Abstract
Regulatory T cell responses to infectious organisms influence not only immunity and immunopathology, but also responses to bystander antigens. Mice infected with the gastrointestinal nematode parasite Heligmosomoides polygyrus show an early Th2-dominated immune response (days 7–14), but by day 28 a strongly regulatory profile is evident with antigen-specific IL-10 release and elevated frequency of CD4+ T cells bearing surface TGF-β. CD4+CD25+ T cells from infected mice show enhanced capacity to block in vitro effector T cell proliferation. CD4+CD25+ cell numbers expand dramatically during infection, with parallel growth of both CD25+Foxp3+ and CD25+Foxp3– subsets. CTLA-4 and glucocorticoid-induced tolerance-associated receptor, also associated with regulatory T cell function, become more prominent, due to both expanded CD25+ cell numbers and increased expression among the CD25– population. Both intensity and frequency of CD103 expression by CD4+ T cells rise significantly, with greatest expansion among CD25+Foxp3+ cells. While TGF-β expression is observed among both CD25+Foxp3+ and CD25+Foxp3– subsets, it is the latter population which shows higher TGF-β staining following infection. These data demonstrate in a chronic helminth infection that Foxp3+ regulatory T cells are stimulated, increasing CD103 expression in particular, but that significant changes occur to other populations including expansion of CD25+TGF-β+Foxp3– cells, and induction of CTLA-4 on CD25– non-regulatory lymphocytes.
Keywords: Mesenteric lymph node, Parasitic helminth, T cells
Introduction
Long-lived helminth parasite infections develop an intriguing balance with the immune system of their host 1, 2. Their continued survival in an immunologically sufficient environment may be ascribed in part to interference with immune activation and attack 3, 4. However, there is increasing evidence that the infected host develops a form of immunological ‘tolerance’ to parasite antigens, which may selectively mute certain effector mechanisms 5. The possibility that susceptibility to helminth infections may be mediated, in part, by regulatory T cells (Treg) is supported by recent work showing that antibody treatment to Treg markers results in heightened anti-parasite responsiveness and clearance of adult worms in Litosomosoides sigmodontis infection 6.
Immunological down-modulation during infection is also important in protecting the host from the more pathological outcomes of infection. In the case of schistosomiasis, egg production can cause hepatic granulomatous disease in chronically infected hosts 7. Immunopathology is controlled initially by the regulatory cytokine IL-10, as IL-10-deficient mice succumb to acute liver inflammation 8, while chronic granulomatous fibrosis can be suppressed by T cells transfected with the forkhead box transcription factor p3 (Foxp3) 9, which is functionally associated with Treg activity 10, 11.
The ability of helminth infection to modulate responses to unrelated bystander antigens is well established 12, 13. More recently, it has been recognised that infection can alter the pathological outcome of autoimmune 14, 15 and allergic 16 challenge. Indeed, we recently showed that infection with the murine gastrointestinal nematode Heligmosomoides polygyrus dampens immune responsiveness to unrelated allergens (ovalbumin and the house dust mite antigen Der p1) in a manner dependent upon CD4+CD25+ T cell activity, but independent of the action of IL-10 17. Moreover, CD4+CD25+ T cells from the mesenteric lymph nodes (MLN) of H. polygyrus-infected, allergen-naïve mice were able to confer suppression of allergy when transferred to uninfected, allergen-sensitized recipients, demonstrating their potent regulatory capacity 17.
Two cardinal characteristics associated with human helminthiases are reproduced in the H. polygyrus model of infection. First, this parasite is known to induce a dominant Th2 response 18–23, while secondly it provides an excellent example of generalised down-regulation of immune responsiveness 24–26, attributable in part to the activity of CD4+CD25+ Treg 17. In the present study, we follow in detail the evolution of both Th2 and Treg parameters over the course of infection, with particular focus on CTLA-4 (CD152), glucocorticoid-induced tolerance-associated receptor (GITR), CD103, TGF-β and Foxp3 within both CD4+CD25+ and CD4+CD25– subsets, as well as the functional characteristics of Treg populations.
Results
H. polygyrus generates a typical Th2 response early in infection
H. polygyrus is a natural gastrointestinal nematode parasite of mice, which follows a direct transmission cycle 27. Orally ingested larvae invade the intestinal mucosa, from where, 9–11 days post-infection, they emerge as adult worms 28. Subsequently, in most strains of mice, they survive as chronic, luminal-dwelling infections that may persist for as long as 300 days 29.
To analyse the adaptive immune response to H. polygyrus, we first characterised the cytokine profile of MLN and spleen cells in response to parasite antigen challenge in vitro, over the course of a 10-wk infection. As shown in Fig. 1, by day 7 there is a substantial Th2-type response marked by antigen-specific IL-4, IL-5, IL-9 and IL-13 evident in the MLN. Th2 responsiveness remains for the life of the infection although generally down-modulated after day 21. Splenic responses are slower to evolve but follow a similar pattern of Th2 responsiveness, as previously reported 18–23.
Figure 1.
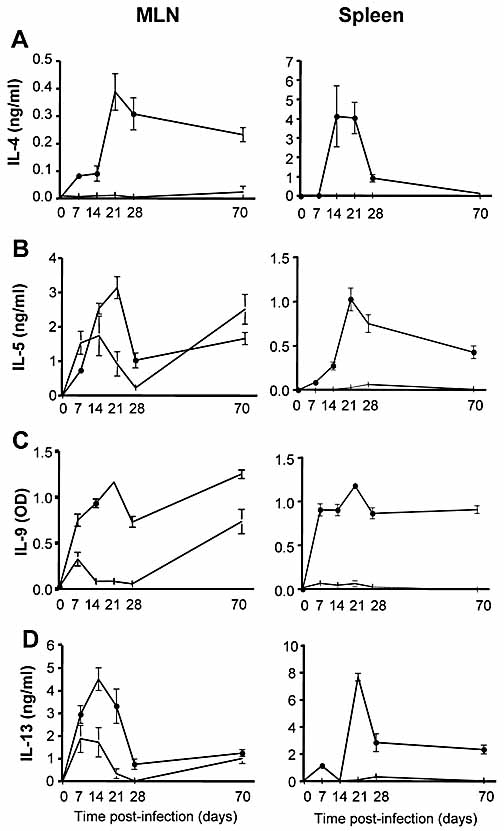
Elevated Th2 cytokine responsiveness in H. polygyrus infection. Parasite antigen-specific cytokine responses are presented from MLNC (left) and spleen cells (right) taken at day 7–70 post-infection. Cells were cultured for 48 h in medium alone (open symbols) or H. polygyrus antigen (solid symbols). Panels (A–D) present respectively IL-4, IL-5, IL-9 and IL-13. Data represent means ± SE from groups of five mice assayed individually; day 0 represents all naïve mice (five for each time point).
