Abstract
5′-AMP-activated kinase (AMPK) plays a key role in the regulation of cellular lipid metabolism. The contribution of vesicular exocytosis to this regulation is not known. Accordingly, we studied the effects of AMPK on exocytosis and intracellular lipid content in a model liver cell line. Activation of AMPK by metformin or 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) increased the rates of constitutive exocytosis by about 2-fold. Stimulation of exocytosis by AMPK occurred within minutes, and persisted after overnight exposure to metformin or AICAR. Activation of AMPK also increased the amount of triacylglycerol (TG) and apolipoprotein B (apoB) secreted from lipid-loaded cells. These effects were accompanied by a decrease in the intracellular lipid content indicating that exocytosis of lipoproteins was involved in these lipid-lowering effects. While AMPK increased the rates of fatty acid oxidation (FAO), the lipid-lowering effects were quantitatively significant even after inhibition of FAO with R-etomoxir. These results suggest that hepatic AMPK stimulates constitutive exocytosis of lipoproteins, which may function in parallel with FAO to regulate intracellular lipid content.
Keywords: AMPK, FM1-43, constitutive exocytosis, Nile red, intracellular lipids
Introduction
AMPK plays a key role in the regulation of cellular metabolism. AMPK is regulated by cellular energy stores and functions at a critical interface between many metabolic pathways [1, 2]. Historically, the primary function of AMPK was to generate ATP when cellular levels are low. More recent studies suggest that AMPK also plays an important role in the regulation of intracellular lipid content [3]. Specifically, activation of hepatic AMPK by the adipocyte-derived hormones stimulates FAO and inhibits synthesis of lipids [4–6]. Similarly, pharmacological activation of AMPK by metformin prevents intracellular accumulation of lipids, and reverses fatty liver in obese humans and mice [7, 8]. The cellular mechanisms responsible for these beneficial effects of AMPK are still poorly understood.
While there has been considerable progress in identifying molecular targets of AMPK involved in the regulation of intracellular lipid content, it has been difficult to study direct cellular effects of AMPK because in intact animals there are significant changes in regulatory hormones and other signaling molecules that accompany a decrease in intracellular lipid content. Thus, the cell-based model used here has specific advantage because it eliminates the effects of other organs.
Vesicular exocytosis plays a key role in the regulation of cellular lipid metabolism. Free fatty acids are chemically modified in the cytosol into biologically inert TG. Subsequently, TG are packaged together with apoB to form hepatic lipoproteins which are secreted through constitutive exocytosis [9]. Consequently, changes in the rates of constitutive exocytosis could modulate the intracellular lipid content in liver cells. Hepatocytes are generally considered to be constitutive secretory cells in which exocytosis occurs at a constant rate. However, our recent studies have demonstrated that liver cells are also capable of regulated exocytosis [10, 11]. Based on these considerations, the purpose of these studies was to assess the potential role of AMPK in the regulation of constitutive exocytosis of lipoproteins.
Materials and Methods
Cell cultures
All studies were performed in HTC cells, a model liver cell line derived from rat hepatoma. Cells were plated and incubated overnight in DMEM (Gibco, Carlsbad CA) supplemented with 10% FBS and 2% penicillin-streptomycin solution (Gemini, Woodland CA). Cells were used within 24 h after plating.
Solutions for imaging
All imaging experiments were performed after washing culture medium with a standard physiologic solution that contained 142 mM NaCl, 4 mM KCl, 1 mM KH2PO4, 2 mM MgCl2, 2 mM CaCl2, 10 mM Hepes, 10 mM D-glucose. The pH was 7.25, and osmolarity was 295–305 mosmol kg−1. All compounds were purchased from Sigma-Aldrich (St. Louis, MO).
Imaging and analysis
For imaging experiments, cells were plated on coverslips in the recording chambers, and incubated overnight in the media as described above. Media was washed with a standard physiologic solution, and cells were visualized through a 40X oil immersion objective (NA= 1.35) and an Olympus IX71 microscope. The images were acquired with a Sensicam QE camera controlled by a SlideBook 3.0 software (Intelligent Imaging Innovations, Denver, CO). Analysis of the fluorescence images was also performed using SlideBook 3.0. FM1-43 fluorescence was measured by drawing a region of interest over the entire cell, and background fluorescence was measured from the regions with no cells. Background fluorescence was subtracted from the cellular fluorescence.
Measurement of exocytosis
The rates of exocytosis were measured using a fluorescent dye N-(3-triethylammoniumpropyl)-4-(4-(dibutylamino) styryl) pyridinium dibromide (FM1-43) as previously described [12]. FM1-43 binds to membranes but does not permeate through lipid bilayers. The dye is not fluorescent in solution, but it becomes fluorescent upon binding to the biological membranes [13]. In the presence of FM1-43 in the extracellular solution, exocytic insertion of vesicles into the plasma membrane results in staining of the vesicle membrane and an increase in FM1-43 fluorescence. Thus, the overall change in FM1-43 fluorescence provides in real time a measure of the sum of all exocytic events [14, 15]. For these experiments, cells were stained with 4 μM of FM1-43 (Molecular Probes, Eugene OR). The fluorescence was excited with an excitation filter (peak 480 nm) and collected with an emission filter (peak 535 nm). FM1-43 fluorescence was measured every 30 sec using exposures of 200 msec. The initial staining of the plasma membrane was used to determine a baseline fluorescence (100%), and the rates of exocytosis (% per min) were calculated from the time course of FM1-43 fluorescence.
To assess whether metformin and AICAR modulate the fluorescent properties of FM1-43, cells were treated with DMSO (20%) for 10 min. Bright-field images confirmed that this manipulation disrupted cell membrane, and the intracellular compartments leaked out. The remaining cell debris were subsequently stained with FM1-43, and exposed to metformin (1 mM) or AICAR (0.5 mM). Both drugs did not change FM1-43 fluorescence (n=14 cells, data not shown) indicating that metformin and AICAR have no effect on the fluorescent properties of FM1-43.
Measurement of cell surface area
Cell surface area was assessed by measuring cell capacitance in the whole-cell configuration of the patch-clamp techniques as previously described [15, 16]. This technique is based on the fact that capacitance of the plasma membrane is proportional to cell surface area (~ 10 fF/μm2). Thus, changes in the capacitance are directly related to the changes in cell surface area. Cells were dialyzed through a patch pipette with a standard intracellular solution that contained 133 mM KCl, 10 mM NaCl, 1 mM EGTA, 0.5 mM CaCl2, 2 mM MgCl2, and 10 mM HEPES/NaOH. The pH was 7.25, and osmolarity was ~ 272 mosmol kg−1. Holding potential was −40 mV. In some experiments to stimulate AMPK, cells were dialyzed with a standard intracellular solution that contained AMP (200 μM, Sigma-Aldrich, St. Louis MO).
