Abstract
Sleep and sleep-dependent learning are impaired in male cocaine users during abstinence, but for female users little is known. Cocaine dependent men (n=12) and women (n=14), and control participants (n=19) participated in this study of sleep and sleep-dependent learning. Cocaine users were assessed at 3, 10 and 20 days of abstinence and controls were studied over one night. Total sleep time, sleep efficiency and overnight motor learning were the main outcome measures. Cocaine dependent men compared to women exhibited deteriorations in sleep time, sleep efficiency, and overnight learning as abstinence progressed from 3 to 20 days. At abstinence day 3, cocaine dependent men and women were no different than control participants in the main outcomes. However, there were significant differences between cocaine men at abstinence day 20 and controls in sleep time and sleep-dependent learning, but no differences between controls and cocaine dependent women. There is growing evidence that sleep disturbances are associated with cocaine abuse and abstinence and have functional consequences that may be relevant to the development of effective treatments. The absence of sleep disturbances in women suggests a need to understand the mechanisms underlying these differences, as such knowledge could lead to novel therapies in cocaine dependence.
KW: cocaine, sleep, gender, cognition, learning
INTRODUCTION
Cocaine dependence is a significant societal problem with a lifetime prevalence of over 30 million persons in the United States affected(Office of Applied Studies - Substance Abuse and Mental Health Services Administration, 2006). Despite biological evidence that women are more vulnerable than men to many aspects of cocaine addiction(Lynch, 2006), the prevalence in men is nearly double that in women(Office of Applied Studies - Substance Abuse and Mental Health Services Administration, 2006). This difference is common to substances of abuse and may largely reflect psychosocial issues that put men at greater risk for substance use and dependence. Nevertheless, there may be biological differences that put men at greater risk as well. All differences are potentially important, as they may inform development of interventions to treat or prevent cocaine dependence, a disorder for which there is no FDA approved pharmacotherapy.
The lack of an FDA approved pharmacotherapy despite numerous trials has led to a greater exploration of the physiological consequences of cocaine dependence. As the physiological consequences of cocaine dependence may make abstinence more difficult, treating these consequences could promote abstinence and hence be helpful in treating this illness. One such consequence could be poor sleep(Johanson et al., 1999; Kowatch et al., 1992; Thompson et al., 1995). Recent evidence has confirmed that sleep disturbances in male cocaine users are significant and possibly long-lasting(Morgan et al., 2008, 2006; Pace-Schott et al., 2005), with a deterioration of sleep and sleep-related cognitive function worsening over 3 weeks of abstinence.
Although sleep in men and women is largely similar(Williams et al., 1974), sex hormones can have strong effects on sleep(Friess et al., 1997; Gambacciani et al., 2005; Montplaisir et al., 2001). Such effects could be most pronounced in pathological conditions where sleep disturbances are prominent and sex hormones play an important role in pathophysiology. Given the well known effects of sex hormones on cocaine dependence and sleep disturbances associated with cocaine dependence(Gawin and Kleber, 1986; Lynch, 2006; Morgan et al., 2008, 2006; Quinones-Jenab, 2006), we hypothesized that sleep in female cocaine users would differ in abstinence from that in men, and that these differences could have functional consequences on sleep-dependent cognitive performance.