In contrast, Th1-type responses are relatively feeble. As shown in Fig. 2A, parasite-specific IFN-γ responses in vitro are only detectable at day 70, when infection is waning. A nascent Th1 reaction at day 7 can be detected at the mRNA level, evident by raised T-box family transcription factor expressed in T cells (T-BET) expression in RT-PCR (Fig. 2B), at the stage when larval parasites are still resident in the intestinal mucosa. However, by day 28, when adult worms are established in the gut lumen, this T-BET has been replaced by the Th2-promoting factor GATA-3 (Fig. 2B). A further reflection of Th2 polarisation is in the isotype balance of anti-H. polygyrus serum antibodies. As previously reported 30, infection stimulates high levels of IgG1, but no detectable IgG2a (Fig. 2C).
Figure 2.
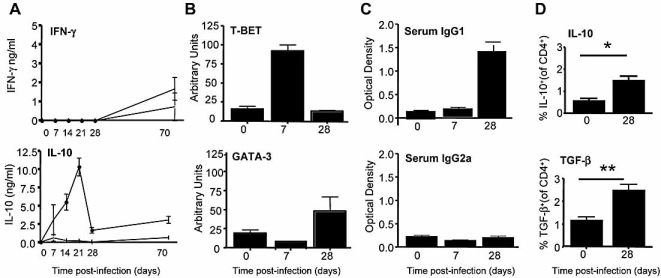
Nascent Th1 and sustained regulatory cytokine responses in H. polygyrus infection. (A) IFN-γ and IL-10 release from MLNC directly stimulated ex vivo, taken at days 7–70 post-infection and cultured for 48 h in medium alone (open symbols) or H. polygyrus antigen (solid symbols). (B) T-BET and GATA-3 real-time PCR in MLN from naïve and infected mice. CD4+ T cells were purified from MLN, RNA was extracted and real-time PCR performed on the resulting cDNA. (C) H. polygyrus-specific serum antibodies of IgG1 and IgG2a isotypes measured by ELISA. (D) Intracellular IL-10 (left) and surface TGF-β (right) staining in naïve and day 28-infected MLN CD4+ T cells determined by flow cytometry. Data represent means ± SE from groups of five mice assayed individually; day 0 represents all naïve mice (five for each time point). *p < 0.05, **p < 0.01.
Regulatory cytokine expression
MLN cells (MLNC) from H. polygyrus mice respond briskly to parasite antigen challenge in vitro with IL-10 release over the course of infection (Fig. 2B). Moreover, ex vivo staining of MLNC from day 28-infected mice shows significant increases in both intracellular IL-10 and surface-bound TGF-β (Fig. 2D). These findings are consistent with other reports of elevated antigen-specific and serum TGF-β in mice infected with H. polygyrus 31.
Expansion of CD25+ T cells during infection
Expression of the IL-2Rα chain, CD25, is a widely used but not exclusive marker for Treg 32. Using flow cytometry, we found that total CD4+CD25+ cell numbers expand dramatically in the draining MLN (Fig. 3A), although total LN cell counts are expanding during the same period. A smaller rise in CD25+ cells occurs in the spleen, particularly from day 21 onwards (data not shown). The intensity of CD25 expression on positive cells does not alter appreciably (Fig. 3B). When considered as a proportion, it can be seen that CD4+CD25+ cells outgrow CD4+CD25– cells from day 7 onwards and remain in significant excess over uninfected controls throughout infection (Fig. 4C).
Figure 3.
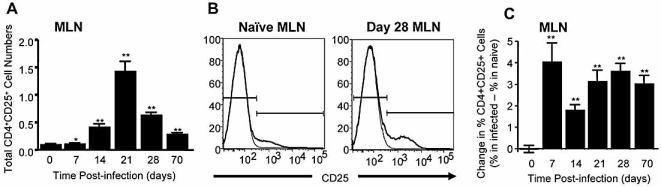
Expansion of CD25-expressing cells within the total CD4+ T cell population during H. polygyrus infection. (A) Total numbers of CD4+CD25+ T cells from day 7–70 post-infection in MLN. (B) Representative histograms of CD25 expression within MLN (left) or splenic (right) CD4+ cells from day 28-infected (black) or naïve (grey) mice; isotype controls are shown as thick black lines. (C) Changes in proportion of CD25-expressing CD4+ T cells relative to total CD4+ cells over 70 days of infection. For each point in time, the percentages of CD4+ T cells which also expressed CD25 among MLNC from infected and naïve animals were determined; the arithmetic difference between infected and naïve in percentage frequency at each time point was then calculated. The mean naïve level was 14.36% (SD = 0.80). For (A, C), Mann–Whitney tests were performed (n.s., no significant difference; *p<0.05, **p<0.01). Data represent means ± SE from groups of five mice assayed individually, compared with naïve mice at the same assay time. Day 0 represents all naïve mice (five for each time point).
Figure 4.
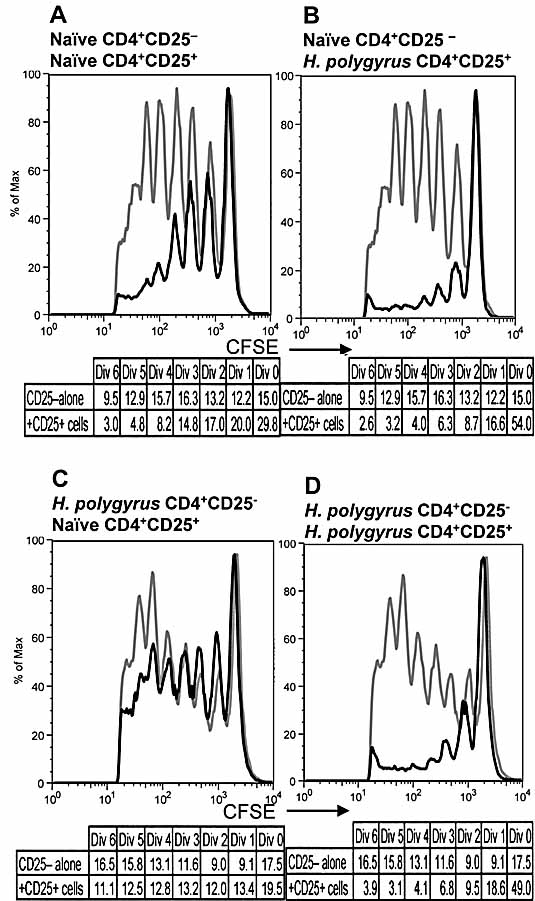
CD4+CD25+ T cells from H. polygyrus-infected mice show more potent suppression of CD25– effector cell proliferation. Proliferation of 5×104 CD4+CD25– T cells in response to in vitro Con A stimulation was measured by CFSE (grey lines), in populations from naïve (A, B) and day 28-infected (C, D) MLNC. In parallel cultures, equal numbers of CD4+CD25+ MLNC were added to the CD4+CD25– CFSE-loaded T cells. MLNC from infected mice (B, D; black lines) show more profound suppression than equal numbers of cells from uninfected controls (A, C; black lines). Tables beneath each graph give percentage of CD25– cells in each round of division when stimulated in the absence (upper row) or presence (lower row) of CD25+ cells. Data represent pooled cells from groups of five mice.