Intracellular lipid accumulation
To increase intracellular lipid content, cells were treated overnight (14 hours) with 50 μM amiodarone (Sigma-Aldrich, St. Louis MO) as described [17]. This manipulation leads to intracellular accumulation of lipids in the absence of changes in cell viability [11].
Measurement of TG secretion
The amount of TG in the media was determined using TLC as described previously [18, 19]. Briefly, intracellular TG were labeled with radioactive glycerol by incubating HTC cells plated on 35 mm Petri dishes with 5 μCi/ml [3H] glycerol for 16 hours. The media was collected, and lipids were extracted using Folch procedure [20]. The total lipid extract was dried, suspended in chloroform, and applied to a thin-layer chromatogram. The TLC plates were developed using a two-solvent system to separate polar lipids with chloroform/methanol/acetic acid/formic acid/water (70:30:12:4:2 by vol), and neutral lipids with petroleum ether/ethyl ether/acetic acid (90:10:1 by vol). The lipids were stained with iodine vapor, and the bands containing TG were identified using known TG standard. The TG bands were cut, and counted using a scintillation counter. To account for the variability in the number of cells, the number of cells in each dish was counted using a hemocytometer, and the total radioactivity was divided with this number.
Western blot analysis of apoB
Media collected from Petri dishes with HTC cells were subjected to electrophoresis on 4–12% continuous gradient polyacrylamide/SDS gels (SDS-PAGE) [21, 22]. Proteins were transferred to Immobilon - P transfer membrane (Millipore, Bedford MA) using a tank system (Owl Separation Systems, Portsmouth NH) with a modified Tris-Cl/Glycine transfer buffer (192 mM glycine, 25mM Tris, pH 8.3) for 18 hours at 35mA with circulating ice water, and packed in frozen cubes for 1 hour at 100mA. Subsequently, proteins were visualized and destained with MemCode Reversible Protein Stain (Pierce, Rockford IL). The membranes were incubated with appropriately diluted anti-rat apoB100 PAb (AbCam ab20737), and horseradish peroxidase-conjugated Donkey anti-rabbit IgG (Jackson ImmunoResearch, Westgrove PA). Immune complexes were revealed with ECL Advance Western Blotting Detection (Amersham Biosciences, UK). Films were scanned by a CanoScan 8400F (Lake Success NY), and the band densities were measured using the NIH Image software (Bethesda MA). Protein concentration was determined by a Lowry method.
Detection of intracellular lipids
Intracellular lipid content was measured with quantitative Nile red fluorescence imaging. Nile red is not fluorescent in solution, but becomes fluorescent after cellular uptake and binding to neutral lipids including TG and cholesterol esters, the major lipid constituents of lipoproteins [23]. This relationship implies that Nile red fluorescence is directly proportional to the amount of intracellular lipids. For these experiments, cells were incubated with 1 μM Nile red (Sigma-Aldrich, St. Louis MO) dissolved in 5 μM pluronic acid (Molecular Probes, Eugene OR) for 1 hour at a room temperature. Prior to measurements, the dye was removed from extracellular solution. Nile red fluorescence was excited through an excitation filter (peak at 535 nm), and collected with an emission filter (peak at 610 nm). To compare the fluorescence intensities from different experiments, all images were acquired with an exposure of 100 msec.
Measurement of SREBP1-c mRNA
Total RNA was isolated from HTC cells using RNA STAT-60 (Tel-Test Inc., Friendswood, TX). Quantitative Real-time PCR (qRT-PCR) was performed using an Applied Biosystems Prism 7900HT sequence detection system as described [24]. SREBP1-c primers were designed using Primer Express Software (PerkinElmer Life Sciences), and validated by analysis of template titration and dissociation curves. Results of qRT-PCR were evaluated by the comparative CT method (user bulletin No. 2, PerkinElmer Life Sciences) using cyclophilin as the invariant control gene.
Measurement of fatty acid oxidation
The rates of FAO were determined by measuring the rates of breakdown of [14C] oleate as described [25]. The experiments were performed in 25-ml Erlenmeyer flasks. Briefly, cell homogenates were incubated in the total volume of 1.25 ml with a modified Krebs-Henseleit bicarbonate buffer (pH 7.4), 0.4 μCi/ml [14C]-oleate, 10% fatty acid free BSA; ATP, CoASH, malate and L-carnitine (final concentrations were 4, 0.05, 0.75 and 0.1 mM, respectively). Reactions were initiated by adding 0.25 ml of a cell homogenate suspended in isotonic KCl, and gassed with a mixture of 95% 02 and 5% CO2. The flasks were shaken at 37°C with 85 oscillations per minute. Reactions were terminated with 0.2 ml of 5% (w/v) perchloric acid. The incorporation of [14C] into total acid-soluble products (ASP) (acetoacetate and P-hydroxybutyrate) and CO2 was determined as previously described [26].
Statistics
Data were expressed as mean ± S.E.M. Results were compared using a two-tailed Student’s t-test on paired and unpaired data, and P values < 0.05 were considered to be statistically significant.
Results
AMPK-dependent exocytosis
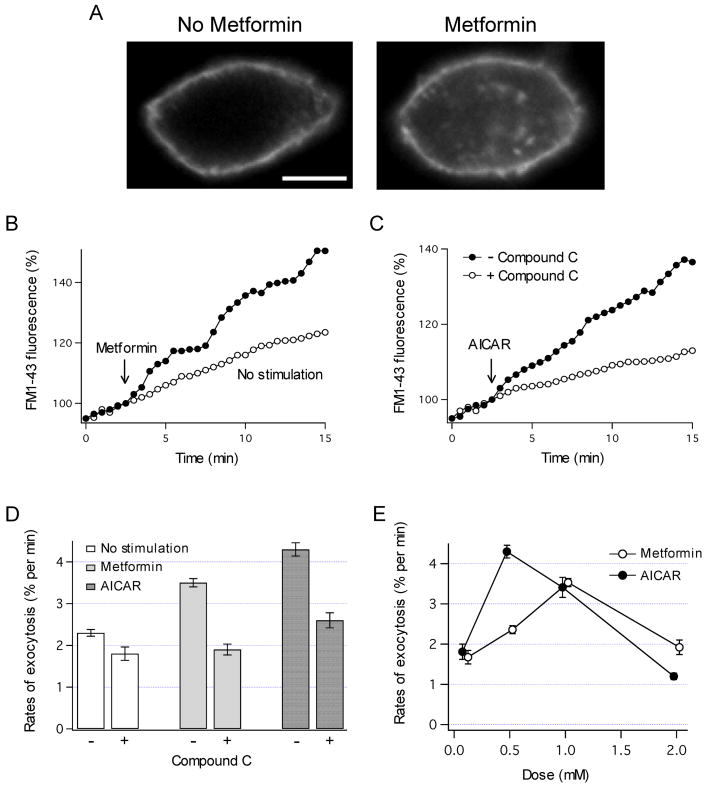
To assess whether AMPK is able to regulate exocytosis, insertion of vesicles into the plasma membrane was monitored before and after stimulation of AMPK activity using FM1-43 fluorescence. Representative images in Fig. 1A illustrate that exposure to metformin markedly increased total cellular FM1-43 fluorescence, and the pattern of fluorescence changed to include both the plasma membrane and the cell interior. Analysis of images in Fig. 1B shows that FM1-43 fluorescence gradually increased in control HTC cells at a constant rate of ~ 2% per min [12]. This continuous increase in FM1-43 fluorescence results from constitutive exocytosis under basal conditions. Activation of AMPK by exposure to metformin (Fig. 1B) or AICAR (Fig. 1C) rapidly increased the rates of exocytosis within minutes of exposure. These effects were related to AMPK since inhibition of AMPK by Compound C prevented an increase in the rates of exocytosis (Fig. 1C and D). Furthermore, both metformin and AICAR increased the rates of exocytosis in a concentration-dependent manner (Fig. 1E). The maximal effects were observed at 0.5 and 1 mM for AICAR and metformin, respectively. Interestingly, at higher drug concentrations the rates of exocytosis decreased. Similar bell-shaped dose-dependence has also been observed for AMPK activation in biochemical studies [27]. These results indicate that AMPK rapidly stimulates constitutive exocytosis in HTC cells.
