperleptinemia is associated with inadequate protein and energy intake and with loss of lean tissue in dialysis patients (8), suggesting a role for leptin in the etiology of malnutrition. Adiponectin –another adipokine that accumulates in kidney disease, regulates energy homeostasis (9) and is inversely related to body mass index and serum albumin in people with CKD (10, 11). Adiponectin may also be associated with a reduction in body weight due to increased energy expenditure (12). Additionally, both leptin and adiponectin have been shown to play a role in glucose homeostasis and insulin action. Peripheral tissue insulin resistance is prevalent in the earlier stages of CKD (13), and potentially an important link in the pathway leading to muscle wasting with CKD.
C-reactive protein (CRP), a well established marker of inflammation and malnutrition in patients with kidney disease (14, 15) is regulated by the adipocyte-derived cytokines tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6) (16). Levels of CRP are elevated in patients with CKD (15, 17) and kidney failure (18). They are directly related with body mass index and percent body fat (19) and inversely related with serum albumin and muscle mass (20). High CRP levels are also associated with all-cause mortality in patients with CKD (21, 22). Increased CRP levels, which reflect the activity of cytokine-mediated inflammatory processes, are associated with protein catabolism and muscle wasting mediated by the ubiquitin-proteosome pathway (23).
The objectives of this study were to examine the relationship between adipocyte-derived proteins leptin and adiponectin, inflammation (as measured by CRP), and insulin resistance (as measured by the Homeostasis Model Assessment –HOMA) with muscle mass (measured as arm muscle area) in stage 3–4 CKD.
Materials and Methods
Study Population
The Modification of Diet in Renal Disease (MDRD) Study (1989 and 1993), was a randomized controlled trial designed to examine the effect of strict blood pressure control and dietary protein restriction on the progression of kidney disease (24). Subjects were 18–70 years of age and had serum creatinine level between 1.4–7.0 mg/dL and 1.2–7.0 mg/dL, for men and women, respectively. Exclusion criteria included history of insulin-dependent diabetes, class III or IV congestive heart failure, renal artery stenosis, kidney transplantation or frequent hospitalizations. Seven hundred and eighty subjects with complete baseline measures were included in this analysis. Baseline glomerular filtration rate (GFR) levels were between 13 and 55 mL/min/1.73 m2, equivalent to CKD stages 3 and 4. This study was approved by the Institutional Review Boards of Tufts-New England Medical Center and the Cleveland Clinic Foundation.
Anthropometric Measures
Anthropometric measurements were performed at baseline. Weight and height were measured using standardized equipment and procedures. Body mass index (BMI) was calculated as weight divided by height square (kg/m2). Mid arm circumference (MAC) was measured using a metal tape. Triceps, biceps and subscapular skinfold thicknesses were measured using a Holtain caliper (Holtain Ltd; Crosswell, Crymych, UK). Mid arm muscle area (AMA) was calculated from MAC and triceps skinfold thickness according to the following equation (25): AMA (cm2) = [(MAC − 3.1416 × triceps skinfold thickness in cm)2/4 × 3.1416]. The arm muscle area measurements reported here are based on bone-free arm muscle area. This is calculated by subtracting a factor of 19.0 for men and 15.5 for women from the AMA equation shown above (25). Arm muscle area was measured at baseline and at one year.
Assays
Samples collected at baseline were used to measure the metabolic factors of interest as follows. Leptin was measured using a commercially available radioimmunoassay kit (Linco Research, St. Louis, MO) with a sensitivity of 0.5 ng/mL. Adiponectin was measured using a commercially available enzyme immunoassay kit (R&D Systems, Minneapolis, MN). Fasting plasma insulin concentrations were measured using Linco specific human antibodies (Linco Research, St. Louis, MO), and glucose using the hexokinase enzymatic method. Insulin resistance was estimated by the Homeostasis Model Assessment (HOMA) method as (fasting glucose in mmol/l × fasting insulin μU/ml)/22.5 (26). High-sensitivity C-reactive protein (CRP) was measured using a Dade Behring BN II nephelometer (Dade Behring, Deerfield, IL) by means of particle-enhanced technology with a detection limit of 1 mg/L. All laboratory measures had coefficients of variations were under 8%.