In vitro suppressive activity by CD25+ Treg
A common test of functional Treg activity is their ability to block the proliferative response of effector T cells to antigen or mitogen stimulation 33. We accordingly assessed whether sorted CD4+CD25+ MLNC from infected animals were able to suppress the proliferation of CFSE-loaded naïve, CD4+CD25– MLNC, responding to Con A. We observed that, on a per-cell basis, CD4+CD25+ MLNC, taken 28 day post-infection, were substantially more suppressive than cells with similar phenotype from naïve animals (Fig. 4A, B).
In parallel experiments we also noted that CD4+CD25– T cells from infected mice showed greater resistance to proliferative inhibition, and indeed were largely refractory to suppression by CD4+CD25+ MLNC from naïve mice (Fig. 4C). However, CD4+CD25– effectors from H. polygyrus-infected animals remained susceptible to inhibition by the more ‘activated’ regulatory cells from infected mice (Fig. 4D, E).
Foxp3 expression levels remain relatively constant during infection
A key marker for natural Treg is the transcription factor Foxp3, which is expressed by the majority of CD25+ T cells 10, 11, 34. As previously reported 17, we found that H. polygyrus infection results in a modest increase in CD4+CD25+Foxp3+ cells (from 7.4% of all CD4+ cells at day 0 to 9.7% at day 28; Fig. 5A). Expansion occurs rapidly following infection (Fig. 5B), although, due to parallel expansion of CD25+Foxp3– cells in infection, there is in fact a small diminution in the proportion of CD4+CD25+ cells which express Foxp3 (Fig. 5C). It was also apparent that the intensity of Foxp3 expression within the Foxp3+CD4+CD25+ T cell population does not increase (Fig. 5D). Thus, the increment in CD25+ cells during infection is not accompanied by preferential Foxp3 induction and represents an expansion of both CD4+CD25+Foxp3+ and CD4+CD25+Foxp3– T cells. This latter phenotype may represent either an activated effector cell (which could be stimulated in the environment of chronic infection), or a Foxp3– ‘adaptive’ or inducible Treg.
Figure 5.
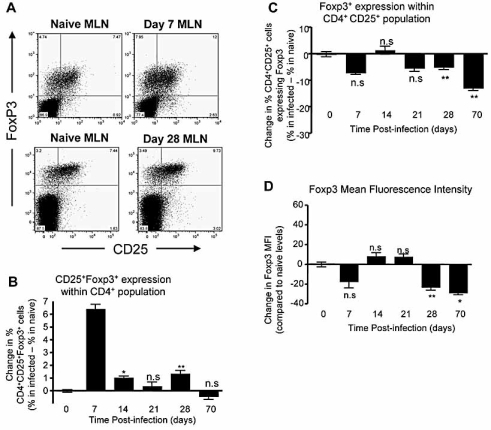
CD4+ T cell Foxp3 expression levels remain relatively constant during H. polygyrus infection. (A) Bivariate flow cytometry analysis of CD25 and Foxp3 expression in CD4+ T cells in naïve (far left), day 7-infected (left) and naïve (right) and day 28-infected (far right) MLNC. (B) Time course of expression of CD25+Foxp3+ as percentage of total CD4+ cells in MLN from 7–70 days of infection. The mean naïve level was 6.43% (SD = 2.27). (C) Foxp3+ T cells as proportion of total CD4+CD25+ T cells in naïve and day 28-infected MLN. The mean naïve level was 67.2% (SD = 17.1). (D) Foxp3 expression intensity over the course of infection. The percentage difference in MFI relative to uninfected values for Foxp3 was calculated. For (B–D) data represent means ± SE from groups of five mice assayed individually; day 0 represents all naïve mice (five for each time point). Mann–Whitney tests were performed (n.s., no significant difference; *p<0.05, **p<0.01).
Increased frequency of CTLA-4 and GITR expression during infection
Two surface markers closely associated with the Treg phenotype are CTLA-4 35 and GITR 36. Both were measured in MLN and spleen cells over the 70-day time course. At day 28, for example, CTLA-4 is expressed by 23% of CD4+ T cells, compared to 12% in naïve controls (Fig. 6A). This increase is represented by a relatively constant per-cell level within the CD4+CD25+ population, together with a significant rise in expression among CD4+CD25– cells (Fig. 6A). Moreover, within the CD4+CD25+ compartment (Fig. 6B) CTLA-4 staining increases more than twofold among the Foxp3– subset, while actually declining in Foxp3+ cells. A similar, though less marked, trend is seen with GITR staining; overall GITR expression within the CD4+ T cell population rises from 8.5% in naïves to 13.8% in day 28-infected mice (Fig. 6C). Again there is a significant rise in the proportion of CD4+CD25– cells expressing GITR, and within the CD4+CD25+ subset the expansion in GITR+ cells occurs with the Foxp3– population (Fig. 6D). These changes are sustained over the longer term course of infection (Fig. 6E, F). Thus, the uplift in CTLA-4 and GITR is observed primarily, if not totally, within cells of a non-regulatory phenotype.
Figure 6.
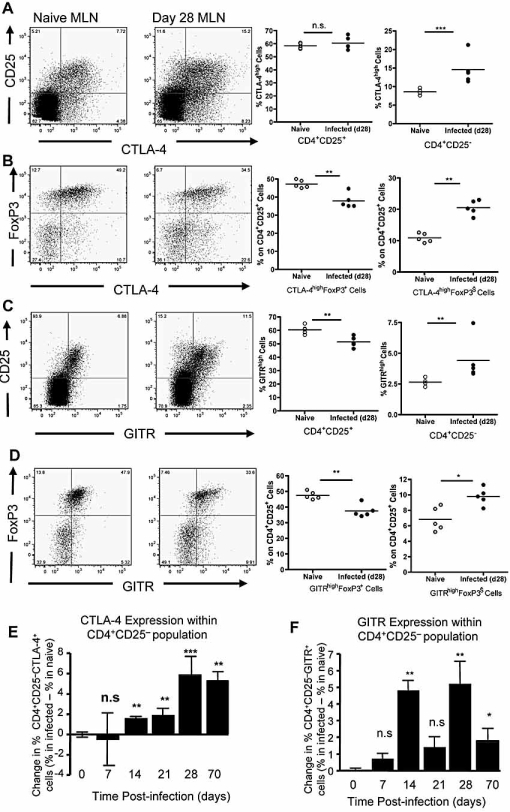
CTLA-4 and GITR expression levels increase on CD4+CD25– cells, but not on CD4+CD25+ cells, during H. polygyrus infection. (A) CTLA-4 expression measured by flow cytometry on MLNC. (Left panels) CTLA-4 and CD25 bivariate plot from naïve and day 28-infected mice; (right panels) percentage of CTLA-4high T cells in the CD4+CD25+ and CD4+CD25– subsets. (B) CTLA-4 expression measured by flow cytometry on MLNC. (Left panels) CTLA-4 and Foxp3 bivariate plot from naïve and day 28-infected mice; (right panels) percentage of CTLA-4high T cells in the CD25+Foxp3+ and CD25+Foxp3– subsets. (C) GITR expression, measured as for CTLA-4 in Fig. 7A. (D) GITR expression, measured as for CTLA-4 in Fig. 7B. (E) Frequency of CTLA-4 expression in CD4+CD25– cells over the course of infection. Mean naïve level = 6.58% (SD = 1.82). (F) Frequency of GITR expression in CD4+CD25– cells over the course of infection. Mean naïve level = 5.45% (SD = 4.75). Data represent means ± SE from groups of five mice assayed individually. Mann–Whitney tests were performed (n.s., no significant difference; *p<0.05, **p<0.01, ***p<0.001.
