Abstract
Dentin is a mesenchymal tissue, and, as such, is based on a collagenous matrix that is reinforced by apatite mineral. Collagen fibrils show piezoelectricity, a phenomenon that is used by piezoresponse force microscopy (PFM) to obtain high-resolution images. We applied PFM to image human dentin with 10-nm resolution, and to test the hypothesis that zones of piezoactivity, indicating the presence of collagen fibrils, can be distinguished in dentin. Piezoelectricity was observed by PFM in the dentin intertubular matrix, while the peritubular dentin remained without response. High-resolution imaging of chemically treated intertubular dentin attributed the piezoelectric effect to individual collagen fibrils that differed in the signal strength, depending on the fibril orientation. This study supports the hypothesis that peritubular dentin is a non-collagenous tissue and is thus an exception among mineralized tissues that derive from the mesenchyme.
Keywords: atomic force microscopy, piezoelectricity, dentin, collagen
INTRODUCTION
In vertebrate animals, hard tissues such as bone, dentin, and cementum are generated by mesenchymal cells that express a matrix of fibrillar collagen type I, which subsequently mineralizes through the introduction of apatite nanocrystals into the fibrils and into the extrafibrillar space among fibrils. Peritubular dentin is considered an exception among mineralized tissues, since analysis of recent data has indicated that it forms from an extracellular matrix rich in glutamic acid (Weiner et al., 1999; Gotliv et al., 2006). The difficulty of achieving a defined biochemical analysis of this tissue derives from its small size. Peritubular dentin is defined as the tissue of increased mineral content that surrounds dentin tubules. It extends about 1 to 2 μm. Thus far, it has been observed only in the teeth of larger mammals, and appears to be absent in rodents (Boyde, 1984; Magne et al., 2002; Gotliv et al., 2006). The nanomechanical properties suggest that at least 60 vol% of apatite mineral is present in peritubular dentin (E-modulus, 36.1 GPa), while the bulk intertubular dentin (E-modulus, 20.6 GPa) contains only about 45 vol% (Marshall et al., 2001a). The function of peritubular dentin is unclear, but it may be mechanical, since the increased stiffness of peritubular dentin can increase stresses around tubules and thus affect crack propagation.
There are two contrary models that describe the development of peritubular dentin. It has been suggested that the increased mineral content around the tubules is the effect of continuous mineral deposition from the pulpal fluid, based on the observation that the collagen matrix of the intertubular dentin continues into the peritubular region (Dai et al., 1991). In contrast, Gotliv et al. (2006) provided a detailed analysis of the tubule area in bovine dentin, using time-of-flight secondary ion mass spectrometry, revealing that proteins rich in glumatic acid are predominant in peritubular dentin, while the quantity of amino acids characteristic of collagen, e.g., proline and hydroxyproline, was minor. This led to the conclusion that collagen is either absent or present in only low quantities in peritubular dentin; hence, a specialized non-collagenous matrix must be secreted by the odontoblasts to mineralize the tubule wall to a higher degree than the intertubular dentin. Earlier, Takuma and Eda (1966) showed staining differences between intertubular and peritubular dentin. Ultrastructural differences were observed by Goldberg et al. (1978), using electron microscopy. Thomas (1984) reported on the presence of a lamina limitans, referring to tubular structures that appeared on dentin specimens when demineralized and treated with collagenase. These structures were rich in glycosaminoglycans and were not susceptible to digestion with collagenase, and therefore most likely represented peritubular dentin. Developing dentin was studied by Jones and Boyde (1984), who described structural and biochemical differences between peritubular and intertubular dentin after papain digestion of predentin.
Piezoresponse force microscopy (PFM), a recent advancement of atomic force microscopy (AFM), facilitates the visualization of piezoelectric domains in materials, and can quantify the local piezoelectric constant with nanometer resolution (Gruverman et al., 1998; Rodriguez et al., 2006). Application of a modulating voltage to a conductive AFM tip results in an alternating field at the tip that induces local contraction and dilation of the substrate if it is piezoelectric. An image is created when the induced mechanical vibration of the material is translated into a two-dimensional map of piezoelectric constants in picometer/volt units. Mineralized tissues are known to exhibit piezoelectricity (Fukada, 1995). Initially, hydroxyapatite mineral was assumed to be the component that causes this phenomenon; however, it is now known that the non-centrosymmetric structure of the collagen molecule is responsible for the piezoelectric behavior of collagen fibrils (ElMessiery et al., 1979). This study tested whether individual collagen fibrils are the source of piezoelectricity in dentin, and whether PFM is a useful tool for clarifying if collagen is indeed absent in the peritubular region.