Statistical Analysis
Statistical analysis was carried out using SPSS for Windows version 14.0 (SPSS Inc., Chicago, IL). Data are reported as mean and standard deviation (SD) for normally distributed continuous data, median and interquartile range (IQR) for non-normally distributed continuous variables, and percentages for categorical data. Severely positively skewed variables (leptin, HOMA, CRP, and transferrin) were log transformed for analyses. Baseline subject characteristics by gender-specific quartiles of arm muscle area are presented for demographic, anthropometric, metabolic and nutritional variables. Differences in baseline characteristics were tested using one-way analysis of variance.
Because BMI is strongly associated with the study outcome (arm muscle area) as well as with the metabolic factors of interest (leptin, adiponectin, HOMA, and CRP), we first assessed the univariate associations between BMI and these factors using linear regression analysis. We then performed partial correlations, adjusting by gender and BMI, to examine the relationships between arm muscle area and selected metabolic and nutritional variables. Finally, we used multivariable linear regression analysis to examine the effect size or degree of association between arm muscle area and the predictor variables. For this purpose, we constructed separate regression models for each metabolic factor of interest: leptin (Model 1), adiponectin (Model 2), insulin resistance or HOMA (Model 3), and CRP (Model 4); adjusting for age, gender, BMI, dietary energy and protein intake, serum albumin concentrations and GFR, as independent variables. These covariates were chosen because of their known association with arm muscle area and based on a P <0.1 in univariate associations with arm muscle area.
We also performed separate multivariable logistic regression models using low arm muscle area (defined as a value < 25th percentile) as the dependent variable and each of the metabolic factors of interest, adjusting for previously defined covariates. We used this conservative cut-off for arm muscle area because there are no reference data in people with CKD. Finally, multivariable linear and logistic regression models were repeated using standardized arm muscle area (by percent body weight) at baseline as the dependent variable to account for body size.
Longitudinal Analysis
The mean difference in arm muscle area at 1-year was calculated as arm muscle area at 1-year minus arm muscle area at baseline. Since there was no significant difference at 1-year we did not perform multivariable analyses. The study cohort was divided into tertiles based on mean difference in arm muscle area and levels of the metabolic factors of interest were compared among tertiles using analysis of variance.
Results
Baseline Characteristics
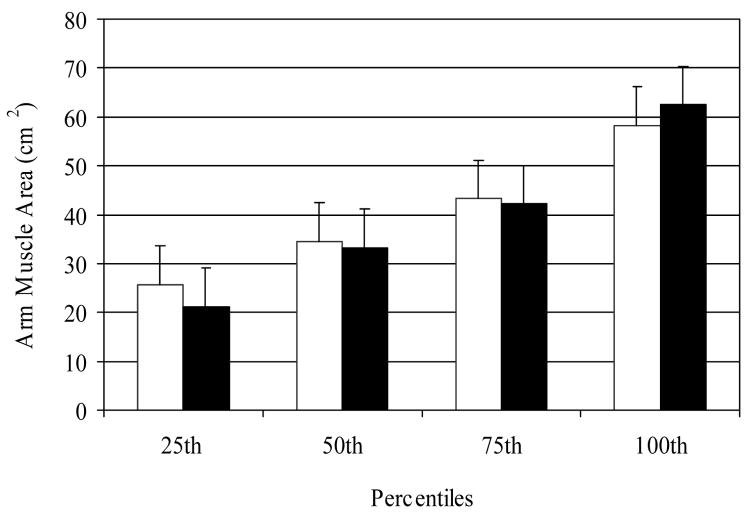
Sixty two percent of the MDRD study participants were men and 85% were white. Mean age was 52 ± 12 years and GFR level was 33 ± 12 mL/min/1.73m2. Baseline mean arm muscle area in men was 46 ± 12 cm2 while in women it was 29 ± 12 cm2. Gender-specific distribution of baseline arm muscle area is shown in Figure 1. Mean adiponectin was 12.6 ± 7.0 μg/mL, and median (IQR) leptin was 9.0 (13.6) ng/mL, HOMA was 2.4 (2.0), and CRP was 2.3 (4.9) mg/L. Baseline subject characteristics by gender-specific quartiles of arm muscle area are shown in Table 1. Participants in the highest quartile of arm muscle area were significantly older; had higher BMI; had higher levels of triglycerides and LDL-cholesterol and lower levels of HDL-cholesterol; and had higher protein intake and GFR levels. Participants with higher arm muscle area also had higher blood levels of serum bicarbonate, glucose, insulin, HOMA, CRP and leptin, and lower adiponectin levels.