CD103 and TGF-β expression is raised in frequency and intensity by infection
We also examined expression of CD103 (the integrin αEβ7) and of the regulatory cytokine TGF-β in the CD4+ populations. In the MLN, there were significant increases in the proportion of CD4+CD25+ T cells which express CD103, returning to control levels by day 70 of infection (Fig. 7A); a similar pattern was observed in splenic populations (data not shown). While most CD103-expressing cells were in the CD4+CD25+ subset, there were also small but significant increases in the frequency of CD103+ among CD25– cells. Moreover, infection induced a substantial upshift in intensity of CD103 expression, reaching levels approximately 50% higher than in naïve populations (Fig. 7B).
Figure 7.
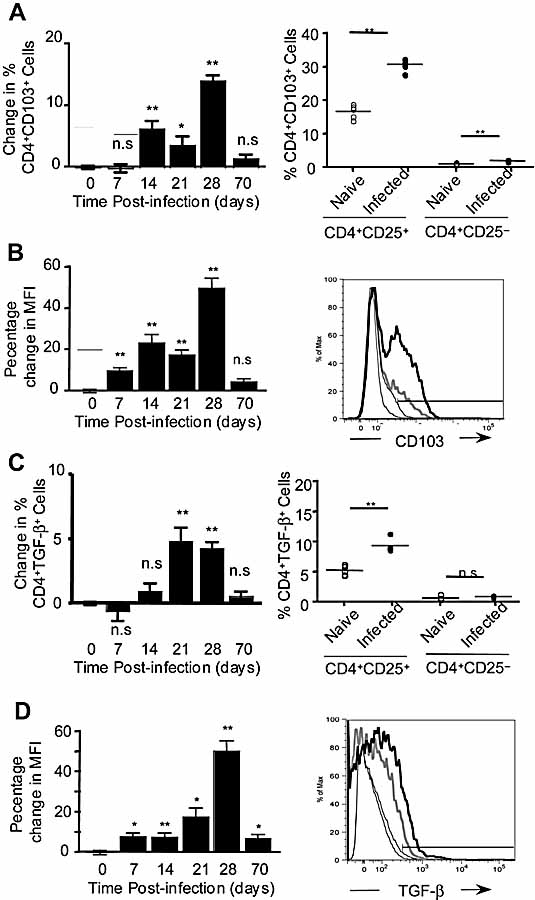
CD103 and TGF-β expression is raised in frequency and intensity by H. polygyrus infection. MLNC were stained for CD4, CD25, CD103 and TGF-β and analysed by flow cytometry. For CD103 and TGF-β, the percentages within total CD4+CD25+ T cell populations were calculated, as well as the percentage change in MFI for each infected group compared to the corresponding naïve group; Mann–Whitney tests were performed (n.s., no significant difference; *p<0.05, **p<0.01). Data represent means ± SE from groups of five mice assayed individually. Day 0 represents all naïve mice (five for each time point). (A) CD103+ T cell numbers over the time course (left), and as proportion of total CD4+CD25+ or CD4+CD25– T cells in naïve and day 28-infected MLNC (right). Over the time course, mean naïve level = 18.3% (SD = 4.09). (B) CD103 expression levels over the time course (left) and in representative MLNC from naïve (grey line) and day 28-infected (thick black line) mice (right); isotype controls are shown as thin black lines. (C) TGF-β+ cell numbers over the time course (left), and as proportion of total CD4+CD25+ or CD4+CD25– T cells in naïve and day 28-infected MLNC (right). Over the time course, mean naïve level = 8.01% (SD = 2.67). (D) TGF-β expression levels over the time course (left), and in representative MLNC from naïve (grey line) and day 28-infected (thick black line) mice (right); isotype controls are shown as thin black lines.
A similar profile was observed for surface-bound TGF-β. A modest, but significant, rise in TGF-β staining occurred in both MLN and splenocytes, peaking at day 21–28; thus by day 28 nearly 10% of all CD4+CD25+ T cells were TGF-β+, although no change was seen in the very low levels of TGF-β among CD4+CD25– cells (Fig. 7C). There was also a measurable rise in fluorescence intensity, evident only in the CD4+CD25+ population, over the first 28 days of infection (Fig. 7D).
CD25+Foxp3+ cells express higher CD103, and CD25+Foxp3– cells show raised TGF-β expression
Since the CD4+CD25+ population from infected mice displayed increases in both CD103 and TGF-β, co-staining was performed for these markers and for the Foxp3 transcription factor. These analyses showed, firstly, that in naïve MLNC approximately two-thirds of the CD103+ cells are Foxp3+ (Fig. 8A), as are a similar proportion of the TGF-β+ cells (Fig. 8B). However, infection generated a substantial and significant increase in CD25+CD103+Foxp3+ cells (Fig. 8A), while the frequency of TGF-β+Foxp3+ cells did not differ between naïve and infected mice (Fig. 8B). Moreover, we found that both CD103 and TGF-β staining occurs on a significant subset of CD25+Foxp3– cells, and indeed comparison of naïve and infected MLN shows significant increases in CD103+Foxp3– (Fig. 8A) and in TGF-β+Foxp3– cells (Fig. 8B).
Figure 8.
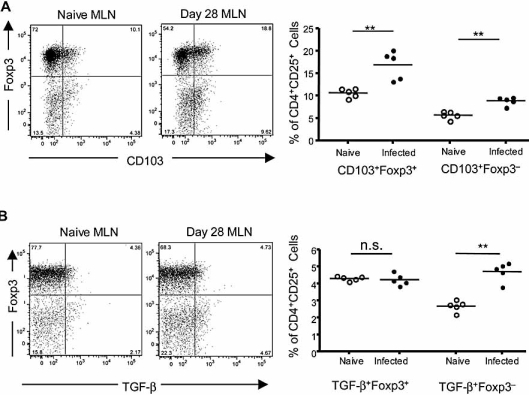
By day 28 of infection, CD103+Foxp3+ cells increase whilst TGFβ+Foxp3+ do not. Cells were stained for CD4, CD25, CD103, Foxp3 and TGF-β. Levels of CD103, TGF-β and Foxp3 expression were determined within the CD4+CD25+ population. Mann–Whitney tests were performed (n.s., no significant difference; **p<0.01). (A) CD103 expression plotted by bivariate analysis against Foxp3 staining, in naïve and day 28-infected MLNC (left), and percentage expression of CD103+Foxp3+ and CD103+Foxp3– cells within CD25+ T cell populations (right). (B) Surface TGF-β expression plotted by bivariate analysis against Foxp3 staining, in naïve and day 28-infected MLNC (left), and percentage expression of TGF-β+Foxp3+ and TGF-β+Foxp3– cells within CD25+ T cell populations (right).