MATERIALS & METHODS
Human third molars with documented history were extracted after informed patient consent was obtained according to protocols approved by the University of California, San Francisco Committee on Human Research. Patients were between 22 and 34 yrs old. Teeth were sterilized by γ-radiation and stored in deionized water at 4°C until prepared. We prepared sagittal mid-coronal sections of 9 teeth (thickness < 1 mm) by polishing through a series of SiO2 papers and with water-based diamond paste to 0.25 μm (Buehler, Lake Bluff, IL, USA). Ultrasonic treatment in water for 10 sec was used to clean the surface. Two groups (n = 3 each) of specimens were investigated: (1) polished only; and (2) polished and etched with10 vol% citric acid for 15 sec and subsequently treated with an aqueous solution of 1 vol% sodium hypochlorite (NaOClaq) for up to 150 sec. The NaOClaq treatment was applied for the non-specific removal of layers of non-collagenous and collagenous proteins. As reported earlier (Habelitz et al., 2002), this procedure removes non-collagenous proteins initially and facilitates the visualization of individual collagen fibrils by atomic force microscopy (AFM).
Dentin sections were glued to metal disks and imaged by AFM (Autoprobe M5, Park Scientific Instruments, Sunnyvale, CA, USA) operating in the PFM mode with conductive cantilevers. The PFM technique is based on the detection of electromechanical responses of a piezoelectric sample to an electrical modulation applied to the conductive AFM tip in contact with the sample surface, as has been explained in detail (Alexe and Gruverman, 2004). While in contact, an ac modulation voltage, Vtip = Vdc + Vaccosωt, is applied between a conductive probing tip in contact with the sample surface and a conductive substrate, Vtip = resulting voltage at the tip, Vdc = direct current voltage applied for topographic imaging, and Vac = voltage from alternate current applied to the conductive tip. The applied modulation voltage induces a local mechanical vibration, if the sample is piezoelectric, by inducing the converse piezoelectric effect. The voltage-induced contraction and dilation of the substrate correspond to the amplitude of the signal, which becomes a function of the voltage Vac applied to the tip, allowing for the calculation of the effective piezoelectric constant at any tip-substrate contact point. The resulting image is a plot of piezoelectric constants in pm/V units over a defined area of the substrate. The unique strength of PFM is that both vertical and lateral components of surface displacement can be measured (Alexe and Gruverman, 2004), providing information on both normal (vertical) and in-plane (lateral) components of the electromechanical response. This allows for the generation of images based on either vertical or lateral deformation of the substrate, referred to as VPFM and LPFM, respectively. While vertical PFM (VPFM) images are based on the electromechanical response at 90 degrees from the surface, lateral PFM (LPFM) images are constructed based on the lateral component of substrate deformation and the electromechanical response in the horizontal plane.
The size of the tip-sample contact area is enlarged, due to the conductive coating of the tip. Hence, the lateral resolution that can be achieved in PFM is reduced after tip coating and is usually on the order of 5 to 10 nm. We obtained images by applying an AC modulation voltage (2.0 V, 15 kHz) to the Pt-coated Si tips (MikroMasch, Tallinn, Estonia; NSC 14, resonant frequency ~ 150 kHz, spring constant k ~ 4.5 N/m). All samples were scanned under dry conditions in contact mode at scan sizes between 3 and 25 μm. Four image types were simultaneously derived from the topographic, error, vertical PFM, and lateral PFM modes.
RESULTS
Atomic force microscopy showed a characteristic dentin surface after polishing with 0.25-μm diamond paste (Fig. 1). Intertubular dentin constituted the bulk of the specimen, intersected by tubules of about 1 μm diameter. (Figs. 1a, 1b, and 1c were obtained from the topographic, VPFM, and LPFM data, respectively.) In the topographic image, a thin ring (~ 1 μm wide) with decreased surface roughness surrounding each tubule was recognizable and could be attributed to peritubular dentin. Both PFM modes showed a moderate piezoelectric signal from the intertubular dentin, with an effective piezoelectric coefficient between 0.15 and 0.25 pm/V. No detectable piezoresponse was obtained from a zone of about 1 μm around the tubule attributed to peritubular dentin (dark area). The tubule itself showed strong piezoresponse in the VPFM mode, which was due to an artifact generated by the sudden change in height at the tubule wall. The LPFM mode was not susceptible to this artifact and showed no piezoelectricity in the tubule. AFM images of dentin from another tooth in topographic and PFM modes were compared at higher resolution (Fig. 2). While peritubular dentin could barely be distinguished from intertubular dentin by topography (Fig. 2a), PFM showed a clear demarcation between the two (Fig. 2b). A thin rim of about 1 μm, which was not piezoactive, surrounded each tubule.