METHODS
Participants
Actively using, treatment-seeking, crack-cocaine dependent participants were recruited from the community for participation in long-term inpatient research studies that also offered standard of care individual and group therapy. Diagnosis of cocaine dependence was confirmed by the Structured Clinical Interview for DSM-IV (SCID-IV) and a positive urine test for cocaine metabolites; non-substance related psychiatric co-morbidity was a criterion for exclusion. Of the 33 potential participants (14 women and 19 men) who met criteria for participation in this study, 26 (12 women and 14 men) participated. Control participants (9 female and 10 male) with no psychiatric illness were also recruited from the community. Participants underwent clinical screening including medical and psychiatric history, physical examination, and laboratory tests as part of their admission to the research unit where the study took place. All participantwere free from major medical conditions and none had been prescribed psychoactive medications in the past 6 months. No participant reported signs or symptoms of sleep apnea, restless leg syndrome, periodic leg movements during sleep, or somnambulism when assessed during clinical interview or as reported on the Harvard Sleep Disorders Screening Questionnaire (Morgan et al. 2006). All participants provided informed consent for participation in this Institutional Review Board approved study. Cocaine dependent women had all had at least three consecutive menstrual cycles of 26–30 days duration not taking hormonal contraceptives just prior to study start. Study participants received $50 for their participation in this study.
Current use of cocaine, alcohol, and marijuana were assessed by the Time Line Follow Back method for all participants. Use of other addictive substances except nicotine and caffeine in the past 90 days was exclusionary for all participants, and use of marijuana or cocaine was exclusionary for control participants. None of the control participants, 11 (out of 12) of the female cocaine dependent, and 13 (out of 14) of the male cocaine dependent participants were smokers. Mean (± S.D.) number of cigarettes smoked per day among the smokers was 14 ± 9 (men) and 9 ± 7 (women, difference not statistically significant).
Procedures
Setting
Cocaine dependent participants were admitted to a 12-bed state psychiatric research facility - a full-service inpatient psychiatric unit with a highly structured daily routine, including individual and group therapy in a fully co-educational setting. Female participants were admitted to the hospital while in the menstrual or early follicular phase of the menstrual cycle. At the time of hospital admission, assessment of last cocaine use was made and research study assessments were scheduled. Participants participated in substance abuse treatment and other unit activities while enrolled in the study. All meals and snacks were provided on the caffeine-free unit and four-times per day “fresh-air” breaks allowed smokers to smoke at the same times each day. All participants were checked by staff at least once every 15 minutes while on the unit, and were monitored constantly if off the unit for protocol related testing or “fresh-air” breaks. Daytime napping was not permitted prior to sleep assessment and was strictly enforced by unit staff. Urine toxicology screens were administered three-times per week.
Control participants were studied as outpatients on two consecutive weekdays (one intervening night). Participants were instructed to maintain their typical bedtimes, and were instructed not to drink caffeine after 5pm on the first day or alcohol at anytime during the two study days (control participants reported caffeine use and absence of alcohol use during the study on a questionnaire). Menstrual phase at the time of testing varied in female controls.
Motor Sequence Task (MST)
On the 3rd and 10th full day of abstinence from cocaine, and on a day between the 17th and 23rd day of cocaine abstinence (hereafter referred to as Abstinence Day 20), cocaine dependent participants were trained on a version of the motor sequence task and were re-tested 24 hours later to assess motor learning(Walker et al., 2002) (n.b. the 3rd day of abstinence could have been the 1st, 2nd, or 3rd day of hospital admission depending on the participant’s self report of last cocaine use). Training and re-test were administered approximately 1 hour after one of the four daily “fresh air” breaks when smoking was allowed. In the motor sequence task the participant is required to type a 5-digit sequence (e.g. 4-1-3-2-4) repeatedly on a computer keyboard with their non-dominant hand as quickly and as accurately as possible. Each testing session consists of twelve 30-second trials separated by 30-second rest periods. Each participant was trained on three of four distinct versions of the task that were used (i.e. for Day 3,10, and 20 of abstinence) that do not exhibit cross-over learning(Morgan et al., 2006). Control participants were each trained on one version of the tasks used and were tested in the same environment as the cocaine dependent participants. Versions of the task used and the order in which they were administered was varied from participant to participant. Overnight learning was measured in the typical fashion by comparing the number of correctly typed sequences in the last two trials of the initial session with that in the first two trials of the retest session, and has been shown to be sleep dependent in young, healthy adults(Walker et al., 2002).