Discussion
It is now evident that Treg are active at many points in the control of immune responses against pathogens 37–41. CD4+CD25+ Treg block protective immunity in animal models of malaria 42 and filariasis 6, but are also required to minimise pathology caused by the response to pathogen invasion 43–45. This critical balance between benefit and harm is played out against a backdrop of pathogens which are likely to have evolved strategies to favour Treg priming, recruitment and survival 41. For the host, the optimal regulatory response may permit residual parasite survival, so providing ongoing antigenic stimulation without disease 38. Thus, whether considering susceptibility, pathology or immunity to pathogens, the contribution of Treg populations can prove decisive.
Helminth parasitic diseases are typically slowly evolving and chronic in nature, often associated with immune down-regulation 1, 5. Evidence from human lymphatic filariasis, onchocerciasis and schistosomiasis records a down-modulation of immunity which is consistent with the activity of Treg-like cells, involving IL-10, TGF-β and CTLA-4 1, 46–49. In animal models of filariasis 50 and schistosomiasis 51, 52, Treg phenotype populations develop following infection, whilst in infection with the murine gastrointestinal nematode H. polygyrus 17, functional regulation by CD4+CD25+ T cells suppresses the bystander response to an allergic provocation. In the current study, we also show that this phenotype is enhanced, following infection, in its ability to suppress the polyclonal proliferative response of CD25– effector cells. However, the origin and specificity of the CD4+C25+ population generated by this or other helminth infections have yet to be delineated.
CD25 (IL-2Rα) is not a unique marker for Treg, being also expressed on activated effector T cells 33. For this reason we analysed additional functional and phenotypic markers, including the transcription factor Foxp3 10 and the inhibitory/stimulatory co-receptors CTLA-4 35 and GITR 36, 53. Expression of Foxp3, as well as the production of the suppressive cytokines IL-10 and TGF-β, are considered to distinguish natural or adaptive Treg subsets 54.
Broadly, natural Treg arise during the normal process of maturation in the thymus, are selected on the basis of specificity for self antigens, express surface CD25 and Foxp3, and employ cell contact-dependent suppressive mechanisms. In contrast, adaptive Treg are thought to develop from naïve (CD25–, Th0) mature peripheral populations in response to specific stimulatory conditions such as sub-optimal signalling from accessory cells. Adaptive Treg include those designated Tr1 55 and Th3 56, produce IL-10 or TGF-β, and have induced CD25 expression. Whether these cells also initiate Foxp3 expression is controversial. No induction could be found either in Tr1 induced in vitro with IL-10, or in vivo by intranasal tolerisation 57. However, in vitro CD4+CD25– T cells can be induced to express CD25 and Foxp3 following stimulation with TGF-β 58–60, and in a T cell receptor-transgenic, thymectomised mouse, de novo Foxp3 expression was induced in a regulatory CD25+ population exposed to low-dose antigen delivered by osmotic pump 61. Hence, Foxp3 expression delimits a subset containing all naturally arising Treg and, possibly, a proportion of adaptive Treg.
In H. polygyrus infection, there is a preferential expansion of CD25-expressing cells without a proportional increase in Foxp3+ cells, and yet the functional regulatory activity of the CD25+ T cell population is greatly amplified in infected mice. Hence, the increment in CD25+Foxp3– T cell numbers is unlikely to represent effector cell expansion alone. A plausible hypothesis is that many of the CD25+Foxp3– cells are adaptive Treg, with specificity for parasite antigens, which have arisen from naïve precursors with induction of expression of CD25 rather than that of Foxp3. In addition, there is good evidence from our work and that of others, that production of IL-10 and TGF-β is substantially heightened in H. polygyrus infection 17, 31.
Treg-derived IL-10 is a major determinant in systems where Th1 immune responses are protective, such as murine infection with Leishmania major 38. In schistosome infections which drive dominant type-2 responsiveness, however, only a small proportion of the IL-10 emanates from CD25+Foxp3+ T cells 51, 52, and even though IL-10 is important in the overall control of immune pathology in schistosomiasis, granuloma modulation is IL-10-independent 8. Moreover, IL-10 is a critical promoter of strong Th2 responses in many helminth systems 62, and unlike the Th1 setting does not fulfil a purely down-regulatory role. This is confirmed in the case of H. polygyrus, as the ability of CD25+ Treg to suppress airway allergy in infected mice is undiminished by anti-IL-10R antibody, while MLNC from IL-10-deficient infected mice transfer suppression of allergy into uninfected animals 17. Hence, in the mouse at least, IL-10 does not appear to be a primary mechanism for helminth-associated Treg function.
TGF-β, however, remains a credible candidate for the functional Treg product in this system, with respect to both the induction and survival of Treg 63, and the down-modulation of effector T cell populations 64. We found significantly raised surface TGF-β staining over the course of infection, and others have reported parasite antigen-specific TGF-β release in similar experiments 31. Most recently, Doligalska and colleagues 65 have reported that anti-TGF-β antibody treatment greatly reduces egg production and worm survival in mice, indicating an important role for TGF-β in parasite immune evasion. Interestingly, in our experiments, increased TGF-β was observed within the CD25+Foxp3– subset, and not among CD25+Foxp3+ cells. Hence, the cells induced to express TGF-β may be adaptive Treg most similar to the Tr1/Th3 type described in other systems, in particular the TGF-β-secreting cells derived from the MLN of orally tolerised mice 56.
We also observed a marked increase in CTLA-4 expression among T cells from infected mice. CTLA-4 is an inhibitory component of the co-stimulation machinery associated with T cell receptor signalling, and may act by competing for ligand with the CD28 stimulatory receptor, and by raising the activation threshold of T cells through the immunological synapse 66. Hence, when CTLA-4 is blocked by antibody treatment in vivo, parasite expulsion is accelerated 67. Interestingly, the more substantial upshift in CTLA-4 staining was seen on CD25– cells, and this change occurred later in the course of infection than the expansion in CD25+ cell numbers. It is plausible, therefore, that the CD25–CTLA-4+ phenotype represents an anergic effector cell, as postulated in both human 48, 49 and mouse 6 helminth infections, which develops subsequent to and under the influence of the more rapidly arising Treg response to infection.
GITR, originally identified as a marker up-regulated on Treg, is a receptor thought to activate both regulatory and effector cells on ligation. In H. polygyrus infection, levels of GITR expression increase albeit less than observed for CTLA-4. In particular, CD25– T cells as well as Foxp3–CD25+ cells show small but significant uplifts in the frequency of GITR expression. The induction of GITR on the CD25– population is similar to that observed in the tissue helminth infection, L. sigmodontis 6.
