Abstract
Human lung mast cells (HLMC) express the Ca2+-activated K+ channel KCa3.1, which plays a crucial role in their migration to a variety of diverse chemotactic stimuli. KCa3.1 activation is attenuated by the β2-adrenoceptor and the adenosine A2A receptor through a Gs-coupled mechanism independent of cyclic AMP. Prostaglandin E2 promotes degranulation and migration of mouse bone marrow-derived mast cells through the Gi-coupled EP3 prostanoid receptor, and induces LTC4 and cytokine secretion from human cord blood-derived mast cells. However, PGE2 binding to the Gs-coupled EP2 receptor on HLMC inhibits their degranulation. We show that EP2 receptor engagement closes KCa3.1 in HLMC. The EP2 receptor-specific agonist butaprost was more potent than PGE2 in this respect, and the effects of both agonists were reversed by the EP2 receptor antagonist AH6809. Butaprost markedly inhibited HLMC migration induced by chemokine-rich airway smooth muscle-conditioned media. Interestingly, PGE2 alone was chemotactic for HLMC at high concentrations (1 µM), but was a more potent chemoattractant for HLMC following EP2 receptor blockade. Therefore, the Gs-coupled EP2 receptor closes KCa3.1 in HLMC and attenuates both chemokine- and PGE2-dependent HLMC migration. EP2 receptor agonists with KCa3.1 modulating function may be useful for the treatment of mast cell-mediated disease.
Keywords: Chemotaxis, Ion channel, KCa3.1, Mast cell, Prostaglandin E2
Introduction
Mast cells are tissue-dwelling cells derived from bone marrow progenitors. They are present in all organs throughout the human body, both at mucosal surfaces and within connective tissues. Mast cells play a major role in tissue homeostasis, host defence and the pathophysiology of many diverse diseases 1. These include pulmonary fibrosis, rheumatoid disease and atherosclerosis, but they are most commonly associated with allergic disease due to their activation by allergen 2. In many diseases mast cells re-locate to specific compartments within tissue. This is typified in asthma where mast cells migrate into the airway epithelium 3, airway smooth muscle (ASM) 4 and submucosal glands 5. This places activated mast cells in direct contact with these dysfunctional airway elements, allowing the specific delivery of detrimental cell–cell signals. Drugs that inhibit this tissue relocation by preventing mast cell migration may prove particularly effective in the treatment of mast cell-mediated disease.
Human mast cells express the intermediate conductance Ca2+-activated K+ channel KCa3.1, which plays a critical role in their migration to diverse chemotactic stimuli 6, and to a lesser extent in their degranulation 7, 8. Drugs that directly block this channel or which close it indirectly therefore have potential as novel therapies for mast cell-dependent disease. KCa3.1 in human lung mast cells (HLMC) is closed by both salbutamol and adenosine via the β2-adrenoceptor and A2A adenosine receptors, respectively 9, 10, which are both Gs-coupled G protein-coupled receptors (GPCR). In keeping with a critical role for KCa3.1 in HLMC migration, adenosine also inhibits HLMC chemotaxis via the A2A receptor 10.
PGE2 is a prostanoid with four specific GPCR, designated EP1–4. EP2 and EP4 couple to Gs, EP3 couples predominantly to Gi although two isoforms also couple to Gs, while EP1 receptors mobilise intracellular Ca2+, probably through Gq 11. PGE2 therefore has diverse biological activities depending on the receptors it interacts with and the cells expressing them. With respect to mast cells, mouse mast cells express the EP3 receptor whose activation induces both degranulation and chemotaxis 12, 13. Human cord blood-derived mast cells, express both EP2 and EP3 receptors. In these cells, PGE2 enhances degranulation and PGD2 production in cells primed by IL-4, an effect mediated through the EP3 receptor. However, following FcɛRI-dependent activation PGE2 inhibits human cord blood-derived mast cell LTC4, PGD2, IL-5 and TNF-α production via the EP2 receptor but has no effect on degranulation 14. In contrast, several studies have shown that PGE2 consistently inhibits degranulation and eicosanoid production by HLMC, an effect mediated via the EP2 receptor 15–17. This receptor also appears to dominate PGE2-dependent signalling on mast cells within the human asthmatic lung as PGE2 markedly attenuates both the early and late phase airway response to allergen challenge 18.
Since KCa3.1 in HLMC is closed by Gs-coupled β2-adrenoceptors and A2A adenosine receptors, we hypothesised that activation of the Gs-coupled EP2 receptor would also close KCa3.1 in these cells. Furthermore, if PGE2 was to close KCa3.1, then it should inhibit HLMC chemotaxis. To test this hypothesis we have used the patch-clamp technique to investigate the effects of PGE2 on HLMC ion channel function, and investigated the effect of PGE2 on HLMC migration induced by asthmatic ASM-conditioned medium.
Results
PGE2 alone does not open KCa3.1
We initially examined whether PGE2 opens KCa3.1 in HLMC under resting baseline conditions. HLMC are typically electrically silent at rest and it is possible that PGE2 could open either KCa3.1 or another channel as reported previously for adenosine 10. No significant change in current amplitude was observed in seven cells following the addition of 10−5 M PGE2 (current changed from 1.22±0.54 to 1.42±0.85 pA at +40 mV; p=0.678).