Figure 1.
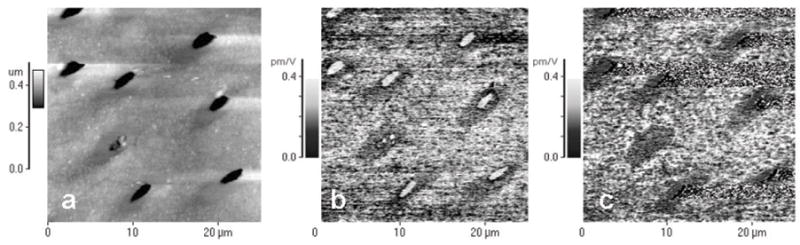
AFM images of human dentin obtained with conductive tips with modulated voltage applied. (a) Topographic image; (b) vertical piezoresponse force mode (VPFM); and (c) lateral piezoresponse force mode (LPFM). Piezoelectric constant reached values of up to 0.25 pm/V (bright areas) and is absent around tubules in the peritubular dentin (dark area).
Figure 2.
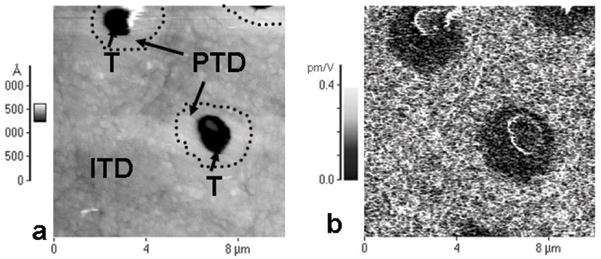
AFM images of human dentin obtained with conductive tips with modulated voltage applied. (a) Topographic image shows 2 dentin tubules (T) surrounded by a brighter rim, attributed to the peritubular dentin (PTD) and the intertubular dentin matrix (ITD). (b) Piezoresponse force image of the same location, showing piezoelectricity of the intertubular dentin, while tubules and the peritubular region do not exhibit a piezoelectric signal (dark).
When intertubular dentin was imaged exclusively at a higher resolution, little contrast was obtained from the topographic mode, due to the low roughness of this area (Fig. 3a). Small areas of piezoelectric activity (Figs. 3b, 3c) were revealed by the piezoelectric response mode. These areas were predominantly circular and of the order of 100 to 300 nm in diameter. To identify the origin of piezoelectricity in intertubular dentin, and to verify whether this effect is associated with collagen fibrils, we used a protocol that facilitated the visualization of collagen fibrils by topography (Marshall et al., 2001b; Habelitz et al., 2002). Dentin specimens were therefore etched and subsequently treated with NaOClaq. Scanning of etched and NaOClaq-treated dentin revealed individual collagen fibrils by contact-mode AFM, as shown in the image derived from the height signal (Fig. 4a), while PFM images (Figs. 4b, 4c) of the same area showed strong piezoelectricity. Both vertical and lateral PFM revealed piezoelectric linear structures associated with the fibrillar structure of collagen in the topographic mode. Interestingly, fibers that showed a strong signal in VPFM mode (bright) lacked pronounced piezoactvity in LPFM and vice versa, indicating that fibril orientation in relation to scanning direction plays an important role in inducing shear piezoelectricity by PFM.
Figure 3.
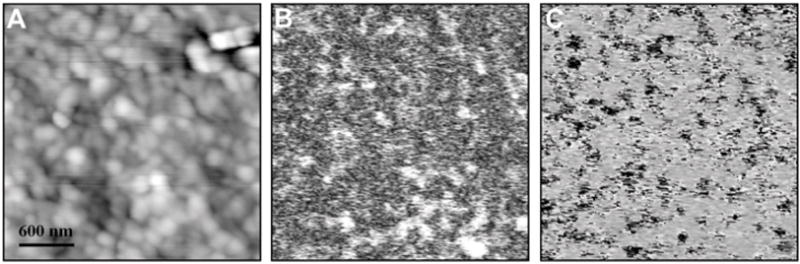
AFM images of intertubular dentin based on (a) topography, (b) VPFM, and (c) VPFM. Small areas around 100 to 300 nm of piezoelectricity can be distinguished.
Figure 4.