Sleep Assessment
On the night of the 3rd, 10th, and 20th day of abstinence from cocaine, or on the night following motor sequence task training for control participants, sleep was assessed using the Nightcap sleep monitor(Ajilore et al., 1995). Participants went to sleep in their own rooms at their accustomed time, between 9:30pm and 11:30pm, and were allowed to sleep ad libitum until 7:30am (hence all participants had at least 8 hours available for sleep each night, and time spent in bed was otherwise controlled by the participants). All participants attached the Nightcap themselves after being trained and tested by staff on how to do so.
The Nightcap is a two-channel recording device that distinguishes wake, REM sleep, and non-REM sleep. One channel of the Nightcap monitors eye movement and the other monitors body movements. The eyelid sensor consists of a disposable adhesive-backed piezoelectric film that is placed on the upper eyelid and detects movements of the eye and lid. The body movement sensor is a cylindrical, multipolar mercury switch that detects head rotations. These sensors are connected by 1 meter cables to the main Nightcap unit, a 7 cm × 11.5 cm × 2.5 cm case containing signal detectors, A/D converters, a clock, an RS-232 serial port (for downloading data) and a microprocessor with 32 Kbyte of RAM powered by an internal 9-V battery. The mount for the disposable eyelid sensor and the body movement sensor are contained in a bandana that is worn by the participant on the head. The recording unit is placed on the participant’s nightstand or under the pillow and can be carried easily to go to the bathroom, etc. without disconnecting or turning off the recorder. Total sleep time (TST) was the number of minutes of sleep measured by the Nightcap on each of the study nights. Sleep efficiency was measured by the Nightcap as the percentage of time spent in sleep out of the total time spent in bed between lying down to sleep and final awakening and is therefore a function of sleep latency (the time required to fall asleep), total sleep time, and time spent awake after the onset of sleep.
Statistical Analysis
Cocaine dependent women vs. men across abstinence
Linear Mixed Effect(Laird and Ware, 1982) models were implemented using SAS, version 8 software (SAS institute, Cary NC) to assess differences in total sleep time, sleep efficiency, and motor sequence task learning. Sex (male vs. female cocaine users) was the between subject factor and Abstinence Day (3, 10, and 20) was the within subjects factor. Log transformation was applied to make the distribution of all the outcome variables symmetric.
Cocaine dependent women vs. men vs. control subjects
One-way ANOVA was used to assess differences in the outcome measures among three groups: cocaine dependent women at abstinence day 20, cocaine dependent men at abstinence day 20, and control participants. Foreseen practical limitations to the execution of the study led to male and female control subjects being treated as a single group a priori. This was considered non-ideal but acceptable because there is no evidence that they would differ in these measures. In retrospect, they were well-matched (TST: 413 ± 30 vs. 414 ± 20 minutes, SE: 86 ± 4% vs. 91± 4%, MST: 1.1 ± 0.6 vs. 0.9 ± 0.7 sequences for males and females, respectively) and treating them as separate (post-hoc) had no effect on the results. Abstinence day 20 was chosen as the point of comparison to the single night of control data because previous work indicated that the greatest difference between cocaine dependent persons and non-substance dependent persons would occur at or beyond two and a half weeks of abstinence(Morgan et al., 2006).
All post-hoc pair-wise comparisons were assessed with the Tukey-Kramer test.
RESULTS
Subject Characteristics
Subject characteristics are shown in Table 1. Cocaine dependent men and women were well-matched in age and education. There were no statistically significant differences between cocaine dependent men and women in amount of cocaine, alcohol, or cannabis used in the past 30 days, or in the Fagerstrom Test of Nicotine Dependence. Control subjects averaged about 5 years younger than cocaine dependent participants, and were somewhat more educated.
Table 1.