PGE2 closes KCa3.1 in the presence of the KCa3.1 opener 1-EBIO
We then examined whether PGE2 closes KCa3.1. Because PGE2 might potentially inhibit many IgE-dependent cell activation pathways that could reduce cytosolic-free Ca2+, and thus reduce KCa3.1 activity indirectly, we concentrated on studying the effects of PGE2 on KCa3.1 currents that were induced by the KCa3.1 opener 1-EBIO 8–10. This compound opens KCa3.1 with a half-maximal value of about 30 µM for heterologously expressed KCa3.1, with a maximal effect at about 300 µM 19. 1-EBIO is specific for KCa3.1 in HLMC and opens it by enhancing the channels sensitivity to [Ca2+]i 19. Thus at 100 µM EBIO, maximal K+ currents are achieved in the presence of 100 nM free Ca2+, which is below the resting [Ca2+]i of most cell types including HLMC 8.
In cells in which KCa3.1 had been activated by 1-EBIO, addition of PGE2 (10−8–10−5 M) produced a rapid (within 30 s) dose-responsive inhibition of channel activity with an associated positive shift in membrane potential (Fig. 1A–E). PGE2 10−5 M suppressed the KCa3.1 current in >90% of cells (Fig. 1B). Thus, addition of 10−5 M PGE2 reduced KCa3.1 membrane current at +40 mV from 155.4±20.9 to 92.6±13.7 pA (p=0.0001, n=22 cells)(Fig. 1B), with a corresponding shift in reversal potential (Vm) from −69.5±2.8 to −56.3±3.9 mV (p=0.0004) (Fig. 1C). Half maximal suppression (IC50) of KCa3.1 by PGE2 occurred at approximately 4.0×10−7 M (calculated from six cells). Importantly, the effect of PGE2 was partially reversed within 1 min by removing it from the recording solution (current post PGE2 49.6±20.8 pA, post wash 103.4±34.5 pA, p=0.046; Vm post PGE2 −44.1±11.7 mV, post wash −72.1±6.81 mV, p=0.028, n=5) (Fig. 1D and E), indicating that non-specific “rundown” was not responsible for the effects seen.
Figure 1.
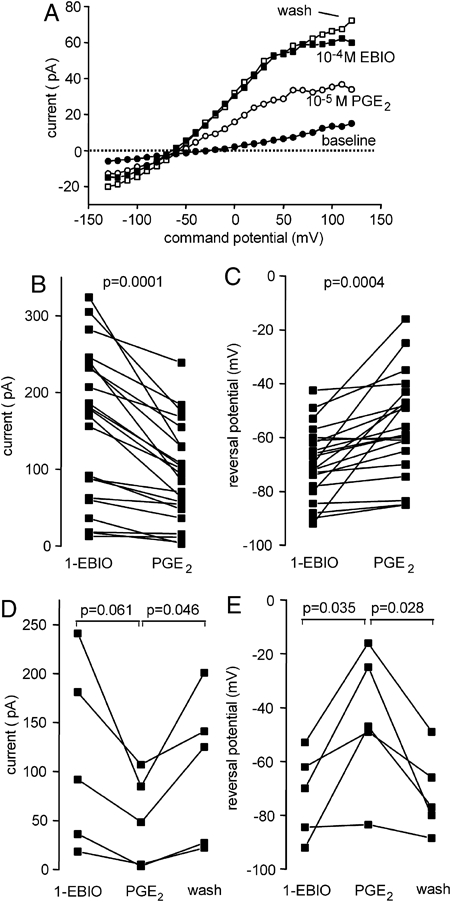
PGE2 closes KCa3.1 in HLMC in a consistent and reversible manner. (A) Current–voltage curve demonstrating suppression of a 1-EBIO-induced KCa3.1 current by 10−5 M PGE2 and partial reversal of the effect following removal of PGE2 (wash). (B) Suppression of KCa3.1 current measured at +40 mV by 10−5 M PGE2 (n=22 cells). (C) Shift in whole-cell current reversal potential (Vm) by 10−5 M PGE2 (n=22 cells). (D) KCa3.1 current measured at +40 mV after addition of 1-EBIO, suppression by 10−5 M PGE2 and then reversibility of suppression following removal of PGE2 (wash) (n=5 cells). (E) Whole-cell current reversal potential (Vm) after the addition of 1-EBIO, a depolarising positive shift in response to 10−5 M PGE2, and then reversibility following removal of PGE2 (wash) (n=5 cells).
KCa3.1 modulation by PGE2 is mediated via EP2 receptors
To examine whether the effects of PGE2 were mediated via EP2 prostanoid receptors we examined the effects of EP2 receptor agonists/antagonists. First, we examined the effects of the selective EP2 receptor agonist butaprost. Butaprost mimicked the effects of native PGE2 in a dose-dependent manner (Fig. 2A–D). At a concentration of 10−5 M, butaprost reduced the KCa3.1 current from 146.0±18.3 pA to 61.2±6.1 pA (p=0.00006, n=20) (Fig. 2B) with a corresponding shift in reversal from −67.5±1.4 to −54.4±3.2 mV (p=0.00006) (Fig. 2C). Half maximal suppression (IC50) of KCa3.1 by butaprost occurred at approximately 2.1×10−7 M (n=6 cells) (Fig. 2D).
Figure 2.