Fig. 1.
AMPK-dependent exocytosis. (A) HTC cell was stained with FM1-43, and the fluorescent images were taken under control conditions (left panel) and 20 min after exposure to metformin (1 mM, right panel). Note that metformin increased FM1-43 fluorescence. Scale bar is 5 μm. (B) FM1-43 fluorescence was measured in a cell stimulated with metformin (1 mM, at arrow). A representative recording from different cell with no stimulation is also shown. (C) HTC cell was exposed to AICAR (0.5 mM, at arrow). AICAR caused a similar increase in FM1-43 fluorescence, and this increase was inhibited by pretreatment with 20 μM Compound C for 5 min. (D) The rates of exocytosis were measured with or without Compound C. Metformin- and AICAR-dependent exocytosis were inhibited by Compound C (P < 0.001 for both). The number of cells assayed was from 10 to 76. (E) The rates of exocytosis were measured after exposures to different concentrations of metformin and AICAR (0.1 – 2 mM). The number of cells was from 4 to 16.
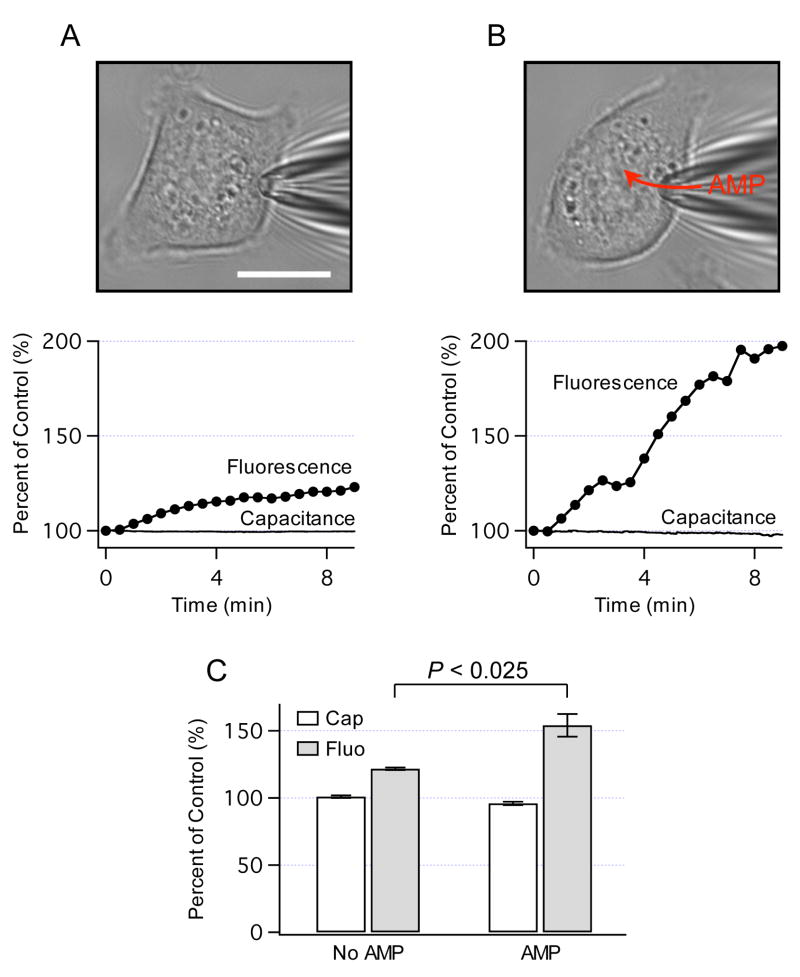
To assess whether AMPK-dependent exocytosis increases cell surface area, cell capacitance was measured using the patch-clamp techniques as described [15]. Figure 2A shows a cell in the whole-cell configuration (top panel). After breaking membrane under a tip of the pipette, the cell was dialyzed with a standard intracellular solution, and the capacitance and FM1-43 fluorescence were simultaneously monitored. A representative recording in Fig. 2A (lower panel) illustrates that FM1-43 fluorescence gradually increased at a rate of ~ 2% per min, while the capacitance remained constant throughout the recording. To activate AMPK in the patch-clamp experiments, HTC cells were dialyzed with AMP (Fig. 2B, top panel). AMP is the most potent endogenous activator of AMPK [27]. Intracellular dialysis of 200 μM AMP rapidly increased FM1-43 fluorescence at a rate of ~ 8% per min, and caused only a small negative change in the capacitance (Fig. 2B, lower panel). The data summarized in Fig. 2C show that increases in FM1-43 fluorescence were not accompanied with similar changes in the capacitance, and that AMP significantly increased cellular FM1-43 fluorescence. Because there was no net increase in cell surface area and the capacitance is a measure of the balance between exocytosis and endocytosis, these findings are consistent with the hypothesis that HTC cells exhibit constitutive membrane trafficking which actively replaces cell membrane through constitutive exocytosis but maintains cell surface area constant. Our recent studies have demonstrated that constitutive exocytosis in HTC cells is balanced with compensatory endocytosis, thus providing further support for this hypothesis [12]. In summary, these observations provide support for the concept that AMPK is directly involved in the regulation of constitutive membrane trafficking in HTC cells.
Fig. 2.