Baseline subject characteristics
| Cocaine Men | Cocaine Women | Control Subjects | |
|---|---|---|---|
| N | 12 | 14 | 19a |
| Ageb | 37 ± 5 | 36 ± 6 | 31 ± 11 |
| Education (years)b | 12 ± 2 | 12 ± 2 | 15 ± 2 |
| African-American/Caucasian/Hispanic (N) | 4/7/1 | 9/5/0 | 2/15/2 |
| Cocaine use (grams; last 30 days)c | 33 ± 30 | 22 ± 15 | 0 ± 0 |
| Alcohol use (drinks; last 30 days)c | 54 ± 90 | 75 ± 125 | 8 ± 8 |
| Cannabis use (joints last 30 days)c | 0 ± 13 | 0 ± 9 | 0 ± 0 |
| Fagerstrom Test of Nicotine Dependencec | 6 ± 2 | 5 ± 3 | 0 ± 0 |
Control subjects were 9 women and 10 men
Mean ± S.D.
Median ± semi-interquartile range
Cocaine dependent females and males across abstinence
Sleep Efficiency
An overall main effect on sleep efficiency was observed (F5,54 = 4.17, p < 0.003). Sleep efficiency in women was greater than in men (p < 0.02) and there was a significant sex by days abstinent interaction (p < 0.03) such that sleep efficiency in men decreased across abstinence whereas sleep efficiency in women did not; indeed, sleep efficiency differed between men and women in post-hoc tests only at 20 days abstinent (p < 0.02; Figure 1). This difference was present despite men spending less time in bed as abstinence progressed (from 443±25 min at 3 days abstinent to 393±20 min and 357 ± 22 min at 10 and 20 days abstinent respectively) and was due to increasing sleep onset latency (11 ± 3 min at 3 days abstinent, 20 ± 7 min and 20 ± 4 min at 10 and 20 days abstinent) and increasing time awake after sleep onset (24 ± 5, 35 ± 12, 37 ± 9 min). In women, time in bed (441 ± 21, 423 ± 14, 428 ± 13 min), sleep onset latency (19 ± 3, 16 ± 2, 12 ± 2 min), and time awake after sleep onset (25 ± 8, 34 ± 8, 26 ± 7 min) changed little or improved (sleep latency) from 3 to 20 days abstinent.
Figure 1.
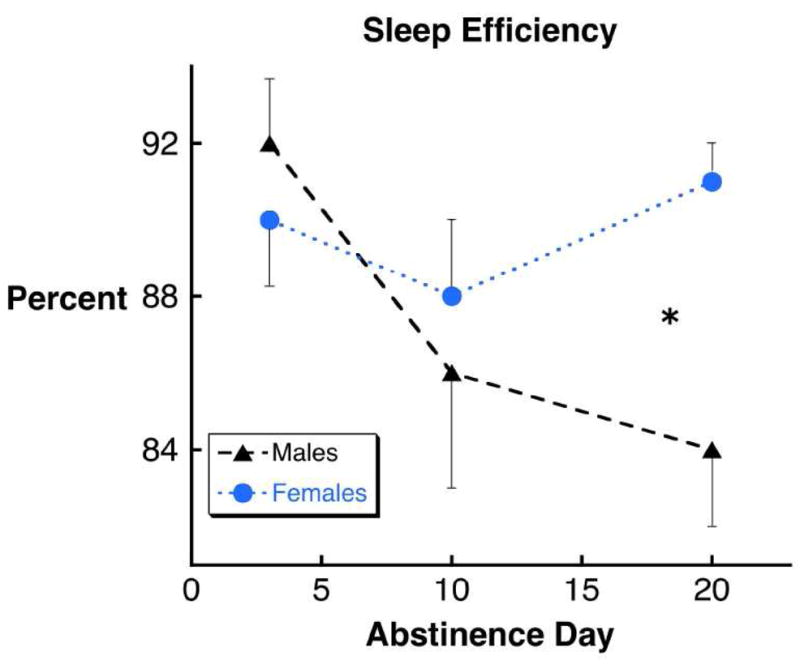
Sleep efficiency decreased in male but not female cocaine dependent persons from 3 to 20 days of abstinence (see text) and was significantly worse in men at 20 days abstinence (*, p < 0.02)(Error bars indicate SEM).