Figure 1.
Distribution of baseline arm muscle area measures for men (open bars) and women (closed bars) in the MDRD Study cohort studied. Error bars represent SD.
Table 1.
Baseline Subject Characteristics
| Quartiles of Arm Muscle Area (cm2) | ||||
|---|---|---|---|---|
| Characteristic | [7.2–37.7] N=195 |
[20.5–44.1] N=195 |
[27.0–53.2] N=195 |
[34.8–88.7] N=195 |
| Age (years) | 49.5 ± 13.5 | 52.7 ± 12.7 | 51.1 ± 11.7 | 53.9 ± 10.9a |
| Body Mass Index (kg/m2) | 23.8 ± 3.0 | 25.9 ± 3.4 | 27.2 ± 2.9 | 31.3 ± 4.0a |
| Protein Intake (g/kg/d) | 0.9 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.3a |
| Energy Intake (kcal/kg/d) | 26.7 ± 6.8 | 26.3 ± 6.8 | 26.2 ± 7.6 | 25.8 ± 8.5 |
| Triglycerides (mg/dL) | 121 (81) | 138 (121) | 136 (116) | 152 (150)a |
| LDL-cholesterol (mg/dL) | 143 ± 39 | 144 ± 40 | 154 ± 42 | 147 ± 44a |
| HDL-cholesterol (mg/dL) | 42 ± 15 | 41 ± 16 | 39 ± 13 | 37 ± 12a |
| Albumin (g/L) | 4.0 ± 0.3 | 4.0 ± 0.4 | 4.0 ± 0.4 | 4.0 ± 0.3 |
| Transferrin (mg/dL) | 272 (61) | 268 (60) | 267 (50) | 266 (59) |
| GFR (mL/min 1.73 m2) | 29.4 ± 11.2 | 32.1 ± 12.6 | 34.1 ± 12.1 | 34.7 ± 11.7a |
| Proteinuria (g/d) | 0.3 (1.2) | 0.3 (1.7) | 0.2 (1.5) | 0.3 (1.5) |
| Bicarbonate (mmol/L) | 22 ± 4 | 24 ± 4 | 23 ± 4 | 23 ± 3a |
| Glucose (mg/dL) | 86 ± 15 | 93 ± 22 | 92 ± 28 | 97 ± 31a |
| Insulin (μU/L) | 10.0 (6) | 10.0 (7) | 11.0 (8) | 14.0 (10)a |
| Leptin (ng/mL) | 7.8 (10.9) | 8.8 (12.1) | 9.0 (15.5) | 10.8 (20.9)a |
| Adiponectin (μg/mL) | 14.5 ± 7.4 | 14.1 ± 7.1 | 11.9 ± 6.5 | 9.8 ± 5.7a |
| HOMA | 2.0 (1.5) | 6.5 (1.9) | 2.4 (2.1) | 3.1 (2.6)a |
| CRP (mg/L) | 1.6 (4.0) | 2.4 (4.3) | 2.1 (4.3) | 3.8 (6.4)a |
Data are mean ± SD or median (IQR). GFR, Glomerular Filtration Rate; HOMA, Homeostasis Model Assessment; CRP, C-reactive protein; adjusted for gender.
Overall P value < 0.05
Conversion to SI units: Multiply by 0.01129 to covert triglycerides in mmol/L, by 0.02586 to convert LDL- and HDL-cholesterol in mmol/L, by 0.01 to covert transferrin in g/L, by 0.05551 to convert glucose in mmol/L, and by 7.175 to convert insulin in pmol/L.
BMI and Arm Muscle Area
BMI was significantly associated with arm muscle area (r=0.64), leptin (r=0.37), adiponectin (r=−0.33), CRP (r=0.44), and HOMA (r=0.43); all P values <0.001. A model with BMI as the only independent variable explained 41% of the variability in arm muscle area.