AMPK and cell surface area. (A) Bright-field image of HTC cell in the whole-cell configuration of the patch-clamp technique (top panel). The cell was stained with FM1-43, and then dialyzed with a standard intracellular solution through a patch pipette. Simultaneous recordings of cell capacitance (measure of cell surface area) and FM1-43 fluorescence are shown in bottom panel. The values are expressed as percent of control of the initial values at the beginning of recordings (100%). Scale bar is 15 μm. (B) Representative cell stained with FM1-43 was dialyzed with a standard intracellular solution that contained AMP (200 μM, top panel). Bottom panel shows that AMP caused FM1-43 fluorescence to increase ~ 4-times faster than without AMP. (C) Capacitance and FM1-43 fluorescence were measured 10 min after intracellular dialysis without AMP (n=4 cells) and with AMP (n=6 cells). Note that AMP significantly increased FM1-43 fluorescence but not the capacitance.
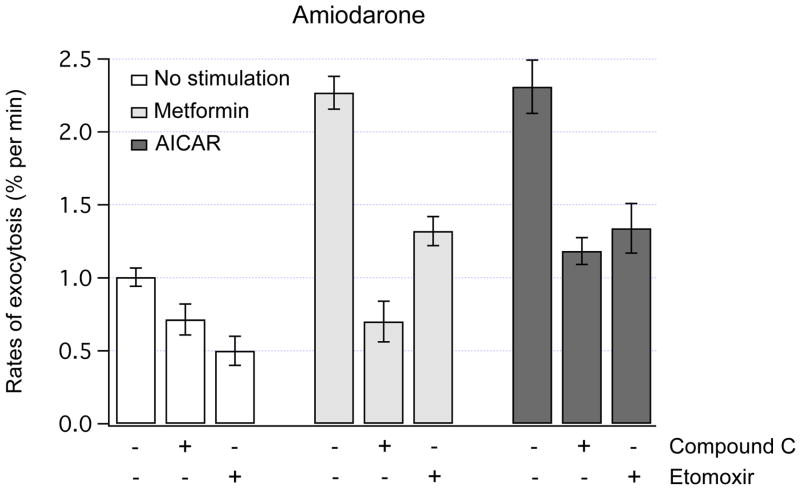
To assess whether AMPK has an effect on exocytosis in the presence of increased levels of intracellular lipids, FM1-43 fluorescence was measured in amiodarone-treated cells. Our recent studies have demonstrated that overnight treatment with amiodarone leads to intracellular accumulation of lipids in HTC cells [11]. In amiodarone-treated cells, the rates of basal exocytosis decreased from ~ 2% per min to ~ 1% per min (compare Fig. 1D and Fig. 3). Similar to the findings in control cells, metformin and AICAR rapidly increased the rates of exocytosis by ~ 2-fold, and these stimulatory effects were potently inhibited by Compound C (Fig. 3).
Fig. 3.
AMPK-dependent exocytosis in lipid-loaded cells. The rates of exocytosis were measured using FM1-43 fluorescence in amiodarone-treated HTC cells. Amiodarone increases intracellular lipid content in HTC cells by ~ 2-fold [11]. Acute exposure to metformin (1 mM) or AICAR (0.5 mM) potently increased the rates of exocytosis (P < 0.001 for both). Pretreatment with 20 μM Compound C (5 min) to inhibit AMPK did not significantly change the rates of exocytosis under basal conditions, but markedly decreased the rates of exocytosis evoked by metformin or AICAR (P < 0.001 for both). Pretreatment with R-etomoxir (100 μM, 1 hour) to inhibit FAO decreased the rates of basal exocytosis (P < 0.006). Note that metformin and AICAR stimulated exocytosis even in the presence of R-etomoxir (P < 0.01 for both). The number of cells analyzed was from 7 to 46.
AMPK is known to stimulate FAO in liver cells [3]. To determine whether FAO is involved in the effects of AMPK on exocytosis, FM1-43 fluorescence was measured after inhibition of FAO with R-etomoxir. Exposure to 100 μM R-etomoxir for 1 hour potently inhibited FAO in amiodarone-treated HTC cells (not shown). The data in Fig. 3 illustrate that although R-etomoxir decreased the rates of exocytosis under basal conditions by ~ 50%, it did not prevent metformin or AICAR from stimulating exocytosis in these lipid-loaded cells. Furthermore, the rates of exocytosis remained elevated even after overnight incubations with metformin (2.2 ± 0.2% per min, n=27, P < 0.001) or AICAR (2.3 ± 0.2% per min, n=24, P < 0.001). These data suggest that activation of AMPK leads to stimulation of constitutive exocytosis, which persists after overnight exposures to AMPK activators, and is independent of FAO.
AMPK-dependent secretion of TG and apoB
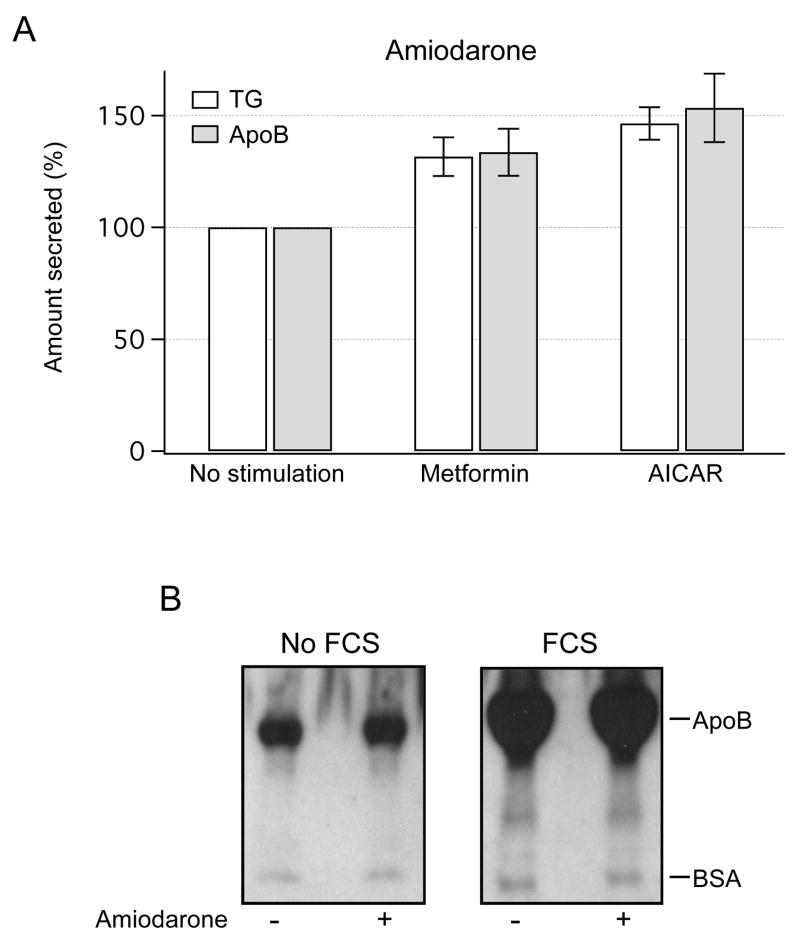
Constitutive exocytosis mediates secretion of lipoproteins. Thus, to assess whether AMPK regulates secretion of TG, the amount of TG in the media was measured after overnight exposure to metformin or AICAR. Both drugs increased the amount of TG secreted from amiodarone-treated HTC cells by ~ 30–50% (Fig 4A).