Total Sleep Time
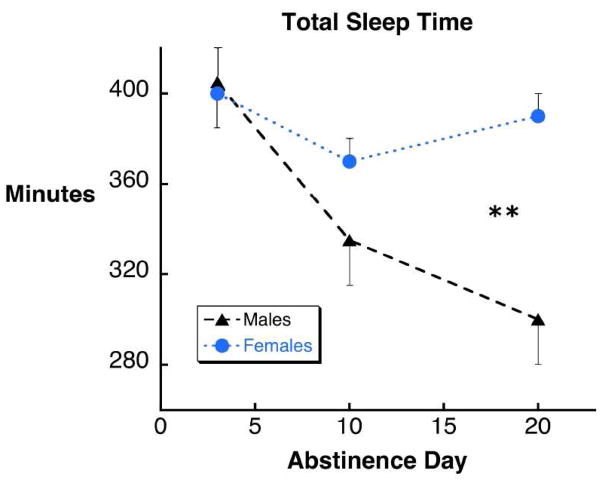
There was an overall main effect on total sleep time (F5,54 = 3.88, p < 0.005). Total sleep time decreased significantly as abstinence progressed from 3 to 20 days (p < 0.003). This change was apparent only in male participants, and total sleep time was significantly less in male participants than female participants at 20 days abstinence (p < 0.002; Figure 2).
Figure 2.
The decrease in total sleep time from 3 to 20 days of abstinence in cocaine dependent persons (see text) was most pronounced in male subjects with a statistically significant difference between men and women at 20 days abstinent (**, p< 0.002).
Motor Sequence Task Learning
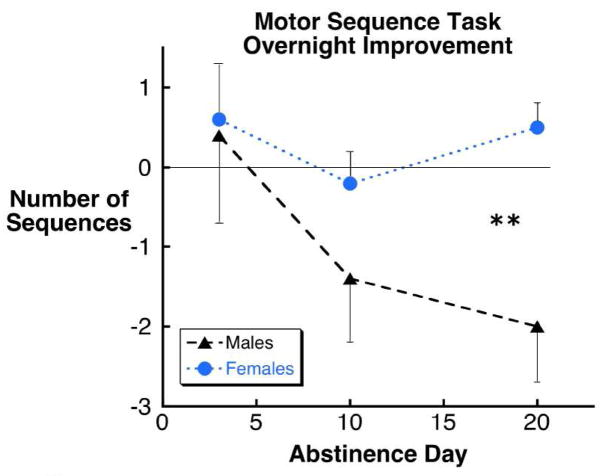
An overall main effect on motor sequence task learning was observed (F5,54 = 3.01, p < 0.02). Strong statistical trends for sex difference (p < 0.08), difference with number of days abstinent (p < 0.08) and sex by days abstinent interaction (p< 0.06) were present with women performing better than men, and men’s performance deteriorating with progressive abstinence, such that men performed significantly worse at 20 days abstinence than women (p < 0.01; Figure 3).
Figure 3.
Male cocaine dependent participants showed overnight worsening at 20 days abstinence on the motor sequence task that was significantly worse than females (**, p < 0.01).