Correlates of Arm Muscle Area
Since the univariate associations were strongly confounded by BMI, partial correlation analyses adjusted for gender and BMI were used to examine the univariate relationships between arm muscle area and demographic, clinical, metabolic and nutritional variables (Table 2). Arm muscle area was positively correlated with energy intake, serum albumin, and GFR, with a trend towards a positive association with dietary protein intake. Arm muscle area was negatively associated with adiponectin, leptin, and CRP.
Table 2.
Correlates of Arm Muscle Area
| Variable | ra | P value |
|---|---|---|
| Age (years) | −0.05 | 0.20 |
| Protein Intake (g/kg/d) | 0.06 | 0.07 |
| Energy Intake (kcal/kg/d) | 0.10 | 0.01 |
| Triglycerides (mg/dL) | −0.02 | 0.53 |
| LDL cholesterol (mg/dL) | 0.04 | 0.91 |
| HDL cholesterol (mg/dL) | 0.01 | 0.77 |
| Albumin (g/L) | 0.08 | 0.02 |
| Transferrin (mg/dL)b | −0.04 | 0.29 |
| GFR (mL/min 1.73 m2) | 0.07 | 0.05 |
| Proteinuria (g/d) | −0.02 | 0.63 |
| Bicarbonate (mmol/L) | 0.02 | 0.50 |
| Glucose (mg/dL) | −0.03 | 0.37 |
| Insulin (μU/L) | 0.02 | 0.60 |
| Leptin (ng/mL)b | −0.26 | <0.001 |
| Adiponectin (μg/mL) | −0.10 | 0.01 |
| HOMAb | 0.01 | 0.78 |
| CRP (mg/L)b | −0.15 | <0.001 |
Partial correlations adjusted for gender and body mass index (BMI)
Transferrin, leptin, Homeostasis Model Assessment (HOMA), and C-reactive protein (CRP) were log transformed
Determinants of Arm Muscle Area
In separate multivariable linear regression analyses adjusting for previously described covariates, there was an independent inverse relationship of leptin and CRP with arm muscle area, a trend toward significance for adiponectin, and no relationship with HOMA (Table 3). After adjusting for other variables in the model, a doubling of leptin and CRP levels were associated with mean reductions in arm muscle area equivalent to 4.77 cm2 (95%CI= −4.12 to 5.41) and 1.86 cm2 (1.39 to 2.32), for leptin and CRP, respectively.
Table 3.
Multivariable Linear Regression Models for Determinants Arm Muscle Area (cm2)
| Parameter | Model 1a | P value | Model 2a | P value | Model 3a | P value | Model 4a | P value |
|---|---|---|---|---|---|---|---|---|
| B (95% CI)b | B (95% CI)b | B (95% CI)b | B (95% CI)b | |||||
| Leptin (ng/mL)c | −6.9 (−8.7, −5.1) | <0.001 | -- | -- | -- | -- | -- | -- |
| Adiponectin (μg/mL) | -- | -- | −0.1 (−0.2, 0.01) | 0.07 | -- | -- | -- | -- |
| HOMAc | -- | -- | -- | -- | −0.3 (−2.5, 1.9) | 0.80 | -- | -- |