Fig. 4.
AMPK-dependent secretion of TG and apoB. (A) The amount of TG and apoB secreted into the media from amiodarone-treated HTC cells was measured using TLC and Western blot analysis, respectively. TG content and apoB band densities were normalized to the values obtained in cells with no stimulation (100%). Overnight incubation with metformin (1 mM) or AICAR (0.5 mM) increased TG content in the extracellular media (P < 0.03 for metformin, P < 0.02 for AICAR, n=7). Both drugs also increased the amount of apoB in the media (P < 0.04 for both, n=3). (B) Control and amiodarone-treated HTC cells were incubated overnight without serum (No FCS) or with serum (FCS), and the media was analyzed for the presence of apoB. Representative blots show that FCS increases the amount of apoB in the media. Positive controls for secretion of albumin were obtained using anti-BSA antibodies.
To assess the effects of AMPK on secretion of apoB, the amount of apoB in the media was measured using Western blot analysis. Initially, to ascertain that apoB secretion in HTC cells can be regulated, cells were incubated overnight in the presence or absence of serum. Representative blots in Fig. 3B show that serum potently increased the apoB content, suggesting that HTC cells are able to regulate secretion of apoB. Notably, overnight exposures to metformin or AICAR significantly increased the amount of apoB secreted from amiodarone-treated HTC cells (Fig. 3A). These results are consistent with the hypothesis that AMPK, in addition to stimulation of FAO, is also capable of stimulating constitutive exocytosis of lipoproteins.
AMPK-dependent regulation of intracellular lipid content
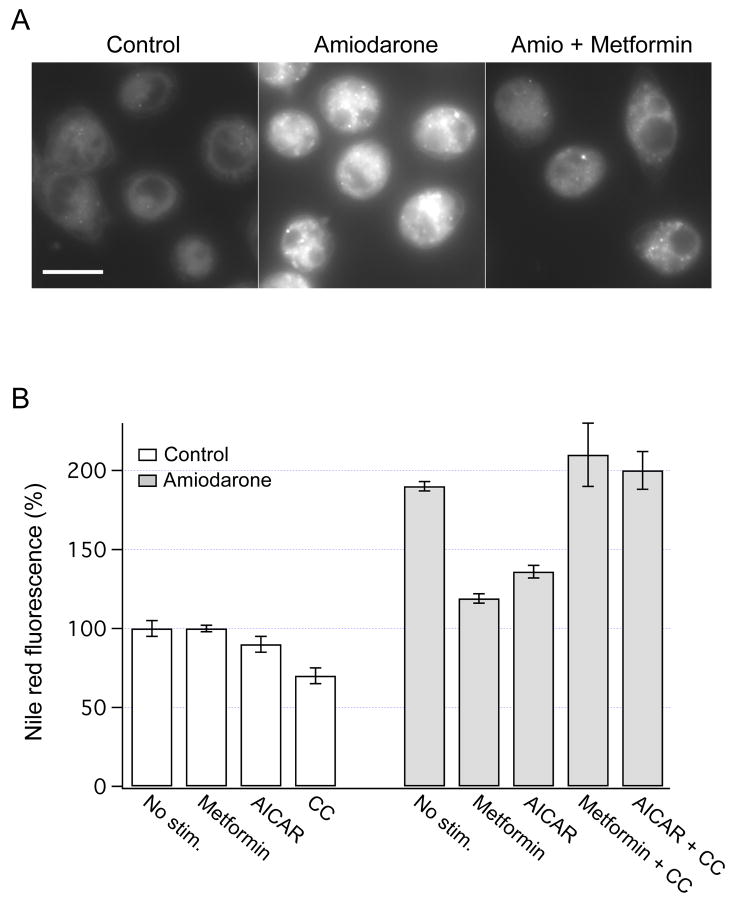
Increased secretion of lipids is anticipated to lower intracellular lipid content. To assess whether AMPK regulates intracellular lipid content, Nile red fluorescence was measured in the presence of metformin or AICAR. Representative images in Fig. 5A illustrate that overnight exposure to metformin decreased intracellular lipid content in amiodarone-treated HTC cells. Similar results were obtained with AICAR. As shown in Fig. 5B, both metformin and AICAR did not change intracellular lipid content in control cells, but markedly lowered the lipid content in amiodarone-treated cells. Inhibition of AMPK by Compound C completely abolished the lipid-lowering effects of metformin and AICAR (Fig. 5B). Thus, AMPK is directly involved in the regulation of intracellular lipid content in HTC cells.
Fig. 5.
Effects of AMPK on intracellular lipid content. (A) HTC cells were stained with Nile red, and the fluorescence images were obtained in control cells (left panel), and after overnight incubation with amiodarone (50 μM, middle panel). The presence of metformin (1 mM) in the incubation media decreased intracellular lipid content (right panel). Scale bar is 15 μm. (B) Nile red fluorescence was measured in control and amiodarone-treated HTC cells after overnight exposures to metformin (1 mM), AICAR (0.5 mM) or Compound C (CC, 20 μM). Similar to previous reports, overnight exposure to amiodarone led to a ~2-fold increase in Nile red fluorescence (P < 0.001, n=34) [11]. Metformin and AICAR significantly decreased the lipid content in amiodarone-treated cells (P < 0.001 for both). The fluorescence was expressed relative to the values obtained in control cells (100%). The number of cells used in these experiments was from 14 to 176.