Comparisons to Control Subjects
Cocaine dependent males and females at 20 days abstinence and controls were compared. Statistically significant differences were observed for total sleep time (F2,36 = 6.59, p < 0.004), and motor sequence task learning (F2,36 = 9.11, p < 0.0001), and a statistical trend was observed for sleep efficiency (F2,36 = 2.90, p < 0.07). Cocaine dependent males had significantly less total sleep time (299 ± 20 vs. 413 ± 20 minutes; p < 0.003) and worse overnight motor learning (−2.0 ± 0.67 vs. +1.0 ± 0.45 additional sequences; p < 0.0003) than control participants, and as apparent in Figures 1–3, significantly less total sleep time (p < 0.002), lower sleep efficiency (p < 0.03) and worse overnight motor learning (p < 0.006) than cocaine dependent females. No differences between cocaine dependent females and control participants were observed. Separating the control group in male and female cohorts had no effect on these results: cocaine dependent males had significantly less total sleep time and worse overnight learning than male controls (p < 0.01 and p< 0.005 respectively) and female controls (p < 0.001, p < 0.01).
DISCUSSION
The present findings are the first indication of a functional sex difference in sleep in persons with substance dependence of any kind. The drops in sleep efficiency, total sleep time, and sleep-dependent procedural learning in male subjects are consistent with prior work (for review see(Morgan and Malison, 2007)), and the present work confirms these findings in a relatively large sample of treatment seeking, cocaine dependent persons. However, the lack of such drops in female participants is unprecedented, as cocaine dependent women had not been appreciably included in objective sleep studies previously.
The possibility that male and female cocaine users have different sleep characteristics during abstinence and across the menstrual cycle is a reasonable hypothesis given the evidence that progesterone affects circadian rhythms and sleep(Baker and Driver, 2007; Cagnacci et al., 1996; Moore-Ede et al., 1982), and is markedly elevated during the luteal phase. However, the finding is still somewhat surprising as healthy men and women matched for age tend to have similar sleep(Voderholzer et al., 2003; Williams et al., 1974) (but see also e.g. (Fukuda et al., 1999; Goel et al., 2005)), and fluctuations of neurosteroid levels within the menstrual cycle of healthy women have only modest effects on polysomnographic sleep measurement(Baker and Driver, 2007; Steiger, 2003). Nevertheless, there is considerable and growing evidence that progesterone has striking effects on sleep in susceptible populations such as postmenopausal women and men.
In postmenopausal women, progesterone may improve sleep efficiency and self-reported sleep quality(Gambacciani et al., 2005; Montplaisir et al., 2001). In men, progesterone administration decreases latency to slow-wave sleep, and increases non-REM sleep time(Friess et al., 1997), consistent with the increased levels of the GABAA receptor active metabolite allopregnanolone(Majewska et al., 1986). Such effects are consistent with human and animal studies that show that progesterone and related neurosteroids have hypnotic effects, with decreased sleep latency, increased non-REM sleep, and in some respects a benzodiazepine-like effect on the sleep EEG profile(Rupprecht, 2003; Steiger, 2003) (but without the hangover effects on next morning cognitive performance(Gron et al., 1997)). Although hormone levels were not measured at the relevant time points in this study, all female subjects entered the study in the menstrual or early follicular phase. Hence it is likely that the 20 days abstinent time point reflected luteal phase levels of progesterone in most of the female participants. That is, progesterone levels were likely highest and most different from male participants at 20 days abstinent. If so, then it is possible that the sleep-promoting effects of progesterone and its metabolites may have offset in female participants the deteriorating sleep and sleep-dependent learning seen in male participants. Although somewhat speculative, this interpretation would be consistent with work suggesting a protective role of progesterone in cocaine dependence(Evans and Foltin, 2006; Fox et al., 2008; Sinha et al., 2007; Sofuoglu et al., 2002; Sofuoglu et al., 1999; Terner and de Wit, 2006), and would support the study of progesterone or related compounds as possible therapies for cocaine dependence.