Treg may act either, or both, at the induction stages of the immune response and at the inflammatory phase in the tissues. In H. polygyrus infections, priming to allergens is unaffected and infection-generated Treg transferred into fully primed mice suppress inflammation in the lung 17. If infection-induced Treg act primarily at the site of inflammation, this may be reflected in the pattern of homing marker expression 68, 69. In this context, the marked up-regulation of CD103 on the infected CD4+CD25+ T cell subset may have particular functional significance. CD103 is a homing marker and is expressed on 20–30% of Treg in lymphoid organs 53. CD103, therefore, may not be a mechanistic mediator of suppression, but rather a pre-requisite for Treg to traffic into, and remain at, sites of inflammation. In a model of leishmaniasis, CD103 is induced and maintained on Treg following or just prior to their arrival in inflamed tissues 70. Hence, CD103 does not define a lineage of CD25+ Treg with distinct properties, but rather a subset capable of homing into the site of infection. The expression of CD103 is positively regulated by TGF-β 71. Surface TGF-β levels increase during infection, and this may provide a mechanism by which CD103 is continuously up-regulated.
In conclusion, we show here that H. polygyrus induces significant phenotypic changes in distinct subsets of cells, including one with functional suppressive properties and some characteristic markers associated with Treg. It may be significant that the Foxp3-expressing population is only one of the players in the system, and we cannot yet distinguish whether ‘natural’ and ‘adaptive’ Treg expand independently in the context of this infection, or the evolution of the adaptive phenotype is dependent upon the pre-existing natural Treg population. Further characterisation of these regulatory cells, their antigen specificity and their mechanisms is therefore imperative, as is the analysis of the potentially anergic effector T cell population which we have postulated. Nematode infections are of particular importance since they affect over two billion people worldwide, mostly in poverty-stricken regions where numerous other infections are rife. Unravelling the effects of dampened immune responses, due to increased regulatory mechanisms triggered by worms, on disease progression and outcome in co-infected people would open new avenues for treatment, control and eradication of these prevalent diseases.
Materials and methods
Mice and parasites
Female BALB/c mice, 6–8 wk of age and maintained in individually ventilated cages, were infected with 200 H. polygyrus bakeri infective L3 larvae using a gavage tube. In time-course experiments, a matched group of naïve animals was analysed at each time point taken.
H. polygyrus antigen and ELISA
H. polygyrus antigen was prepared by homogenising adult worms in PBS, which was centrifuged (13 000 × g, 10 min); the supernatant was filtered (0.2 µm Millex) and stored at 1.5 mg/mL at –80°C. Antigen-specific antibody responses were determined by ELISA. Multisorp (Nunc) plates were coated with 5 μg/mL H. polygyrus antigen in 0.06 M carbonate buffer pH 9.6, overnight at 4°C. Plates were blocked with 5% BSA (fraction V, Gibco) for 2 h at 37°C. Sera were diluted in TBS/0.05% Tween and added to wells overnight at 4°C. Antigen-specific IgG isotypes were detected with HRP-conjugated goat anti-mouse IgG1 (1070-05, Southern Biotechnology) and anti-IgG2a (1080-05), with ABTS peroxidase substrate (50-62-00, KPL).
In vitro restimulation and cytokine assays
Unfractionated LN and spleen cells were cultured at 1×107/mL in 96-well plates (3799, Costar) for 48 h in RPMI 1640 medium (Gibco), 10% FCS, 1% l-glutamine, 1% penicillin/streptomycin (supplemented RPMI 1640) in the presence of 10 μg/mL H. polygyrus antigen or 1 μg/mL Con A (Sigma). Cytokines in supernatants were measured by ELISA according to suppliers’ guidelines. Capture antibodies for IL-4 (11B11, 4 μg/mL), IL-5 (TRFK5, 2 μg/mL), IL-10 (JES5-2A5, 4 μg/mL) and IFN-γ (R46A2, 3 μg/mL) were produced in-house or by Pharmingen. Capture antibody for IL-13 (38213, 2 μg/mL) was from R&D Systems, and that for IL-9 (229.4, 5 µg/mL) kindly provided by Dr. Melanie Leech (IIIR, Edinburgh). Biotinylated detection antibodies were from Pharmingen: IL-4 (BVD6-24G2, 5 μg/mL), IL-5 (TRFK4, 2 μg/mL), IL-9 (D9302C12, 1 μg/mL), IL-10 (SXC-1, 2 μg/mL) and IFN-γ (XMG1.2, 0.5 μg/mL), or for IL-13, from Peprotech (rabbit polyclonal, cat. No. 500-P178Bt, 0.1 μg/mL).
Flow cytometry
LN and spleen cell suspensions were prepared for flow cytometry at 1×107/mL in supplemented RPMI 1640. Antibodies were diluted in PBS, 0.5% BSA (Sigma), 0.05% sodium azide, and added to cell suspensions (1×106–2×106 total cells) for 20 min at 4°C. For detection of CD4+CD25+ and CD4+CD25– cells, rat anti-mouse CD4 (L3T4, clone RM4-5, IgG2a, 1/100) and anti-CD25 (clone PC61, IgG1, CALTAG, 1/100) monoclonal antibodies were used. For intracellular IL-10 and CTLA-4 staining, cells were permeablised in cytofix/cytoperm, washed in perm/wash buffer (Pharmingen) and stained with rat anti-mouse IL-10 (JES5-16E3, rat IgG2b, 1/50) or CTLA-4 (UC10-4F10-11, 1/10) for 30 min. For staining Foxp3, cells were permeablised in cytofix/cytoperm for 1 h, washed in perm/wash buffer (eBiosciences) and stained with rat anti-mouse Foxp3 (FJK-16s, rat IgG2a, eBiosciences, 1/50) for 30 min. Surface-bound TGF-β, CD103 and GITR were detected using rat anti-mouse TGF-β1 (A75-3, IgG2a, 1/25), CD103 (M290, IgG2a, 1/100) and GITR (DTA-1, IgG2a, produced in-house, 1/250). Analysis was performed on an LSR II flow cytometer using FlowJo software (Tree Star). All fluorochrome-labeled antibodies were obtained from BD Pharmingen, unless otherwise stated.
CD4+ T cell enrichment and quantitative PCR
For CD4+ cell purification, cell suspensions were incubated at 1×108/mL with 10 μL CD4 (L3T4) microbeads (130-049-201, Miltenyi Biotech) per 107 cells and separated on MACS MS columns with pre-separation filters. RNA was recovered from purified CD4+ cells by the addition of Trizol (Invitrogen, 1 mL/106 cells), and extracted following the manufacturer's protocol. RT-PCR was performed with 1 μg RNA using murine Moloney leukaemia virus reverse transcriptase (Stratagene) and oligo(dT) primers (Promega). Transcript quantification was performed by real-time RT-PCR, using the LightCycler (Roche Molecular Biochemicals), with β-actin for normalisation.