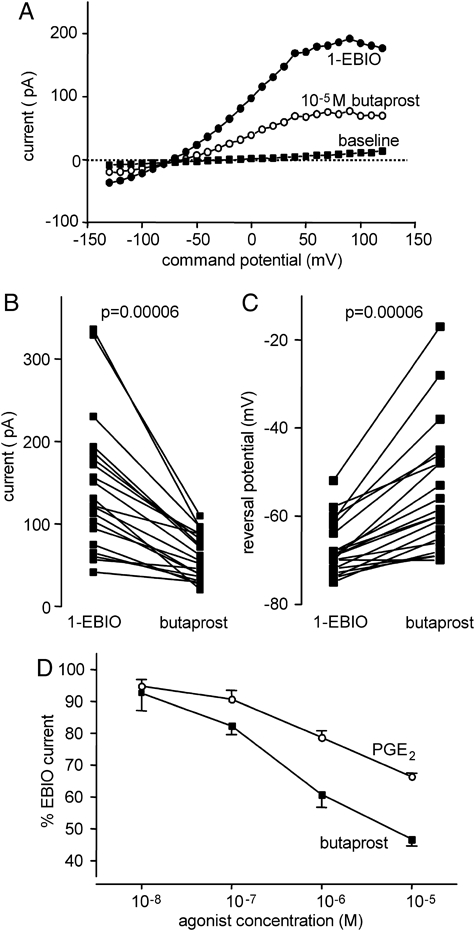
The EP2 receptor agonist butaprost closes KCa3.1 in HLMC. (A) Current–voltage curve demonstrating suppression of a 1-EBIO-induced KCa3.1 current by 10−5 butaprost. (B) Suppression of KCa3.1 current measured at +40 mV by 10−5 M butaprost (n=20 cells). (C) Shift in whole-cell current reversal potential (Vm) by 10−5 M butaprost (n=20 cells). (D) Concentration–response curves for KCa3.1 suppression by PGE2 and the EP2 agonist butaprost (mean±SEM of five cells for each).
The suppression of KCa3.1 by 10−5 M PGE2 was partially reversed by the competitive EP1 and EP2 receptor antagonist AH6809 (Fig. 3A–C). Thus, in experiments studying AH6809 at a concentration of 10−5 M, current at +40 mV was 112±14.3 pA post PGE2, increasing to 160.9±23.2 pA post AH6809 (p=0.011, n=10 cells) (Fig. 3B). There was however no significant shift in reversal potential in these experiments explained by the fact that significant KCa3.1 currents remained following PGE2 application (Vm post PGE2 −60.9±2.1 mV, post AH6809 −63.6±1.8 mV, p=0.17) (Fig. 3C). We also examined the effect of AH6809 on the selective EP2 agonist butaprost. The suppression of KCa3.1 by 10−5 M butaprost was also partially reversed by 10−5 M AH6809 (Fig. 3D–F). Current at +40 mV was 58.5±8.3 pA post butaprost, increasing to 111.6±15.9 pA post AH6809 (p=0.0004, n=12) (Fig. 3E). There was also a significant shift in reversal potential (Vm post butaprost −48.1±5.8 mV, post AH6809 −61.0±3.7 mV, p=0.0004) (Fig. 3F). In the presence of AH6809, PGE2 had no effect on KCa3.1 currents that had been induced by 1-EBIO (current at +40 mV 67.0±17.2 pA post 1-EBIO, 66.2±15.7 post AH6809, 62.7±13.7 pA post PGE2, n=7)(Fig. 4A). Similarly, KCa3.1 currents did not appear in resting cells to which AH6809 was added prior to PGE2 (current 6.9±0.9 pA at baseline, 5.8±0.6 pA post AH6809, 6.3±1.1 pA post PGE2, n=6) (Fig. 4B). The EP1 and EP3 receptor agonist 17-phenyl trinor PGE2 did not close KCa3.1 after activation with 1-EBIO (current at +40 mV post 1-EBIO 128.3±94.9 pA, post 17-phenyl trinor PGE2 127.8±90.4 pA, p=0.9567, n=6) and did not open KCa3.1 in resting cells (baseline current at +40 mV 5.70±0.88 pA, current post 17-phenyl trinor PGE2 5.36±0.949 pA, p=0.202, n=5). These results exclude a role for EP1, EP3 or EP4 receptors in KCa3.1 modulation. Taking the data together, it can be concluded that suppression of KCa3.1 by PGE2 is mediated by the EP2 receptor.
Figure 3.