Several limitations of this study must be considered, most importantly the lack of hormonal data, data beyond one menstrual cycle, or data from women at different menstrual phases relative to abstinence that could have distinguished abstinence effects from menstrual phase effects. In addition, participants in this study had no restrictions on sleep outside of the three experimental days in this study. Although napping was not permitted on those three days, subjects could choose their own bedtime within the restrictions of inpatient unit. Furthermore, polysomnographic (PSG) sleep recording was not done, but rather an ambulatory sleep monitor was used that has shown good correlation with PSG-measured total sleep time and sleep efficiency(Ajilore et al., 1995). Although the Nightcap sleep monitor is minimally intrusive, it is possible that the first night of recording may have been subject to a first night effect. However, in this study such an effect would have been more likely to diminish the observed results than augment them. Although time in bed was not fixed in this naturalistic study, male subjects spent less time in bed as abstinence progressed, so the drop in sleep efficiency was not a result of more time spent in bed. Rather, the drop in total sleep time observed in male subjects was greater than the drop in time in bed, leading to the deterioration in sleep efficiency, and suggesting severely compromised sleep.
Another potential limitation is the non-statistically significant age difference between controls and cocaine dependent participants. As sleep time and efficiency tend to deteriorate with age (Williams et al. 1974), the 5-year difference in age might have had some effect on the results. In addition, unrecognized differences in why cocaine dependent men and women seek treatment may have influenced the results. For example, men in this study may have been more likely to seek treatment because of some awareness of the deleterious neurophysiological effects of chronic cocaine, whereas women may have been more likely to seek treatment to escape an abusive relationship or other untenable social situation. Such a difference could have amplified the apparent difference in sleep and learning observed between men and women; unfortunately, detailed assessment of these possible factors was not performed.
There is now evidence that sleep disturbance associated with cocaine dependence and abstinence has functional consequences and may be relevant to the development of effective treatments(Morgan and Malison, 2007; Morgan et al., 2008, 2006). The absence of this finding in women with cocaine dependence suggests that therapies that target sleep or cognitive consequence of cocaine use may be more helpful in men than women, and points to a possible direction in the development of such treatments. Indeed, in the absence of such treatments, there is some evidence that women may respond better to treatment than men. Two studies that reported gender differences in treatment outcomes in cocaine users found that women, despite having similar or worse prognostic factors (e.g. severity of use, comorbidity, demographics), were more likely to remain abstinent and/or have reduced cocaine use at 6-month follow-up (Kosten et al., 1993; Weiss et al., 1997). Hence, the current findings should be confirmed in future polysomnographic sleep studies and the mechanism of this sex difference should be explored.
Acknowledgments
Funding for this study was provided by NIH grants P50-DA16556(RS); K02-DA17232(RTM); K12RR17594(PTM); and UL1-RR024139(RS); and the Connecticut Department of Mental Health and Addiction Services (DHMAS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajilore O, Stickgold R, Rittenhouse CD, Hobson JA. Nightcap: laboratory and home-based evaluation of a portable sleep monitor. Psychophysiology. 1995;32:92–98. doi: 10.1111/j.1469-8986.1995.tb03410.x. [DOI] [PubMed] [Google Scholar]
- Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–622. doi: 10.1016/j.sleep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Soldani R, Laughlin GA, Yen SS. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol. 1996;80:25–29. doi: 10.1152/jappl.1996.80.1.25. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Fox H, Hong K, Paliwal P, Morgan P, Sinha R. Altered levels of sex and stress steroid hormones assessed daily over a 28-day cycle in early abstinent cocaine dependent females. Psychopharmacology (Berl) 2008;195:527–536. doi: 10.1007/s00213-007-0936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friess E, Tagaya H, Trachsel L, Holsboer F, Rupprecht R. Progesterone-induced changes in sleep in male subject s. Am J Physiol. 