AFM images of intertubular dentin that has been etched with citric acid and subsequently treated with NaOClaq solutions to reveal fibrillar collagen type I. (a) Topography; (b) VPFM; (c) LPFM. Piezoresponse is attributed to collagen fibrils and has opposite signal strength in VPFM and LPFM.
DISCUSSION
We applied PFM to untreated and chemically treated dentin substrates, to identify piezoelectric regions in human dentin. Intertubular dentin provided a relatively strong piezoelectric response, while the peritubular dentin remained without a piezoresponse signal. Surface treatments revealed that the piezoelectricity of intertubular matrix was associated with collagen fibrils. We therefore attribute the lack of piezoelectricity in peritubular dentin to the absence of collagen fibrils, confirming earlier observations by different methods (Thomas, 1984; Weiner et al., 1999; Gotliv et al., 2006).
Dentin originates from the expression and secretion of a type-I collagenous matrix and a series of non-collagenous proteins, including phosphoproteins and proteoglycans, through the neural-crest-derived odontoblasts. This specialized cell develops a process that is several millimeters long, but only approximately 1 micrometer in diameter. The odontoblast process embeds itself into the extracellular matrix and remains there during and after mineralization, creating the characteristic tubules in dentin. Peritubular dentin lines the tubules surrounding the odontoblastic processes, and mineralizes simultaneously with the mineralizing front of the intertubular dentin. Our studies support the hypothesis that peritubular dentin is a non-collagenous tissue and a product of the secretion of specialized proteins that facilitate the mineralization of the tubule wall. This finding corroborates the dual secretion model established by Linde (1989), who proposed the expression and secretion of two different matrix compositions, e.g., that phosphoprotein and Gla-proteins are predominantly located at the mineralizing front, while the bulk of the predentin consists of type I collagen and proteoglycans that hydrolyze during mineralization. These spatial and timely differences in matrix composition may be responsible for the generation of the two types of mineralized tissues observed: the intertubular dentin, based on collagen fibrils; and the peritubular dentin, formed by non-collagenous and glutamic-acid-rich proteins. Both phosphoproteins and Gla-proteins have a high affinity for calcium ions and can induce apatite nucleation, suggesting an inductive role in mineralization, most likely giving rise to the higher degree of mineralization in peritubular dentin.
The number of tubules in dentin increased from the DEJ toward the pulp, from about 20,000 to above 50,000 per mm2, respectively (Marshall et al., 1997). In the same way, the amount of peritubular dentin increased from about 3 vol% at the DEJ to at least 60 vol% close to the pulp (Gotliv et al., 2006). Hence, protein expression of the odontoblast shifted from being predominantly collagen at early dentinogenesis to increasingly glutamic-acid-rich proteins of the peritubular dentin toward the end of crown formation. Peritubular dentin appeared to be less prominent in the root, but it is unclear what triggers these shifts in gene expression of the odontoblasts, depending on the location.
Topographic images of etched and NaOClaq-treated dentin juxtaposed to PFM images of the same location revealed that the piezoresponse of collagen fibrils depended on the fibril orientation. The origin of the directional dependence of piezoresponse may be associated mainly with the molecular orientation of tropocollagen within fibrils. Collagen fibrils exhibit shear piezoelectricity, indicating that the displacement of adjacent ions in opposite directions causes the generation of an electrical field. Collagen fibril formation is a self-assembly process, and it has been shown that tropocollagen molecules not only align themselves parallel to each other, but also orient their C- and N-termini in one direction (Prockop and Fertala, 1998). It is suggested that the observed differences in the piezoactivity of individual collagen fibrils may be associated with different or opposing orientations of the tropocollagen in one fibril compared with adjacent ones. Depending on the fibril orientation, the AFM tip will either scan in the direction from the C to the N terminus or the reverse, resulting in shear-induced piezoresponses that are opposed to each other. The effect of molecular orientation on piezoelectricity has been described (Kalinin et al., 2005).
PFM imaging is a suitable and rapid method for the identification of the presence of collagen fibers in tissues at small length scales. Using PFM on human dentin substrates, we have shown that peritubular dentin is a mineralized tissue that does not contain collagen fibrils. This suggests that specific non-collagenous proteins are expressed by the odontoblast process to create the additional rim of higher mineralization in the dentin of larger mammals.
Acknowledgments
Funding was provided by NIH/NIDCR Grant P01DE09859, by UT-Battelle grant DOE DE-AC05-00OR22725, and by National Science Foundation grant DMR02-35632.