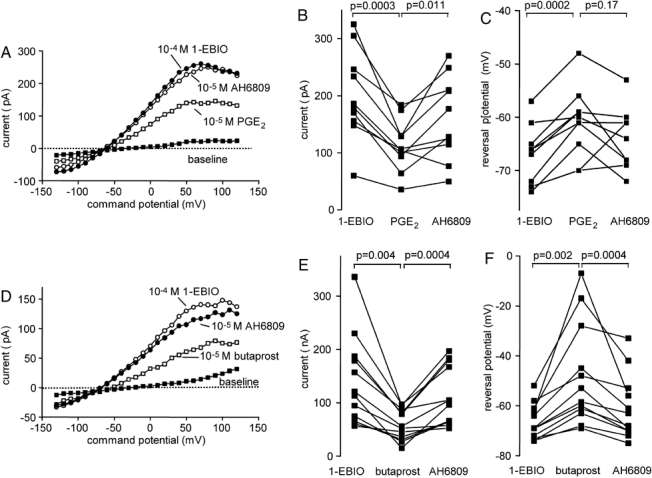
The effect of the EP1/2 receptor antagonist AH6809 on PGE2- and butaprost -dependent closure of KCa3.1. (A) Current–voltage curve demonstrating reversibility of KCa3.1 suppression by PGE2 following administration of the EP1/2 receptor antagonist AH6809. (B) KCa3.1 current measured at +40 mV after addition of 1-EBIO, suppression by 10−5 M PGE2 and then reversibility of suppression following addition of AH6809 (n=10 cells). (C) Whole-cell current reversal potential (Vm) after the addition of 1-EBIO, a depolarising positive shift in response to 10−5 M PGE2, and then following addition AH6809 (n=10 cells). (D) Current–voltage curve demonstrating reversibility of KCa3.1 suppression by butaprost following administration of AH6809. (E) KCa3.1 current measured at +40 mV after addition of 1-EBIO, suppression by 10−5 M butaprost and then reversibility of suppression following addition of AH6809 (n=12 cells). (F) Whole-cell current reversal potential (Vm) after the addition of 1-EBIO, a depolarising positive shift in response to 10−5 M PGE2, and then reversibility following addition AH6809 (n=12 cells).
Figure 4.
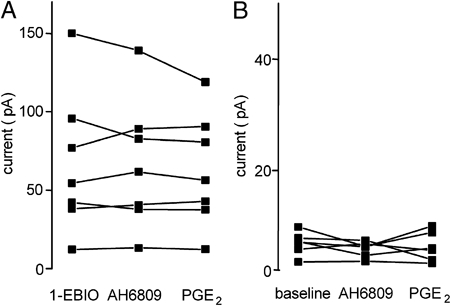
PGE2 is ineffective in the presence of the AH6809. (A) KCa3.1 current measured at +40 mV after addition of 1-EBIO, stability following addition of AH6809, and failure to suppress following subsequent addition of 10−5 M PGE2. (B) KCa3.1 current measured at +40 mV in resting cells, after addition of AH6809, and then following subsequent addition of 10−5 M PGE2 showing failure of KCa3.1 to open.
PGE2 closes KCa3.1 following IgE-dependent activation
Lastly, we confirmed whether PGE2-dependent regulation of KCa3.1 was relevant to KCa3.1 channels that had been opened by anti-IgE-dependent activation. Anti-IgE (1:1000 dilution) opened KCa3.1 in 5/5 cells tested (baseline current at +40 mV 5.8±1.3 pA, baseline Vm −17.8±3.2 mV; post anti-IgE current 54.2±7.6 pA, Vm −62.4±3.0 mV). PGE2 (10−5 M) suppressed the current to 34.3±4.9 pA post PGE2, p=0.005) (Fig. 5A and B) and produced an associated positive shift in Vm to −52.2±4.1 mV, p=0.051) (Fig. 5C). This suppressive effect of PGE2 was partially reversed by AH6809 (current 48.3±6.9 pA, p=0.011; Vm −66.6±4.0 mV, p=0.041) (Fig. 5A and C).
Figure 5.
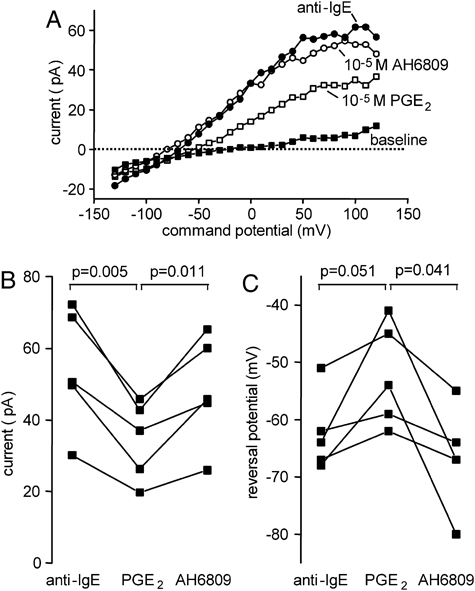
The effect of 10−5 M PGE2 on KCa3.1 currents elicited by anti-IgE-dependent mast cell activation. (A) A representative HLMC demonstrating the development of a KCa3.1 current following anti-IgE-dependent activation, suppression of this by 10−5 M PGE2, and reversal of the PGE2-induced suppression by the EP1/2 receptor antagonist AH6809. (B) KCa3.1 current measured at +40 mV after addition of anti-IgE, suppression by 10−5 M PGE2 and then reversibility of suppression following addition of AH6809 (n=5 cells). (C) Whole-cell current reversal potential (Vm) after the addition of anti-IgE, a depolarising positive shift in response to 10−5 M PGE2, and then reversibility following addition AH6809 (n=5 cells).
PGE2 suppresses HLMC migration through the EP2 receptor
Conditioned medium from asthmatic ASM, which has been activated with TNFα, IFNγ and IL-1β, mediates HLMC chemotaxis predominantly via the CXCL10/CXCR3 pathway with additional contributions from ligands for CXCR1 and CXCR3 20. Inhibition of KCa3.1 by channel blockers markedly suppresses this HLMC chemotaxis 6, as do molecules that close KCa3.1 such as adenosine 10. Migration of HLMC using conditioned medium from asthmatic airway smooth muscle was 2.8±0.9 fold that of medium control (n=4, p=0.046) (Fig. 6A) and this was not inhibited significantly by PGE2 (Fig. 6B). However, the selective EP2 agonist butaprost produced marked inhibition of HLMC migration to ASM-conditioned medium (Fig. 6B). Interestingly, PGE2 was chemotactic on its own, but much less potent than in mouse bone marrow-derived mast cells 13 (Fig. 6C). This HLMC chemotactic activity of PGE2 was markedly increased in the presence of EP1/2 blockade by AH6809 10−5 M (Fig. 6D). HLMC migration to both ASM-conditioned medium and PGE2 itself is therefore attenuated by the EP2 receptor.