| CRP (mg/L)c | -- | -- | -- | -- | -- | -- | −2.7 (−3.9, −1.4) | <0.001 |
| Age (y) | −0.1 (−0.1, 0.04) | 0.58 | −0.1 (−0.1, 0.04) | 0.61 | −0.1 (−0.1, 0.04) | 0.61 | 0.1 (−0.04, 0.1) | 0.55 |
| Gender (women vs. men) | −11 (−12, −9) | <0.001 | −13 (−15, −12) | <0.001 | −14. (−15, −13) | <0.001 | −14 (−15, −12) | <0.001 |
| Body Mass Index (kg/m2) | 2.2 (2.1, 2.4) | <0.001 | 1.9 (1.8, 2.1) | <0.001 | 1.9 (1.8, 2.1) | <0.001 | 2.1 (1.9, 2.2) | <0.001 |
| Energy Intake (kcal/kg/d) | 0.1 (−0.04, 0.2) | 0.18 | 0.1 (−0.02, 0.2) | 0.09 | 0.1 (−0.02, 0.2) | 0.12 | 0.1 (−0.04, 0.2) | 0.21 |
| Protein Intake (g/kg/d) | 0.3 (2.4, 2.9) | 0.83 | −0.2 (−2.9, 2.5) | 0.86 | −0.02 (−2.8, 2.7) | 0.98 | 0.2 (−2.5, 2.9) | 0.85 |
| Albumin (g/L) | 0.2 (0.04, 0.4) | 0.01 | 0.2 (−0.02, 0.3) | 0.09 | 0.2 (0.01, 0.4) | 0.04 | 0.1 (−0.02, 0.3) | 0.09 |
| GFR (mL/min 1.73 m2) | 0.02 (−0.03, 0.1) | 0.36 | 0.03 (−0.02, 0.1) | 0.27 | 0.04 (−0.01, 0.1) | 0.14 | 0.04 (−0.01, 0.1) | 0.14 |
R2 Model 1=0.67; Model 2=0.65; Model 3=0.65; Model 4=0.66
Regression coefficient and 95% confidence interval
Leptin, Homeostasis Model Assessment (HOMA), and C-reactive protein (CRP) were log transformed for analysis
In multivariate logistic regression analyses, higher leptin levels [Odds Ratio and (95% Confidence Interval)= 4.3 (2.2, 8.5), P<0.001) and CRP levels [1.8 (1.2, 2.6), P<0.01]; but not adiponectin [0.9 (0.9, 1.0), P=0.94] or HOMA [1.0 (0.5,2.2), P=0.92] were associated with increased odds of low arm muscle area (defined as <25th percentile). Similar results were obtained when analyses were repeated using baseline arm muscle area standardized by percent body weight instead of absolute arm muscle area (data not shown).
Longitudinal Analysis
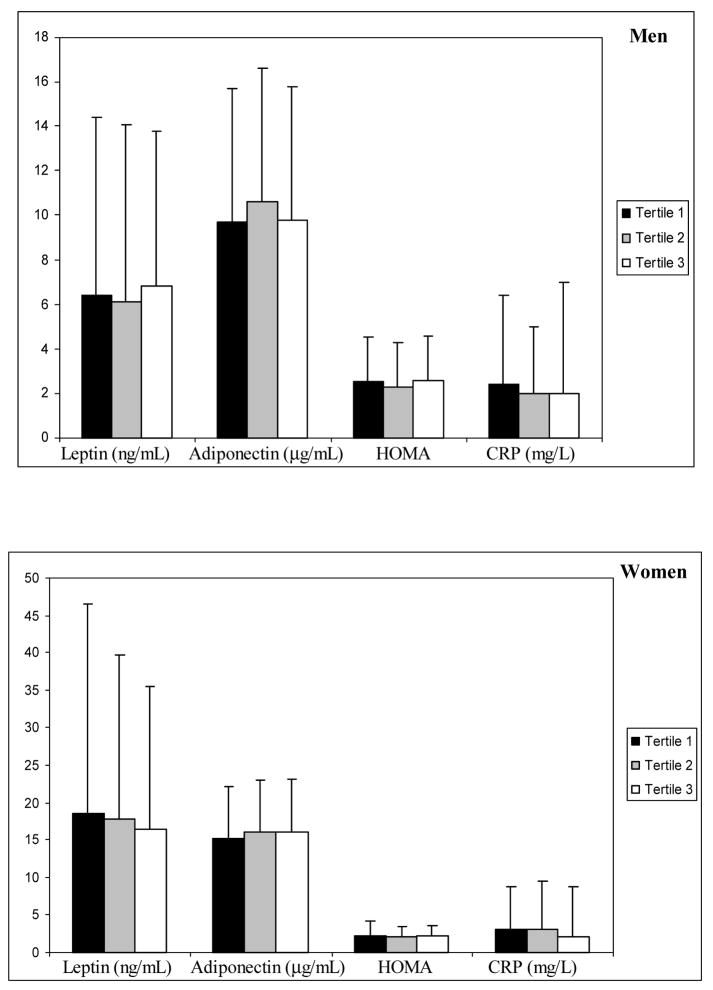
Mean arm muscle area at one year follow up was 45 ± 11 cm2 in men and 29 ± 13 cm2 in women. There was no change in mean arm muscle area compared to baseline (mean differences were −1.0 ± 5.8 cm2 and 0.1 ± 7.8 cm2 for men and women, respectively). There were no differences in the levels of leptin, adiponectin, HOMA and CRP from baseline to one year follow up when assessed by tertiles of mean difference in arm muscle area. In addition, there were no associations between any of these metabolic factors and mean difference in arm muscle area at one year. Studies with longer duration of follow up may be needed to further evaluate these relationships.