Lipogenesis and fatty acid oxidation
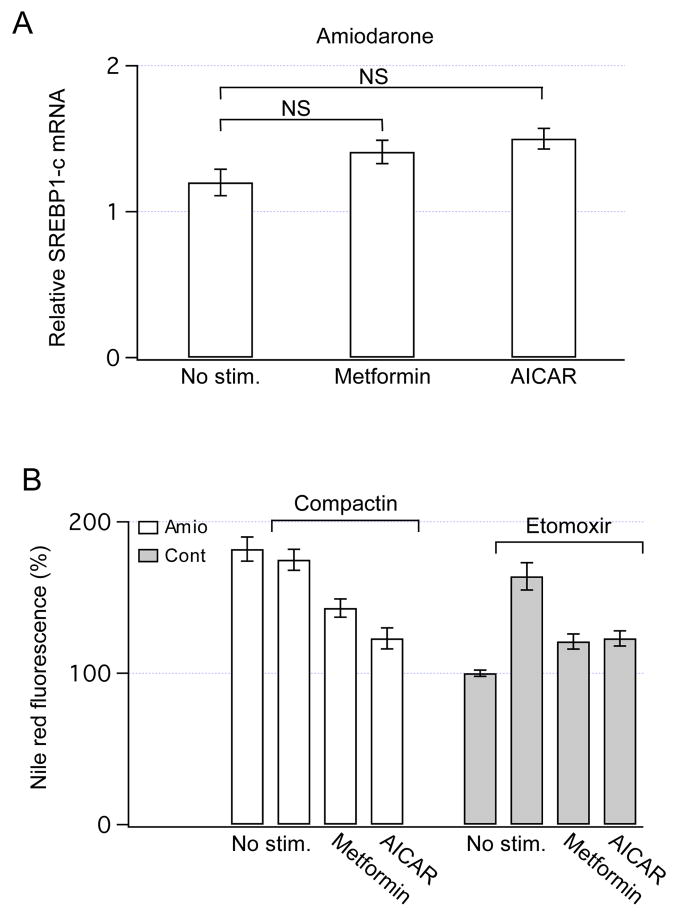
In addition to increased secretion of lipoproteins, AMPK could lower intracellular lipid content by inhibiting lipogenesis through a reduction of expression of sterol regulatory element-binding protein-1 (SREBP-1) [1]. Specifically, an isoform SREBP1-c is directly responsible for synthesis of TG and other lipids in liver cells [28]. Thus, the content of SREBP1-c mRNA was measured after overnight exposure to metformin or AICAR. Both drugs did not significantly increase the expression of SREBP1-c in amiodarone-treated HTC cells (Fig. 6A) indicating that the lipid-lowering effects of AMPK cannot be explained by decreases in the expression of SREBP1-c.
Fig. 6.
The contribution of lipogenesis and FAO to the regulation of intracellular lipid content by AMPK. (A) The expression of SREBP1-c mRNA was measured using qRT-PCR, and the data were normalized to the values obtained in control cells. Overnight exposures to metformin (1 mM) or AICAR (0.5 mM) did not significantly change expression of SREBP1-c in amiodarone-treated HTC cells (n=4). NS - not significant. (B) Nile red fluorescence was measured in amiodarone-treated cells after inhibition of cholesterol synthesis by overnight incubation with compactin (50 μM). Compactin did not change intracellular lipid content (P > 0.53), and both metformin (P < 0.002) and AICAR (P < 0.001) significantly decreased the intracellular lipid content. The number of cells analyzed was from 35 to 116. Nile red fluorescence was also measured after inhibition of FAO by overnight incubation of HTC cells with R-etomoxir (100 μM). R-etomoxir alone increased the intracellular lipid content by ~ 70% (P < 0.001), and the addition of metformin (1 mM) or AICAR (0.5 mM) to the incubation media significantly decreased the intracellular lipid content (P < 0.001 for both). Data were obtained from 14 to 51 cells.
AMPK could also lower the lipid content by blocking the activity of a HMG-CoA reductase, the rate-limiting enzyme for synthesis of cholesterol [29]. To assess whether inhibition of cholesterol synthesis contribute to the lipid-lowering effects of AMPK, Nile red fluorescence was measured after inhibition of the HMG-CoA reductase by compactin. As illustrated in Fig. 6B, even in the presence of compactin, both metformin and AICAR were effective in lowering intracellular lipid content. These data suggest that inhibition of cholesterol synthesis by AMPK is not critical for lowering the intracellular lipid content in HTC cells. Thus, mechanisms other than inhibition of cholesterol synthesis are quantitatively important for the lipid-lowering effects of AMPK.
Stimulation of FAO by AMPK could also lower intracellular lipid content. Consequently, the rates of FAO were measured in the presence or absence of metformin and AICAR. Both drugs increased the rates of FAO by ~ 20–30% (Table 1), consistent with a role for FAO in the lipid-lowering effects. To further assess this hypothesis, intracellular lipid content was measured after inhibition of FAO. Overnight incubation of HTC cells with R-etomoxir per se increased the lipid content by ~ 70% (Fig. 6B). Both metformin and AICAR markedly decreased the lipid content even in the presence of R-etomoxir (Fig. 6B), suggesting that stimulation of FAO is not the dominant mechanism responsible for the lipid-lowering effects of AMPK. In summary, these data provide the evidence that stimulation of constitutive exocytosis of lipoproteins contributes significantly to the regulation of intracellular lipid content by AMPK.
Table 1.
Effects of AMPK on fatty acid oxidation. The rates of FAO were determined by measuring the rates of breakdown of [l4C]-Oleate into acid soluble products (ASP) and CO2. Control and amiodarone-treated HTC cells were exposed to metformin (1 mM) or AICAR (0.5 mM) for 10 min to activate AMPK. The rates were normalized to the values obtained with no stimulation, and the number of experiments is indicated in the parenthesis.
| Rates of ASP formation | ||
|---|---|---|
| Control | Amiodarone-treatment | |
| No stimulation | 100% | 100% |
| Metformin | 122 ± 3% (4) * | 130 ± 8% (3) # |
| AICAR | 128 ± 7% (4) & | 128 ± 4% (3) * |
The P values are:
P < 0.04,
P < 0.01 and
& P < 0.02. Similar results were obtained for CO2 production (not shown).
Discussion
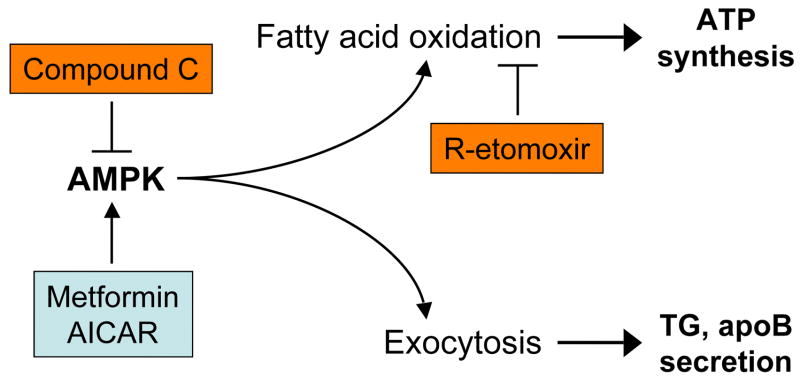
AMPK regulates cellular lipid metabolism in large part through stimulation of fatty acid oxidation. The major finding of these studies is that AMPK also stimulates a previously unrecognized pathway that involves constitutive exocytosis of lipoproteins. This alternative pathway appears to be quantitatively important for intracellular lipid homeostasis. Figure 7 illustrates a proposed model for the regulation of intracellular lipid content by AMPK, emphasizing distinct mechanisms involving oxidation of fatty acids and constitutive exocytosis of lipoproteins.