Figure 6.
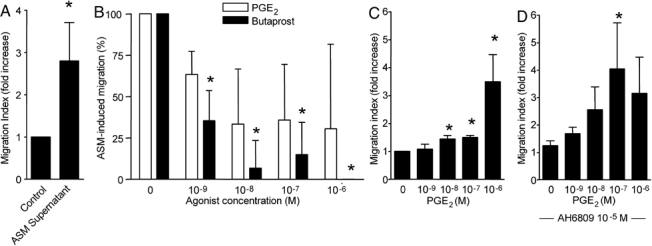
Inhibition of HLMC chemotaxis through EP2 receptor activation. (A) HLMC migration using conditioned medium from asthmatic airway smooth muscle as the chemotactic stimulus. (B) HLMC migration is attenuated significantly by butaprost (n=4) but not PGE2 (n=3) in the presence of ASM-conditioned medium. *p<0.05 compared with control (no PGE2 or butaprost). (C) PGE2 is chemotactic when present in isolation. *p<0.05 compared to control (no PGE2), n=4. (D) PGE2-dependent chemotaxis is enhanced in the presence of the EP1/2 receptor antagonist AH6809 (10−5 M). *p<0.05 compared to control (no AH6809), n=4.
PGE2 attenuates histamine release from cultured HLMC
PGE2 inhibits the degranulation of HLMC freshly isolated from lung tissue. Because the cells used in this study were cultured in stem cell factor (SCF), IL-6 and IL-10, we investigated whether PGE2 also inhibits release from these HLMC. In keeping with observations in freshly isolated HLMC, PGE2 inhibited histamine release dose dependently over the concentration range 10−9–10−6 M (control mean±s.e.m. net histamine release 19.8±3.7% versus 7.5±3.3% with 10−6 PGE2, n=4 donors, p=0.046).
KCa3.1 is not modulated by Gi or Gq-coupled receptors
We have now identified three distinct Gs-coupled receptors that close KCa3.1 in HLMC when exposed to the relevant ligands, and in the case of the β2-adrenoceptor, the inverse agonist ICI 118551 opens the channel 9. In contrast, the Gi-coupled CXCR3 receptor does not couple directly to KCa3.1 6. We have therefore investigated further Gi and Gq agonists known to have biological effects on HLMC. Platelet activating factor (PAF, 10−7 M) (Gi), lysophosphatidic acid (LPA, 10−5 M) (Gq,12/13,i) and UTP (10−4 M)(Gq) did not open KCa3.1 in resting HLMC (n=≥6 cells for each agonist) and did not close KCa3.1 that had been opened by 1-EBIO (n=≥5 cells for each agonist)(data not shown).
Discussion
In this study we have examined the effects of EP2 receptor activation on KCa3.1 ion channel function in HLMC. In keeping with the known effects of the β2-adrenoceptor and A2A adenosine receptor 9, 10, the Gs-coupled EP2 receptor also closes this channel reversibly. Consistent with this effect on the channel, EP2 receptor activation attenuates HLMC migration, and in consequence masks the potential chemotactic activity of PGE2 on HLMC, which is particularly evident when the EP2 receptor is blocked pharmacologically.
PGE2 closed KCa3.1 reversibly following IgE-dependent mast cell activation demonstrating physiological relevance. PGE2 also closed KCa3.1 channels that had been activated by the KCa3.1 opener 1-EBIO. The inhibition of KCa3.1 by PGE2 was reversed both by removing it from the recording solution, and by the addition of the competitive EP1 and EP2 receptor antagonist AH6809 indicating a receptor-mediated mechanism. Since the effects of PGE2 were mimicked by the specific EP2 receptor agonist butaprost but not the EP1 agonist 17-phenyl trinor PGE2, and no effects of native PGE2 were seen in the presence of the EP1/2 receptor antagonist AH6809, we have firm evidence that the suppression of KCa3.1 by PGE2 is mediated via the EP2 receptor.
Because 1-EBIO opens KCa3.1 directly in resting cells by increasing its affinity for Ca2+ 19, it suggests that there is tight coupling between the EP2 receptor and the channel rather than modulation of intracellular signalling pathways. The ability of PGE2 to close KCa3.1 is in keeping with our previous observations that this channel is closed by Gs-coupled β2-adrenoceptors 9 and Gs-coupled adenosine A2A receptors 10. These effects on KCa3.1 are not mimicked by cAMP analogues or the activator of adenylate cyclase forskolin 9, and considering they are seen in whole-cell configuration of the patch-clamp technique, indicate that the most likely mechanism of action is membrane-delimited involving the Gαs or βγ subunits of these GPCR. This view is further supported by the observation that the β2-adrenoceptor inverse agonist ICI-118551 actually opens KCa3.1 9, but Gi agonists such as CXCL10 and PAF, which lower intracellular cAMP, do not directly activate this channel 6.