1997;272:E885–891. doi: 10.1152/ajpendo.1997.272.5.E885. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Honma H, Kohsaka M, Kobayashi R, Sakakibara S, Kohsaka S, Koyama T. Gender difference of slow wave sleep in middle aged and elderly subjects. Psychiatr Clin Neurosci. 1999;53:151–153. doi: 10.1046/j.1440-1819.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Cappagli B, Monteleone P, Benussi C, Bevilacqua G, Vacca F, Genazzani AR. Effects of low-dose, continuous combined hormone replacement therapy on sleep in symptomatic post menopausal women. Maturitas. 2005;50:91–97. doi: 10.1016/j.maturitas.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Gawin F, Kleber H. Abstinence symptomatology and psychiatric diagnosis in chronic cocaine abusers. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Goel N, Kim H, Lao RP. Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol Int. 2005;22:905–915. doi: 10.1080/07420520500263235. [DOI] [PubMed] [Google Scholar]
- Gron G, Friess E, Herpers M, Rupprecht R. Assessment of cognitive performance after progesterone administration in healthy male volunteers. Neuropsychobiology. 1997;35:147–151. doi: 10.1159/000119336. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Roehrs T, Schuh K, Warbasse L. The effects of cocaine on mood and sleep in cocaine-dependent males. Exp Clin Psychopharmacol. 1999;7:338–346. doi: 10.1037//1064-1297.7.4.338. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- Kowatch RA, Schnoll SS, Knisely JS, Green D, Elswick RK. Electroencephalographic sleep and mood during cocaine withdrawal. J Addict Dis. 1992;11:21–45. doi: 10.1300/J069v11n04_03. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Lorrain J, Denesle R, Petit D. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause. 2001;8:10–16. doi: 10.1097/00042192-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Moore-Ede M, Sulzman F, Fuller C. The clocks that time us. Cambridge, MA: Harvard University Press; 1982. [Google Scholar]
- Morgan P, Malison R. Cocaine and sleep: early abstinence. The Scientific World Journal. 2007 doi: 10.1100/tsw.2007.209. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Cocaine, sleep architecture, and visual learning. Addiction. 2008 doi: 10.1111/j.1360-0443.2008.02233.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleep-dependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–249. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Office of Applied Studies -Substance Abuse and Mental Health Services Administration. Results from the 2006 National Survey on Drug Use and Health: National Findings. 2006.
- Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, Hart CL, Clarke D, Morgan A, Hobson JA. Sleep quality deteriorates over a binge--abstinence cycle in chronic smoked cocaine users. Psychopharmacology (Berl) 2005;179:873–883. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V. Why are women from Venus and men from Mars when they abuse cocaine? Brain Res. 2006;1126:200–203. doi: 10.1016/j.brainres.2006.08.109. [DOI] [PubMed] [Google Scholar]
- Rupprecht R. Neuroactive steroids: mechanism of action and neuropsyhopharmacological properties. Psychoneuroendocrinology. 2003;28:139–168. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox H, Hong K, Sofuoglu M, Morgan P, Bergquist K. Sex steroid hormones, stress response and drug craving in cocaine dependent women: implications for relapse susceptibility. Experimental and Clinical Psychopharmacology. 2007;15:445–452. doi: 10.1037/1064-1297.15.5.445. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–435. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hat sukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Steiger A. Sleep and endocrine regulation. Front Biosci. 2003;8:s358–376. doi: 10.2741/1055. [DOI] [PubMed] [Google Scholar]
- Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84:1–13. doi: 10.1016/j.drugalcdep.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Gillin JC, Golshan S, Irwin M. Polygraphic sleep measures differentiate alcoholics and stimulant abusers during short-term abstinence. Biol Psychiatry. 1995;38:831–836. doi: 10.1016/0006-3223(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Voderholzer U, Al -Shajlawi A, Weske G, Feige B, Reimann D. Are there gender differences in objective and subjective sleep measures? A study of insomniacs and healthy controls. Depress Anxiety. 2003;17:162–172. doi: 10.1002/da.10101. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend. 1997;44:35–40. doi: 10.1016/s0376-8716(96)01319-1. [DOI] [PubMed] [Google Scholar]
- Williams RL, Karacan I, Hursch CJ. Electroencephalography (EEG) of Human Sleep: Clinical Applications. New York: John Wiley & Sons; 1974. [Google Scholar]