Fig. 7.
Regulation of intracellular lipid content by AMPK. Activation of AMPK by metformin or AICAR leads to stimulation of fatty acid oxidation, and constitutive exocytosis of lipoproteins. These pathways are independent, and work in concert to modulate intracellular lipid content in liver cells.
Activation of AMPK decreased the intracellular lipid content in amiodarone-treated HTC cells, similar to the results in lipid-loaded HepG2 cells [2]. It is intriguing that the lipid-lowering effects of metformin and AICAR were not observed in control cells. The reason for this quantitative disparity is not known. One possible explanation could be that AMPK-dependent regulation of the intracellular lipid content may be different in the presence of elevated concentration of lipids. Our recent studies provide support for this explanation. Overexpression of CPT1a to stimulate FAO had no effect on the lipid content in control cells, but significantly reduced the lipid content in amiodarone-treated HTC cells [11]. Similar results have been obtained after overexpression of CPT1a in myocytes and pancreatic β-cells [30, 31].
It has been suggested that stimulation of FAO contributes importantly to the lipid-lowering effects of AMPK in vivo [1]. Activation of AMPK in HTC cells also increased the rates of FAO. However, stimulation of AMPK activity was effective in lowering the cellular lipid content even after inhibition of FAO. This was a surprising result suggesting that FAO is not the dominant mechanism responsible for the lipid-lowering effects of AMPK. Notably, a decrease in intracellular lipid content was coupled to secretion of apoB-containing lipoproteins. We used two biochemically independent assays to demonstrate that secretion of lipoproteins is important for the lipid-lowering action of AMPK. Quantitative TLC measurements showed that lipid-loaded HTC cells secrete more TG after exposure to metformin or AICAR. Similarly, Western blot analysis documented a parallel increase in the amount of apoB secreted from these cells. Because TG and apoB are essential constituents of hepatic lipoproteins, these findings are consistent with the hypothesis that AMPK stimulates secretion of lipoproteins.
Direct measurement of exocytosis using FM1-43 fluorescence provides further support for this hypothesis. Exposures to metformin or AICAR rapidly increased the rates of constitutive exocytosis in a concentration-dependent manner. Furthermore, in the continuous presence of these agents, this stimulatory effect persisted after overnight exposures. If constitutive exocytosis of lipoproteins mediates the lipid-lowering effects of AMPK, then the changes in FM1-43 and Nile red fluorescence should be inversely proportional. Increases in the rates of exocytosis evoked by metformin or AICAR were associated with the reduction in intracellular lipid content, and were evident even after inhibition of FAO. Thus, hepatic AMPK may prevent intracellular accumulation of lipids by stimulating constitutive exocytosis of lipoproteins.
Under normal physiologic conditions, hepatic AMPK is activated by the adipocyte-derived hormones leptin and adiponectin. Thus, if similar phenomenon occurs in hepatocytes in situ, then stimulation of lipoprotein secretion by leptin and adiponectin might function to protect the liver from intracellular accumulation of lipids. This implies that AMPK activators should increase the plasma TG levels. Clinical studies have not demonstrated a consistent effect of metformin on plasma TG levels during one year of therapy in patients with non-alcoholic fatty liver disease (NAFLD), despite a reduction in liver fat content [8, 32]. However, many factors other than hepatocyte secretion contribute to the plasma TG levels. In contrast, in this cell-based model where circulating hormones and the contribution of other organs are not a factor, the effects are clear. Multiple lines of evidence suggest that intracellular lipid accumulation is an early and critical event responsible for the development of hepatic insulin resistance in NAFLD [7, 8]. Thus, it is attractive to speculate that decreasing the cellular lipid content through constitutive exocytosis of lipoproteins may contribute to the improvement of hepatic insulin sensitivity.
Assuming that these findings are relevant for the regulation of intracellular lipid content by AMPK, several important points merit emphasis. First, activation of AMPK increased the rates of exocytosis by ~ 2-fold, while the amount of secreted TG and apoB increased by only ~ 30–50%. This quantitative disparity may be due to much higher sensitivity of FM1-43 fluorescence over TLC and Western blot measurements. While FM1-43 fluorescence can be monitored in single cells, detection of TG and apoB require secretion from a large number of cells (millions). Thus, the amount of secreted TG and apoB may have been underestimated. Alternatively, because FM1-43 stains all vesicles that undergo exocytosis, and if AMPK also stimulates constitutive exocytosis of vesicles different from the vesicles loaded with lipoproteins, the rates of lipoprotein exocytosis may have been overestimated. The present studies do not provide clear evidence for the identity of the AMPK-dependent vesicles. In addition to these effects, the discrepancy can also be due to an altered composition of membrane stained with FM1-43 as a consequence of lipoprotein secretion. For example, if a fraction of lipoproteins stained with FM1-43 remains attached to membrane before they leak out, this could lead to an overestimate of FM1-43 fluorescence.
Secondly, labeling of cellular lipids with Nile red is nonspecific. The bulk of Nile red signal comes from cytosolic lipid droplets, a large cytosolic pool that turns over slowly. This large pool is thought to be a primary source of TG used for assembly with apoB [33]. Biochemical studies have shown that the strongest staining with Nile red is obtained for TG and cholesterol esters, the major lipid constituents of lipoproteins [34]. Consequently, Nile red fluorescence should be directly proportional to the amount of intracellular lipids used for the formation of lipoproteins.
Thirdly, the lipid-lowering effects of AMPK may be also mediated through inhibition of lipogenesis. Activation of AMPK did not change significantly the expression of SREBP1-c, and was effective in decreasing the intracellular lipid content after inhibition of cholesterol synthesis with compactin. However, AMPK could lower the concentration of malonyl-CoA, the essential substrate for synthesis of fatty acids [35]. AMPK could also inhibit a mitochondrial sn-glycerol-3-phosphate acyltransferase (mtGPAT), the enzyme that catalyzes the initial and committed step in TG synthesis [36]. In each case, the resulting inhibition of fatty acid supply would be anticipated to decrease secretion of TG. Because AMPK stimulated TG secretion, it is unlikely that any of these pathways are critical for the lipid-lowering effects of AMPK.
Finally, it is acknowledged that although AMPK may stimulate secretion of lipoproteins by stimulating constitutive exocytosis, other mechanisms may also contribute. The assembly of lipoproteins prior to exocytosis is a multi-step complex process that involves participation of different enzymes. For example, AMPK could also inhibit intracellular degradation of apoB, thus providing more apoB for secretion of lipoproteins, as described for oleic acid in HepG2 cells [37]. AMPK could also increase intracellular lipid supply by stimulating lipolysis of cytosolic TG pool through activation of arylacetamide deacetylase (AADA) and triacylglycerol hydrolase (TGH) [33, 38]. The potential contribution of these mechanisms to the lipid-lowering effects of AMPK remains to be defined.