The ability of GPCR to modify KCa3.1 appears to be limited to Gs-coupled receptors as we have not found any evidence that Gi, Gq, or G12/13-coupled receptors modify KCa3.1 activity. In particular blockade of EP1 and EP2 in this study did not uncover any Gi-coupled EP3 effects, and GPCR agonists active on human mast cells such as CXCL10 (Gαi) 6, PAF (Gi), LPA (Gq, −12/13 and i), and UTP (Gαq P2Y2 receptor) do not open or close KCa3.1 in HLMC. It is well established using cell attached, inside-out and outside patch-clamp recording that most classes of GPCR including Gαs couple directly to ion channels through membrane-delimited mechanisms 21, 22. This may lead to either channel opening or channel closing depending on the channel and receptor, and may utilise either the Gα component or specific combinations of βγ subunits 21, 22. A further level of specificity between receptor and channel is likely to be achieved by the close approximation of these proteins in tight membrane-restricted signalling complexes. For example, the β2 adrenoceptor that can modify both KCa1.1 and voltage-gated Ca2+ channel gating is associated with these two channels in a macromolecular complex held together by A-kinase-anchoring proteins 23. It is therefore interesting to speculate that KCa3.1 also localises with the various Gαs receptors that modify its function. This and the exact mechanism by which Gαs modifies KCa3.1 function will be an important area for future research.
Several molecules that attenuate HLMC secretion including PGE2, adenosine and β2-adrenoceptor agonists increase intracellular cAMP 24. The generally held view is that this increase in intracellular cAMP couples to inhibition of secretion, supported by the observation that cAMP analogues and non-specific inhibitors of adenylate cyclase can also attenuate secretion from HLMC 24. However, no mechanism has been identified in mast cells that explains how increases in cAMP inhibit the secretory pathway, and the exclusive role of cAMP in the inhibition of other systems such as smooth muscle relaxation has been challenged 25. Opening of KCa3.1 enhances IgE-dependent Ca2+ influx and degranulation to a submaximal stimulus 8, and its blockade by charybdotoxin attenuates this 7. Thus, while cAMP plays some role in the inhibition of mast cell mediator release, the demonstration that PGE2 also closes KCa3.1 supports the view that cAMP-independent, KCa3.1-dependent mechanisms also contribute in part to EP2-dependent inhibition of HLMC mediator release.
In many diseases the recruitment of mast cells to key tissue structures appears critical for their pathophysiological effects 4, 26, 27. For example, the contribution of mast cells to the disordered airway physiology of asthma is undoubtedly facilitated by their migration into the airway epithelium 3, submucosal glands 5 and ASM 4. Inhibition of their migration and subsequent microlocalisation within these structures might therefore offer a novel approach to therapy. Blockade of KCa3.1 markedly inhibits HLMC migration in response to a number of diverse chemotactic stimuli including conditioned medium from activated asthmatic ASM 6. The ability of PGE2 to close KCa3.1 suggested that it should also inhibit HLMC migration. However, its ability to inhibit HLMC migration in response to ASM-conditioned medium was variable and did not reach statistical significance. In contrast, the selective EP2 receptor agonist butaprost, which was more potent at closing KCa3.1 than PGE2, produced a marked and consistent inhibition of HLMC migration. PGE2 was also chemotactic at high concentrations when used alone, but was more potent as a chemoattractant when EP2 receptors were blocked. This indicates that there are competing pro-migratory and anti-migratory signals when PGE2 is present, mediated by the EP2 receptor (inhibitory) and most probably the EP3 receptor (pro-migratory). These findings are in marked contrast to those in mouse bone marrow-derived mast cells in which PGE2 in isolation is a potent chemoattractant. This chemotactic activity is mediated through the EP3 receptor, and the lack of inhibition can be attributed to the absence of EP2 receptors in mouse bone marrow-derived mast cells 13. In human cord blood-derived mast cells, which express both EP2 and EP3 receptors, PGE2 is not a chemoattractant 13, compatible with an inhibitory role for the EP2 receptor. In human lung therefore, PGE2 is not likely to act as a HLMC chemoattractant, in keeping with its anti-inflammatory activities in this tissue 18.
In humans PGE2 inhibits the early and late asthmatic airway response to allergen challenge 18. The ability of PGE2 to close KCa3.1 provides a mechanism through which it is able to achieve these effects. It will be of great interest to investigate whether PGE2 has similar effects on lung T-cell KCa3.1 function, cytokine secretion and migration, and whether it can inhibit KCa3.1-dependent ASM proliferation 28. Drugs that block KCa3.1 are also in development as anti-inflammatory treatments 29. Our demonstration that the EP2 receptor closes KCa3.1 in HLMC provides further support for the development of specific EP2 receptor agonists for the treatment of mast cell-mediated pulmonary disease.
Materials and methods
Reagents
We used the following reagents: SCF, IL-6 and IL-10 (R&D, Abingdon, UK); goat polyclonal anti-human IgE, PGE2, AH6809, butaprost, PAF, LPA, UTP (Sigma, Poole, Dorset, UK); 17-phenyl trinor PGE2 (Cayman Chemical Company, Ann Arbor, Michigan, US); mouse IgG1 mAb YB5.B8 (anti-CD117) (Cambridge Bioscience, Cambridge, UK); sheep anti-mouse IgG1 Dynabeads (Dynal, Wirral, UK); Dulbecco's Modified Essential Medium (DMEM)/glutamax/HEPES, antibiotic/antimycotic solution, MEM non-essential aminoacids, and fetal calf serum (Life Technologies, Paisley, Scotland, UK).