PCR amplifications were in 10 μL, containing 1 μL cDNA, 1.2 μL MgCl2 (25 mM), 0.3 μL (10 μM) primers and 1 μL LightCycler-DNA SYBRGreen-I mix (10×). For Foxp3 amplification the QuantiTech SYBRGreen PCR Kit (Qiagen) was used, and PCR amplifications were performed in 10 μL, containing 1 μL cDNA, 0.5 μL (10 μM) primers and 5 μL SYBRGreen mix. The amplification of β-actin (5′-TGGAATCCTGTGGCATCCATGAAAC-3′, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′), T-bet (5′-GCCAGGGAACCGCTTATATG-3′, 5′-GACGATCATCTGGGTCACATTGT-3′), Smad-7 (5′-GCATTCCTCGGAAGTCAAGAGG-3′, 5′-TGCGGTTGTAAACCCACACG-3′), Foxp3 (5′-CCTGGCCTGCCACCTGGGATCAA-3′, 5′-TTCTCACAACCAGGCCACTTG-3′) and Gata-3 (5′-CTACGGTGCAGAGGTATCC-3′, 5′-GATGGACGTCTTGGAGAAGG-3′) was performed as follows: 30 s denaturation at 95°C, 5 s annealing at 55°C and 12 s elongation at 72°C, for 40–60 cycles. For T-bet the acquisition temperature was reduced to 84°C. Conventional curve analyses were performed according to the LightCycler instruction kit. Products were run on agarose to ensure that no genomic DNA amplification occurred.
CD25 enrichment and suppression assay
For CD4+CD25+ cell enrichment, CD4+ cells were negatively isolated using sheep anti-rat IgG beads (M540, Dynal) and biotinylated anti-MAC1 (0.5 μL/107 cells, M1/70.15), anti-CD8α (0.5 μL/107 cells, 53-6.72), anti-MHC class II (1 μL/107 cells, M5114), anti-B220 (0.5 μL/107 cells, RAB632) and anti-GR1 (3.33 μL/107 cells, RB6-8C5, BD Pharmingen). Antibody-bound beads and cell solutions were separated on a magnetic particle concentrator (Dynal MPC). Positive selection of CD25+ cells then employed PE-conjugated anti-CD25 (130-091-013, Miltenyi Biotech) and PE microbeads (130-048-801, Miltenyi Biotech), on MACS LS separation columns with pre-separation filters. CD25– cells obtained were stained with CFSE (5 μM, Sigma) and cultured with or without 5×104 CD4+CD25+ cells for 4 days in a 1:1 ratio, with 1×105 irradiated CD4– APC and in the presence of 1 μg/mL Con A.
Statistics
Mann–Whitney test was used for all statistical comparisons; p values less than 0.05 were considered significant. To compare the percentage of cells positive for the surface and intracellular markers studied, the difference in frequency of the marker was calculated between each infected group and its naïve group. For comparing intensity levels, the difference in each infected group compared to its naïve group was calculated as a percentage increase/decrease compared to the baseline naïve values.
Acknowledgments
C.A.M.F. was supported by an MRC Postgraduate Studentship, M.S.W. by a Wellcome Trust Prize Studentship, M.D.T. by an MRC Programme Grant (jointly held by Judith E. Allen and R.M.M.) and R.M.M. by a Wellcome Trust Research Leave Award and Programme Grant. DTA-1 cell line was kindly provided by Shimon Sakaguchi. The IL-9-secreting hybridoma cell line was kindly provided by Professor E. Schmitt; the IL-9 capture antibody was kindly donated by Richard Grencis and Melanie Leech.
Glossary
Abbreviations:
- Foxp3:
forkhead box transcription factor p3
- GITR:
glucocorticoid-induced tolerance-associated receptor
- MLNC:
MLN cells
- T-BET:
T-box family transcription factor expressed in T cells
References
- 1.Maizels RM, Yazdanbakhsh M. Regulation of the immune response by helminth parasites: Cellular and molecular mechanisms. Nat. Rev. Immunol. 2003;3:733–743. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 2.Mountford AP, Trottein F. Schistosomes in the skA balance between immune priming and regulation. Trends Parasitol. 2004;20:221–226. doi: 10.1016/j.pt.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Harnett W, Goodridge HS, Harnett MM. Subversion of immune cell signal transduction pathways by the secreted filarial nematode product, ES-62. Parasitology. 2005;130(Suppl):S63–S68. doi: 10.1017/S0031182005008164. [DOI] [PubMed] [Google Scholar]
- 4.Smith P, Fallon RE, Mangan NE, Walsh CM, Saraiva M, Sayers JR, McKenzie ANJ, et al. Schistosoma mansoni secretes a chemokine binding protein with antiinflammatory activity. J. Exp. Med. 2005;202:1319–1325. doi: 10.1084/jem.20050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor M, Allen JE. Helminth parasites: Masters of regulation. Immunol. Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 6.Taylor M, Le Goff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 2005;174:4924–4933. doi: 10.4049/jimmunol.174.8.4924. [DOI] [PubMed] [Google Scholar]
- 7.Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol. Rev. 2004;201:156–167. doi: 10.1111/j.0105-2896.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 8.Wynn TA, Cheever AW, Williams ME, Hieny S, Caspar P, Kühn R, Müller W, Sher A. IL-10 regulates liver pathology in acute murine schistosomiasis mansoni but is not required for immune down-modulation of chronic disease. J. Immunol. 1998;159:4473–4480. [PubMed] [Google Scholar]
- 9.Singh KP, Gerard HC, Hudson AP, Reddy TR, Boros DL. Retroviral Foxp3 gene transfer ameliorates liver granuloma pathology in Schistosoma mansoni infected mice. Immunology. 2005;114:410–417. doi: 10.1111/j.1365-2567.2004.02083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 11.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 12.Jarrett EEE, Stewart DC. Potentiation of rat reagenic (IgE) antibody by helminth infection. Simultaneous potentiation of separate reagins. Immunology. 1972;23:749–755. [PMC free article] [PubMed] [Google Scholar]
- 13.Kullberg MC, Pearce EJ, Hieny SE, Sher A, Berzofsky JA. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J. Immunol. 1992;148:3264–3270. [PubMed] [Google Scholar]
- 14.Cooke A, Tonks P, Jones FM, O'Shea H, Hutchings P, Fulford AJ, Dunne DW. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- 15.La Flamme AC, Ruddenklau K, Backstrom BT. Schistosomiasis decreases central nervous system inflammation and alters the progression of experimental autoimmune encephalomyelitis. Infect. Immun. 2003;71:4996–5004. doi: 10.1128/IAI.71.9.4996-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang CC, Nolan TJ, Schad GA, Abraham D. Infection of mice with the helminth Strongyloides stercoralis suppresses pulmonary allergic responses to ovalbumin. Clin. Exp. Allergy. 2001;31:495–503. doi: 10.1046/j.1365-2222.2001.01044.x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson MS, Taylor M, Balic A, Finney CAM, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urban JF, Jr., Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc. Natl. Acad. Sci. USA. 1991;88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svetic A, Madden KB, di Zhou X, Lu P, Katona IM, Finkelman FD, Urban JF, Jr., Gause WC. A primary intestinal helminthic infection rapidly induces a gut-associated elevation of Th2-associated cytokines and IL-3. J. Immunol. 1993;150:3434–3441. [PubMed] [Google Scholar]
- 20.Wahid FN, Behnke JM, Grencis RK, Else KJ, Ben-Smith AW. Immunological relationships during primary infection with Heligmosomoides polygyrus: Th2 cytokines and primary response phenotype. Parasitology. 1994;108:461–471. doi: 10.1017/s0031182000076022. [DOI] [PubMed] [Google Scholar]
- 21.Shi HN, Ingui CJ, Dodge I, Nagler-Anderson C. A helminth-induced mucosal Th2 response alters nonresponsiveness to oral administration of a soluble antigen. J. Immunol. 1998;160:2449–2455. [PubMed] [Google Scholar]