Discussion
We explored the relationship of leptin, adiponectin, insulin resistance, and CRP with arm muscle area (a proxy of muscle mass) in a large cohort of patients with stages 3–4 CKD. After adjustment for BMI, higher levels of leptin and CRP were associated with lower arm muscle area. There was a trend toward an inverse relationship of adiponectin with arm muscle area, but no independent association with HOMA. However it must be noted that the most important determinant of arm muscle area in this cohort was BMI and that BMI was also correlated with leptin, adiponectin, CRP and HOMA, thus potentially confounding these relationships.
Bigger body size is usually accompanied by a combination of higher BMI (a result of increased body fatness) and greater muscle mass. This would account for the fact that BMI was the single most important determinant of arm muscle area in this study, and that the univariate associations between the metabolic factors under study and arm muscle area were confounded by their association with BMI. We used arm muscle area measured by skinfold anthropometry as an indicator of the muscular component of lean body mass. This technique has been widely used in population studies because it is simple, affordable and reliable (27). The assessment of arm muscle area based on triceps skinfold thickness and arm circumference does take into account subcutaneous fat (28). However, in a disease condition like CKD, where a mismatch between fat and muscle is possible (29), the use of simple anthropometric techniques may be limited. Therefore, our findings should be taken with caution as alternative measures of body composition may be needed to clearly evaluate the contribution of total and regional body fat to muscle mass in this patient population. Nonetheless, our findings corroborate those observed in kidney failure and underline the possible role of these metabolic factors on muscle mass maintenance in the earlier stages of the disease, as described below.
Leptin and Arm Muscle Area
We found that high serum leptin levels were significantly associated with low arm muscle area, suggesting that leptin may be involved in the development of malnutrition in this patient population. There are some data in support of a role for hyperleptinemia in the development of malnutrition in CKD. In the general population leptin levels are directly associated with fat mass (30) and are believed to regulate adiposity by interacting with putative hypothalamic receptors and altering eating behavior (7). A few studies have demonstrated that hyperleptinemia in dialysis patients is associated with inadequate protein and energy intake and loss of lean tissue (8). Although, there are no studies confirming that leptin is the direct cause of anorexia and malnutrition in kidney disease, the anorexia of uremia has been linked with factors known to regulate appetite including leptin (31). Another potential mechanism by which hyperleptinemia may lead to malnutrition is through cytokine-mediated protein catabolism (32).
Adiponectin and Arm Muscle Area
Interestingly, we found a trend for an inverse association between adiponectin levels and arm muscle area, suggestive of a potentially deleterious effect of high adiponectin levels on nutritional status in CKD. In contrast to findings in the general population (33), high adiponectin levels are associated with increased mortality in patients with CKD (10) and heart disease (34). This may be due to the possible role of adiponectin on increase energy expenditure (12), which in catabolic states like CKD, may lead to accelerated muscle wasting and adverse disease outcomes.
Insulin Resistance and Arm Muscle Area
We did not find an association between insulin resistance (as measured by increased HOMA levels) and arm muscle area, despite the fact that insulin-mediated protein anabolism occurs largely in skeletal muscle (35). It is possible that this cohort of relatively healthy and well nourished patients with CKD stages 3–4 may be less insulin resistant than patients with kidney failure (CKD stage 5); and thus, we were unable to appreciate the effects seen with more severe insulin resistance. It is also possible that subtle increases in protein degradation that could contribute to a slow but sustained reduction in muscle mass might have not been detected with the anthropometric techniques used in this study.