Human mast cell purification and culture
All human subjects gave written informed consent, and the study was approved by the Leicestershire Research Ethics Committee. HLMC were dispersed and purified from macroscopically normal lung (n=14 donors) obtained within 1 h of resection for lung cancer using immunomagnetic affinity selection as described previously 30. Final mast cell purity determined by Kimura stain was >99% and viability determined by Trypan blue was >97%. HLMC were cultured in DMEM/glutamax/HEPES containing antibiotic/antimycotic solution, non-essential amino acids, 10% fetal calf serum, 100 ng/mL SCF, 50 ng/mL IL-6, and 10 ng/mL IL-10 for up to 10 wk as described previously 8, 31.
Electrophysiology
The whole-cell variant of the patch-clamp technique was used 7, 32. Patch pipettes were made from borosilicate fibre-containing glass (Clark Electromedical Instruments, Reading, UK), and their tips were heat polished, typically resulting in resistances of 4–6 MΩ. The standard pipette solution contained (in mM) KCl, 140; MgCl2, 2; HEPES, 10; Na+-ATP, 2; GTP, 0.1 (pH 7.3). The standard external solution contained (in mM) NaCl, 140; KCl, 5, CaCl2, 2; MgCl2,1; HEPES, 10 (pH 7.3). For recording, mast cells were placed in 35-mm dishes containing standard external solution. Whole-cell currents were recorded using an Axoclamp 200A amplifier (Axon Instruments, Foster City, CA, USA), and currents were evoked by applying voltage commands to a range of potentials in 10 mV steps from a holding potential of −20 mV. The currents were digitised (sampled at a frequency of 10 kHz), stored on computer, and subsequently analysed using pClamp software (Axon Instruments). Capacitance transients were minimised using the capacitance neutralisation circuits on the amplifier. Correction for series resistance was not routinely applied. Experiments were performed at 27°C, and the temperature controlled by a Peltier device. Experiments were performed with a perfusion system (Automate Scientific, San Francisco, CA) to allow solution changes, although drugs were added directly to the recording chamber.
PGE2 was dissolved in ethanol to give a stock solution of 10−2 M. Thus, at the maximal concentration PGE2 used (10−5 M), the concentration of ethanol in the recording chamber was 0.1%. This concentration of ethanol did not affect KCa3.1 currents when tested in isolation.
HLMC chemotaxis
HLMC chemotaxis assays were performed using the Transwell system (BD Biosciences, Oxford, UK) with 24-well plates as described previously 6, 20. Conditioned medium from asthmatic ASM that had been activated with TNFα, IL-1β and IFNγ was placed in the lower wells as described previously 20, with appropriate cytokine containing medium in the negative control. PGE2 was added to the bottom wells in the concentration range 10−6–10−3 M. A total of 1×105 HLMC in 100 µL were added to the top well. After incubating the cells for 3 h at 37°C, we counted the number of HLMC in the bottom well using Kimura stain in a haemocytometer. HLMC migration was calculated as the fold increase of migrated cells in the test wells compared with the negative control (no chemoattractant in the lower well) as described previously 6, 20.
Data presentation and statistical analysis
Data are expressed as mean±SEM. unless otherwise stated. Differences between groups of data were explored using Student's paired or unpaired t-test (two-tailed) as appropriate.
Acknowledgments
Supported by the Wellcome Trust, grant number 076264.
Glossary
Abbreviations
- ASM
airway smooth muscle
- EP
E prostanoid receptor
- 1-EBIO
1-ethyl-2-benzimidazolinone
- GPCR
G protein-coupled receptor
- HLMC
human lung mast cell
- LPA
lysophosphatidic acid
- PAF
platelet activating factor
- PG
prostaglandin
Conflict of interest:
The authors declare no financial or commercial conflict of interest.