References
- Alexe M, Gruverman A. Nanoscale characterisation of ferroelectric materials: scanning probe microscopy approach. Berlin: Springer-Verlag; 2004. [Google Scholar]
- Boyde A. Dependence of rate of physical erosion on orientation and density in mineralised tissues. Anat Embryol (Berl) 1984;170:57–62. doi: 10.1007/BF00319458. [DOI] [PubMed] [Google Scholar]
- Dai XF, Ten Cate AR, Limeback H. The extent and distribution of intratubular collagen fibrils in human dentine. Arch Oral Biol. 1991;36:775–778. doi: 10.1016/0003-9969(91)90045-v. [DOI] [PubMed] [Google Scholar]
- ElMessiery MA, Hastings GW, Rakowski S. Effects of measuring system parameters on the frequency/dependence of strain-related potentials in bone. Med Biol Eng Comput. 1979;17:471–475. doi: 10.1007/BF02447060. [DOI] [PubMed] [Google Scholar]
- Fukada E. Piezoelectricity of biopolymers. Biorheology. 1995;32:593–609. doi: 10.1016/0006-355X(95)00039-C. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Genotelle-Septier D, Weill R. Glycoproteins and proteoglycans in the predentin and dentin matrix in the rat: an ultrastructural study. J Biol Buccale. 1978;6:75–90. [article in French] [PubMed] [Google Scholar]
- Gotliv BA, Robach JS, Veis A. The composition and structure of bovine peritubular dentin: mapping by time of flight secondary ion mass spectroscopy. J Struct Biol. 2006;156:320–333. doi: 10.1016/j.jsb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Gruverman A, Auciello O, Tokumoto H. Imaging and control of domain structures in ferroelectric thin films via scanning force microscopy. Ann Rev Mater Sci. 1998;28:101–123. [Google Scholar]
- Habelitz S, Balooch M, Marshall SJ, Balooch G, Marshall GW. In situ atomic force microscopy of partially demineralized human dentin collagen fibrils. J Struct Biol. 2002;138:227–236. doi: 10.1016/s1047-8477(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Boyde A. Ultrastructure of dentin and dentinogenesis. In: Linde A, editor. Dentin and dentinogenesis. Boca Raton: CRC Press; 1984. pp. 81–134. [Google Scholar]
- Kalinin SV, Rodriguez BJ, Jesse S, Thundat T, Gruverman A. Electromechanical imaging of biological systems with sub-10 nm resolution. Appl Phys Lett. 2005;87:53901–53903. [Google Scholar]
- Linde A. Dentin matrix proteins: composition and possible functions in calcification. Anat Rec. 1989;224:154–166. doi: 10.1002/ar.1092240206. [DOI] [PubMed] [Google Scholar]
- Magne D, Guicheux J, Weiss P, Pilet P, Daculsi G. Fourier transform infrared microspectroscopic investigation of the organic and mineral constituents of peritubular dentin: a horse study. Calcif Tissue Int. 2002;71:179–185. doi: 10.1007/s00223-001-2108-5. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Jr, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. J Dent. 1997;25:441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Habelitz S, Gallagher R, Balooch M, Balooch G, Marshall SJ. Nanomechanical properties of hydrated carious human dentin. J Dent Res. 2001a;80:1768–1771. doi: 10.1177/00220345010800081701. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Yucel N, Balooch M, Kinney JH, Habelitz S, Marshall SJ. Sodium hypochlorite alterations of dentin and dentin collagen. Surf Sci. 2001b;491:444–455. [Google Scholar]
- Prockop DJ, Fertala A. The collagen fibril: the almost crystalline structure. J Struct Biol. 1998;122:111–118. doi: 10.1006/jsbi.1998.3976. [DOI] [PubMed] [Google Scholar]
- Rodriguez BJ, Kalinin SV, Shin J, Jesse S, Grichko V, Thundat T, et al. Electromechanical imaging of biomaterials by scanning probe microscopy. J Struct Biol. 2006;153:151–159. doi: 10.1016/j.jsb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Takuma S, Eda S. Structure and development of peritubular matrix in dentin. J Dent Res. 1966;45:683–692. [Google Scholar]
- Thomas HF. The lamina limitans of human dentinal tubules. J Dent Res. 1984;63:1064–1066. doi: 10.1177/00220345840630081101. [DOI] [PubMed] [Google Scholar]
- Weiner S, Veis A, Beniash E, Arad T, Dillon JW, Sabsay B, et al. Peritubular dentin formation: crystal organization and the macromolecular constituents in human teeth. J Struct Biol. 1999;126:27–41. doi: 10.1006/jsbi.1999.4096. [DOI] [PubMed] [Google Scholar]