C-Reactive Protein and Arm Muscle Area
Finally, we found that increased CRP levels were associated with a significant reduction in arm muscle area. Of note, CRP levels in the MDRD cohort were shown to approximate the levels reported in the general population using data from the Third National Health and Nutrition Examination Survey (NHANES III) and the same testing methodology (36). This finding suggests that this cohort may be relatively healthier and better nourished than the general CKD population with the same disease stage. A possible interpretation for these data is that the pathogenesis of malnutrition and muscle wasting in the earlier stages of kidney disease may in fact be associated with low levels of inflammation, thus requiring more attention.
There are three potential limitations of our analyses. First, the cross-sectional nature of this study precludes any assumptions of causality in the relationships examined. However, our results indirectly provide further support for the role of adipokines and inflammation as risk factors of muscle loss in patients with CKD stages 3–4. Second, the results observed may be unique to the MDRD Study cohort of relatively young and healthy patients with predominantly non-diabetic CKD. However, this cohort includes patients with a wide range of kidney function and detailed ascertainment of nutritional status. Third, the use arm muscle area by skinfold anthropometry, a widely used and valid technique in large population studies (27). Our results suggest that in patients with CKD, arm muscle area estimated by anthropometry is heavily confounded by BMI and subcutaneous fat. Accordingly, our univariate associations were confounded by BMI and the relationships between metabolic factors of interest and arm muscle area were unmasked only after adjustment for BMI.
In conclusion, our findings suggest a potential role for adipocyte-derived factors in the development of muscle wasting, a powerful prognostic indicator in CKD. Further studies are needed to understand the mechanisms underlying malnutrition in earlier stages of CKD and to develop preventive interventions.
Figure 2.
Gender distribution of each metabolic factor (leptin, adiponectin, Homeostasis Model Assessment –HOMA, and C-reactive protein –CRP) is shown by tertiles of mean difference in arm muscle area (one year minus baseline). Error bars represent mean (SD) for adiponectin and median (IQR) for log transformed variables (leptin, HOMA, and CRP).
Acknowledgments
This study was supported by UO1 DK 35073 and NIDDK K23 DK67303. This project was also supported in part by the U.S. Department of Agriculture, Agricultural Research Service, under agreement number 58-1950-001. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. The authors gratefully acknowledge the study population who participated in this study. We acknowledge the help of Frederick Van Lente, Ph.D., the Cleveland Clinic Foundation, in splitting and maintaining the frozen samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kopple JD. Pathophysiology of protein-energy wasting in chronic renal failure. J Nutr. 1999;129:247S–251S. doi: 10.1093/jn/129.1.247S. [DOI] [PubMed] [Google Scholar]
- 2.Dwyer JT, Larive B, Leung J, et al. Are nutritional status indicators associated with mortality in the Hemodialysis (HEMO) Study? Kidney Int. 2005;68:1766–76. doi: 10.1111/j.1523-1755.2005.00593.x. [DOI] [PubMed] [Google Scholar]
- 3.Beddhu S, Pappas LM, Ramkumar N, et al. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–72. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 4.Mitch WE, Du J. Cellular mechanisms causing loss of muscle mass in kidney disease. Semin Nephrol. 2004;24:484–7. doi: 10.1016/j.semnephrol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Trayhurn P, Bing C, Wood IS. Adipose tissue and adipokines--energy regulation from the human perspective. J Nutr. 2006;136:1935S–1939S. doi: 10.1093/jn/136.7.1935S. [DOI] [PubMed] [Google Scholar]
- 6.Dagogo-Jack S, Ovalle F, Landt M, et al. Hyperleptinemia in patients with end-stage renal disease undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int. 1998;18:34–40. [PubMed] [Google Scholar]
- 7.Kennedy A, Gettys TW, Watson P, et al. The metabolic significance of leptin in humans: gender-based differences in relationship to adiposity, insulin sensitivity, and energy expenditure. J Clin Endocrinol Metab. 1997;82:1293–300. doi: 10.1210/jcem.82.4.3859. [DOI] [PubMed] [Google Scholar]