References
- 1.Bradding P, Holgate ST. Immunopathology and human mast cell cytokines. Crit. Rev. Oncol. Haematol. 1999;31:119–133. doi: 10.1016/s1040-8428(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 2.Bradding P. Mast cells in asthma. In: Busse WW, Holgate ST, editors. Asthma & Rhinitis. Boston: Blackwell Scientific Publications; 2000. pp. 319–338. [Google Scholar]
- 3.Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, et al. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am. J. Respir. Cell Mol. Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 4.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast cell infiltration of airway smooth muscle in asthma. N. Engl. J. Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 5.Carroll NG, Mutavdzic S, James AL. Increased mast cells and neutrophils in submucosal mucous glands and mucus plugging in patients with asthma. Thorax. 2002;57:677–682. doi: 10.1136/thorax.57.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruse G, Duffy SM, Brightling CE, Bradding P. Functional KCa3.1 K+ channels are required for human lung mast cell migration. Thorax. 2006;61:880–885. doi: 10.1136/thx.2006.060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy SM, Lawley WJ, Conley EC, Bradding P. Resting and activation-dependent ion channels in human mast cells. J. Immunol. 2001;167:4261–4270. doi: 10.4049/jimmunol.167.8.4261. [DOI] [PubMed] [Google Scholar]
- 8.Duffy SM, Berger P, Cruse G, Yang W, Bolton SJ, Bradding P. The K+ channel IKCa1 potentiates Ca2+ influx and degranulation in human lung mast cells. J. Allergy Clin. Immunol. 2004;114:66–72. doi: 10.1016/j.jaci.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Duffy SM, Cruse G, Lawley WJ, Bradding P. Beta2-adrenoceptor regulation of the K+ channel IKCa1 in human mast cells. FASEB J. 2005;19:1006–1008. doi: 10.1096/fj.04-3439fje. [DOI] [PubMed] [Google Scholar]
- 10.Duffy SM, Cruse G, Brightling CE, Bradding P. Adenosine closes the K+ channel KCa3.1 in human lung mast cells and inhibits their migration via the adenosine A2A receptor. Eur. J. Immunol. 2007;37:1653–1662. doi: 10.1002/eji.200637024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J. Biol. Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen M, Solle M, Audoly LP, Tilley SL, Stock JL, McNeish JD, Coffman TM, et al. Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J. Immunol. 2002;169:4586–4593. doi: 10.4049/jimmunol.169.8.4586. [DOI] [PubMed] [Google Scholar]
- 13.Weller CL, Collington SJ, Hartnell A, Conroy DM, Kaise T, Barker JE, Wilson MS, et al. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc. Nat. Acad. Sci. 2007;104:11712–11717. doi: 10.1073/pnas.0701700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood. 2006;107:3243–3250. doi: 10.1182/blood-2005-07-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peachell PT, MacGlashan DW, Jr., Lichtenstein LM, Schleimer RP. Regulation of human basophil and lung mast cell function by cyclic adenosine monophosphate. J. Immunol. 1988;140:571–579. [PubMed] [Google Scholar]
- 16.Kay LJ, Yeo WW, Peachell PT. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br. J. Pharmacol. 2006;147:707–713. doi: 10.1038/sj.bjp.0706664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters SP, Schulman ES, Schleimer RP, MacGlashan DW, Jr., Newball HH, Lichtenstein LM. Dispersed human lung mast cells. Pharmacologic aspects and comparison with human lung tissue fragments. Am. Rev. Respir. Dis. 1982;126:1034–1039. doi: 10.1164/arrd.1982.126.6.1034. [DOI] [PubMed] [Google Scholar]
- 18.Pavord ID, Wong CS, Williams J, Tattersfield AE. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am. Rev. Respir. Dis. 1993;148:87–90. doi: 10.1164/ajrccm/148.1.87. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen KA, Schroder RL, Skaaning-Jensen B, Strobaek D, Olesen SP, Christophersen P. Activation of the human intermediate-conductance Ca(2+)-activated K(+) channel by 1-ethyl-2-benzimidazolinone is strongly Ca(2+)-dependent. Biochim. Biophys. Acta. 1999;1420:231–240. doi: 10.1016/s0005-2736(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 20.Brightling CE, Ammit AJ, Kaur D, Black JL, Wardlaw AJ, Hughes JM, Bradding P. The CXCL10/CXCR3 axis mediates human lung mast cell migration to asthmatic airway smooth muscle. Am. J. Respir. Crit. Care Med. 2005;171:1103–1108. doi: 10.1164/rccm.200409-1220OC. [DOI] [PubMed] [Google Scholar]
- 21.Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol. Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- 22.Kume H, Graziano MP, Kotlikoff MI. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proc. Natl. Acad. Sci. USA. 1992;89:11051–11055. doi: 10.1073/pnas.89.22.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of a Ca2+-dependent BK channel signaling complex by binding to beta2 adrenergic receptor. EMBO J. 2004;23:2196–2205. doi: 10.1038/sj.emboj.7600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weston MC, Peachell PT. Regulation of human mast cell and basophil function by cAMP. Gen. Pharmacol. 1998;31:715–719. doi: 10.1016/s0306-3623(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 25.Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. Beta-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J. Clin. Invest. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue Y, King TEJ, Tinkle SS, Dockstader K, Newman LS. Human mast cell basic fibroblast growth factor in pulmonary fibrotic disorders. Am. J. Pathol. 1996;149:2037–2054. [PMC free article] [PubMed] [Google Scholar]
- 27.Gotis-Graham I, McNeil HP. Mast cell responses in rheumatoid synovium. Association of the MCTC subset with matrix turnover and clinical progression. Arthritis Rheum. 1997;40:479–489. doi: 10.1002/art.1780400314. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd MC, Duffy SM, Harris T, Cruse G, Schuliga M, Brightling CE, Neylon CB, et al. KCa3.1 Ca2+ activated K+ channels regulate human airway smooth muscle proliferation. Am. J. Respir. Cell Mol. Biol. 2007;37:525–531. doi: 10.1165/rcmb.2006-0358OC. [DOI] [PubMed] [Google Scholar]
- 29.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanmugalingam D, Wardlaw AJ, Bradding P. Adhesion of human lung mast cells to bronchial epithelium: evidence for a novel carbohydrate-mediated mechanism. J. Leuk. Biol. 2000;68:38–46. [PubMed] [Google Scholar]
- 31.Duffy SM, Lawley WJ, Kaur D, Yang W, Bradding P. Inhibition of human mast cell proliferation and survival by tamoxifen in association with ion channel modulation. J. Allergy Clin. Immunol. 2003;112:970–977. doi: 10.1016/j.jaci.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv.—Eur. J. Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]