- 22.Finkelman FD, Morris SC, Orekhova T, Mori M, Donaldson D, Reiner SL, Reilly NL, et al. STAT6 regulation of in vivo IL-4 responses. J. Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 23.Mohrs K, Harris DP, Lund FE, Mohrs M. Systemic dissemination and persistence of Th2 and type 2 cells in response to infection with a strictly enteric nematode parasite. J. Immunol. 2005;175:5306–5313. doi: 10.4049/jimmunol.175.8.5306. [DOI] [PubMed] [Google Scholar]
- 24.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat. Med. 2000;6:536–542. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 25.Bashir ME, Andersen P, Fuss IJ, Shi HN, Nagler-Anderson C. An enteric helminth infection protects against an allergic response to dietary antigen. J. Immunol. 2002;169:3284–3292. doi: 10.4049/jimmunol.169.6.3284. [DOI] [PubMed] [Google Scholar]
- 26.Elliott DE, Setiawan T, Metwali A, Blum A, Urban JF, Jr., Weinstock JV. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 27.Monroy FG, Enriquez FJ. Heligmosomoides polygyrus: A model for chronic gastrointestinal helminthiasis. Parasitol. Today. 1992;8:49–54. doi: 10.1016/0169-4758(92)90084-f. [DOI] [PubMed] [Google Scholar]
- 28.Behnke JM, Parish HA. Nematospiroides dubius: Arrested development of larvae in immune mice. Exp. Parasitol. 1979;47:116–127. doi: 10.1016/0014-4894(79)90013-4. [DOI] [PubMed] [Google Scholar]
- 29.Behnke JM. Evasion of immunity by nematode parasites causing chronic infections. Adv. Parasitol. 1987;26:1–71. doi: 10.1016/s0065-308x(08)60294-8. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard DI, Williams DJL, Behnke JM, Lee TDG. The role of IgG1 hypergammaglobulinaemia in immunity to the gastrointestinal nematode Nematospiroides dubius. The immunochemical purification, antigen-specificity and in vivo anti-parasite effect of IgG1 from immune serum. Immunology. 1983;49:353–365. [PMC free article] [PubMed] [Google Scholar]
- 31.Su Z, Segura M, Morgan K, Loredo-Osti JC, Stevenson MM. Impairment of protective immunity to blood-stage malaria by concurrent nematode infection. Infect. Immun. 2005;73:3531–3539. doi: 10.1128/IAI.73.6.3531-3539.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shevach EM. CD4+ CD25+ suppressor T cells: More questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 33.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-β in CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 37.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 38.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 39.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills KHG. Regulatory T cells: Friend or foe in immunity to infection? Nat. Rev. Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 41.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat. Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 42.Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S-I, Yasutomo K. Escape of malaria parasites from host immunity requires CD4+CD25+ regulatory T cells. Nat. Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 43.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus-induced colitis. J. Exp. Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raghavan S, Fredriksson M, Svennerholm AM, Holmgren J, Suri-Payer E. Absence of CD4+CD25+ regulatory T cells is associated with a loss of regulation leading to increased pathology in Helicobacter pylori-infected mice. Clin. Exp. Immunol. 2003;132:393–400. doi: 10.1046/j.1365-2249.2003.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, et al. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 46.King CL, Mahanty S, Kumaraswami V, Abrams JS, Regunathan J, Jayaraman K, Ottesen EA, Nutman TB. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J. Clin. Invest. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoerauf A, Brattig N. Resistance and susceptibility in human onchocerciasis – beyond Th1 vs. Th2. Trends Parasitol. 2002;18:25–31. doi: 10.1016/s1471-4922(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 48.Steel C, Nutman TB. CTLA-4 in filarial infections: Implications for a role in diminished T cell reactivity. J. Immunol. 2003;170:1930–1938. doi: 10.4049/jimmunol.170.4.1930. [DOI] [PubMed] [Google Scholar]
- 49.Babu S, Blauvelt CP, Kumaraswami V, Nutman TB. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: Implications for parasite persistence. J. Immunol. 2006;176:3248–3256. doi: 10.4049/jimmunol.176.5.3248. [DOI] [PubMed] [Google Scholar]
- 50.Gillan V, Devaney E. Regulatory T cells modulate Th2 responses induced by Brugia pahangi third-stage larvae. Infect. Immun. 2005;73:4034–4042. doi: 10.1128/IAI.73.7.4034-4042.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumgart M, Tomkins F, Leng J, Hesse M. Naturally-occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J. Immunol. 2006;176:5374–5387. doi: 10.4049/jimmunol.176.9.5374. [DOI] [PubMed] [Google Scholar]
- 52.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses, and control the magnitude and phenotype of the Th effector population. J. Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 53.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4+CD25+ immunoregulatory T cells: Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 54.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 55.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Kuchroo VK, Inobe J.-I, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: Suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 57.Vieira PL, Christensen JR, Minaee S, O'Neill EJ, Barrat FJ, Boonstra A, Barthlott T, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 58.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25– naïve T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. TGF-β induces a regulatory phenotype in CD4+CD25– T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 60.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J. Exp. Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Helmby H, Grencis RK. Contrasting roles for IL-10 in protective immunity to different life cycle stages of intestinal nematode parasites. Eur. J. Immunol. 2003;33:2382–2390. doi: 10.1002/eji.200324082. [DOI] [PubMed] [Google Scholar]
- 63.Green EA, Gorelik L, McGregor CM, Tran EH, Flavell RA. CD4+CD25+ T regulatory cells control anti-islet CD8+ T cells through TGF-β-TGF-β receptor interactions in type 1 diabetes. Proc. Natl. Acad. Sci. USA. 2003;100:10878–10883. doi: 10.1073/pnas.1834400100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 65.Doligalska M, Rzepecka J, Drela N, Donskow K, Gerwel-Wronka M. The role of TGF-β in mice infected with Heligmosomoides polygyrus. Parasite Immunol. 2006;28:387–395. doi: 10.1111/j.1365-3024.2006.00845.x. [DOI] [PubMed] [Google Scholar]
- 66.Egen JG, Kuhns MS, Allison JP. CTLA-4: New insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 67.McCoy K, Camberis M, Le Gros G. Protective immunity to nematode infection is induced by CTLA-4 blockade. J. Exp. Med. 1997;186:183–187. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huehn J, Hamann A. Homing to suppress: Address codes for Treg migration. Trends Immunol. 2005;26:632–636. doi: 10.1016/j.it.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Siegmund K, Feuerer M, Siewert C, Ghani S, Haubold U, Dankof A, Krenn V, et al. Migration matters: Regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J. Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 71.Robinson PW, Green SJ, Carter C, Coadwell J, Kilshaw PJ. Studies on transcriptional regulation of the mucosal T-cell integrin αEβ7 (CD103) Immunology. 2001;103:146–154. doi: 10.1046/j.1365-2567.2001.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