- 8.Young GA, Woodrow G, Kendall S, et al. Increased plasma leptin/fat ratio in patients with chronic renal failure: a cause of malnutrition? Nephrol Dial Transplant. 1997;12:2318–23. doi: 10.1093/ndt/12.11.2318. [DOI] [PubMed] [Google Scholar]
- 9.Stefan N, Stumvoll M. Adiponectin--its role in metabolism and beyond. Horm Metab Res. 2002;34:469–74. doi: 10.1055/s-2002-34785. [DOI] [PubMed] [Google Scholar]
- 10.Menon V, Li L, Wang X, et al. Adiponectin and mortality in patients with chronic kidney disease. J Am Soc Nephrol. 2006;17:2599–606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- 11.Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16:1091–8. doi: 10.1681/ASN.2004090742. [DOI] [PubMed] [Google Scholar]
- 12.Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Alvestrand A, Smith D, et al. Insulin resistance in uremia. J Clin Invest. 1981;67:563–8. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenvinkel P, Heimburger O, Lindholm B, et al. Are there two types of malnutrition in chronic renal failure? Evidence for relationships between malnutrition, inflammation and atherosclerosis (MIA syndrome) Nephrol Dial Transplant. 2000;15:953–60. doi: 10.1093/ndt/15.7.953. [DOI] [PubMed] [Google Scholar]
- 15.Stenvinkel P, Heimburger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55:1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 16.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 17.Owen WF, Lowrie EG. C-reactive protein as an outcome predictor for maintenance hemodialysis patients. Kidney Int. 1998;54:627–636. doi: 10.1046/j.1523-1755.1998.00032.x. [DOI] [PubMed] [Google Scholar]
- 18.Bergstrom J, Lindholm B. Malnutrition, cardiac disease, and mortality: an integrated point of view. Am J Kidney Dis. 1998;32:834–41. doi: 10.1016/s0272-6386(98)70148-9. [DOI] [PubMed] [Google Scholar]
- 19.Sarnak MJ, Poindexter A, Wang SR, et al. Serum C-reactive protein and leptin as predictors of kidney disease progression in the Modification of Diet in Renal Disease Study. Kidney Int. 2002;62:2208–15. doi: 10.1046/j.1523-1755.2002.00677.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaizu Y, Ohkawa S, Odamaki M, et al. Association between inflammatory mediators and muscle mass in long-term hemodialysis patients. Am J Kidney Dis. 2003;42:295–302. doi: 10.1016/s0272-6386(03)00654-1. [DOI] [PubMed] [Google Scholar]
- 21.Yeun JY, Levine RA, Mantadilok V, et al. C-reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2000;35:469–476. doi: 10.1016/s0272-6386(00)70200-9. [DOI] [PubMed] [Google Scholar]
- 22.Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68:766–72. doi: 10.1111/j.1523-1755.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 24.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 25.Kopple JD, Greene T, Chumlea WC, et al. Relationship between nutritional status and the glomerular filtration rate: results from the MDRD study. Kidney Int. 2000;57:1688–703. doi: 10.1046/j.1523-1755.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Frisancho AR. New norms of upper limb fat muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34:2540–45. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 28.Heymsfield SB, McManus C, Smith J, et al. Anthropometric measurement of muscle mass: revised equations for calculating bone-free arm muscle area. Am J Clin Nutr. 1982;36:680–90. doi: 10.1093/ajcn/36.4.680. [DOI] [PubMed] [Google Scholar]
- 29.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 30.Considine RV. Regulation of leptin production. Rev Endocr Metab Disord. 2001;2:357–63. doi: 10.1023/a:1011896331159. [DOI] [PubMed] [Google Scholar]
- 31.Bergstrom J. Mechanisms of uremic suppression of appetite. J Ren Nutr. 1999;9:129–32. doi: 10.1053/JREN00900129. [DOI] [PubMed] [Google Scholar]
- 32.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171–5. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 34.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 35.Kimball SR, VaryL TC, Jefferson S. Regulation of protein synthesis by insulin. Annu Rev Physiol. 1994;56:321–348. doi: 10.1146/annurev.ph.56.030194.001541. [DOI] [PubMed] [Google Scholar]
- 36.Menon V, Wang X, Greene T, et al. Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis. 2003;42:44–52. doi: 10.1016/s0272-6386(03)00407-4. [DOI] [PubMed] [Google Scholar]