In summary, these results provide support for the concept that activation of hepatic AMPK stimulates a novel pathway, which involves constitutive exocytosis of lipoproteins. These effects are rapid and persist after prolonged exposures to AMPK activators. Notably, AMPK-dependent exocytosis of lipoproteins is independent of FAO, and quantitatively significant for the regulation of intracellular lipid content by AMPK. Thus, pharmacological activation of AMPK represents an attractive target for minimizing the effects of intracellular lipids on insulin-dependent signaling and metabolism.
Acknowledgments
This work is supported in part by National Institute of Health grants (DK43278 and DK46082). We are grateful to Roger Unger for critical reading of the manuscript, and Joyce Repa for help with the qRT-PCR experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- 3.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y, Wang MY, Kakuma T, Wang ZW, Babcock E, McCorkle K, Higa M, Zhou YT, Unger RH. Liporegulation in diet-induced obesity. The antisteatotic role of hyperleptinemia. J Biol Chem. 2001;276:5629–5635. doi: 10.1074/jbc.M008553200. [DOI] [PubMed] [Google Scholar]
- 7.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 8.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 9.Vance JE, Vance DE. Lipoprotein assembly and secretion by hepatocytes. Annu Rev Nutr. 1990;10:337–356. doi: 10.1146/annurev.nu.10.070190.002005. [DOI] [PubMed] [Google Scholar]
- 10.Kilic G, Angleson JK, Cochilla AJ, Nussinovitch I, Betz WJ. Sustained stimulation of exocytosis triggers continuous membrane retrieval in rat pituitary somatotrophs. J Physiol. 2001;532:771–783. doi: 10.1111/j.1469-7793.2001.0771e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puljak L, Pagliassotti MJ, Wei Y, Qadri I, Parameswara V, Esser V, Fitz JG, Kilic G. Inhibition of cellular responses to insulin in a rat liver cell line. A role for PKC in insulin resistance. J Physiol. 2005;563:471–482. doi: 10.1113/jphysiol.2004.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilic G, Doctor RB, Fitz JG. Insulin stimulates membrane conductance in a liver cell line: evidence for insertion of ion channels through a phosphoinositide 3-kinase-dependent mechanism. J Biol Chem. 2001;276:26762–26768. doi: 10.1074/jbc.M100992200. [DOI] [PubMed] [Google Scholar]
- 13.Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Smith CB, Betz WJ. Simultaneous independent measurement of endocytosis and exocytosis. Nature. 1996;380:531–534. doi: 10.1038/380531a0. [DOI] [PubMed] [Google Scholar]
- 15.Kilic G. Exocytosis in bovine chromaffin cells: studies with patch-clamp capacitance and FM1-43 fluorescence. Biophys J. 2002;83:849–857. doi: 10.1016/S0006-3495(02)75213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- 17.Letteron P, Sutton A, Mansouri A, Fromenty B, Pessayre D. Inhibition of microsomal triglyceride transfer protein: another mechanism for drug-induced steatosis in mice. Hepatology. 2003;38:133–140. doi: 10.1053/jhep.2003.50309. [DOI] [PubMed] [Google Scholar]
- 18.Gilham D, Ho S, Rasouli M, Martres P, Vance DE, Lehner R. Inhibitors of hepatic microsomal triacylglycerol hydrolase decrease very low density lipoprotein secretion. Faseb J. 2003;17:1685–1687. doi: 10.1096/fj.02-0728fje. [DOI] [PubMed] [Google Scholar]
- 19.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem. 2000;275:8416–8425. doi: 10.1074/jbc.275.12.8416. [DOI] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Ho SS, Pal S. Margarine phytosterols decrease the secretion of atherogenic lipoproteins from HepG2 liver and Caco2 intestinal cells. Atherosclerosis. 2005;182:29–36. doi: 10.1016/j.atherosclerosis.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Basso F, Freeman LA, Ko C, Joyce C, Amar MJ, Shamburek RD, Tansey T, Thomas F, Wu J, Paigen B, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr Hepatic ABCG5/G8 overexpression reduces apoB-lipoproteins and atherosclerosis when cholesterol absorption is inhibited. J Lipid Res. 2007;48:114–126. doi: 10.1194/jlr.M600353-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Greenspan P, Fowler SD. Spectrofluorometric studies of the lipid probe, nile red. J Lipid Res. 1985;26:781–789. [PubMed] [Google Scholar]
- 24.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 25.McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977;60:265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannaerts GP, Thomas J, Debeer LJ, McGarry JD, Foster DW. Hepatic fatty acid oxidation and ketogenesis after clofibrate treatment. Biochim Biophys Acta. 1978;529:201–211. doi: 10.1016/0005-2760(78)90063-2. [DOI] [PubMed] [Google Scholar]
- 27.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 28.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. Embo J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perdomo G, Commerford SR, Richard AM, Adams SH, Corkey BE, O’Doherty RM, Brown NF. Increased beta-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J Biol Chem. 2004;279:27177–27186. doi: 10.1074/jbc.M403566200. [DOI] [PubMed] [Google Scholar]
- 31.Herrero L, Rubi B, Sebastian D, Serra D, Asins G, Maechler P, Prentki M, Hegardt FG. Alteration of the malonyl-CoA/carnitine palmitoyltransferase I interaction in the beta-cell impairs glucose-induced insulin secretion. Diabetes. 2005;54:462–471. doi: 10.2337/diabetes.54.2.462. [DOI] [PubMed] [Google Scholar]
- 32.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 33.Gibbons GF, Wiggins D, Brown AM, Hebbachi AM. Synthesis and function of hepatic very-low-density lipoprotein. Biochem Soc Trans. 2004;32:59–64. doi: 10.1042/bst0320059. [DOI] [PubMed] [Google Scholar]
- 34.Fowler SD, Brown WJ, Warfel J, Greenspan P. Use of nile red for the rapid in situ quantitation of lipids on thin-layer chromatograms. J Lipid Res. 1987;28:1225–1232. [PubMed] [Google Scholar]
- 35.Velasco G, Geelen MJ, Guzman M. Control of hepatic fatty acid oxidation by 5′-AMP-activated protein kinase involves a malonyl-CoA-dependent and a malonyl-CoA-independent mechanism. Arch Biochem Biophys. 1997;337:169–175. doi: 10.1006/abbi.1996.9784. [DOI] [PubMed] [Google Scholar]
- 36.Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338(Pt 3):783–791. [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon JL, Furukawa S, Ginsberg HN. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J Biol Chem. 1991;266:5080–5086. [PubMed] [Google Scholar]
- 38.Dolinsky VW, Gilham D, Alam M, Vance DE, Lehner R. Triacylglycerol hydrolase: role in intracellular lipid metabolism. Cell Mol Life Sci. 2004;61:1633–1651. doi: 10.1007/s00018-004-3426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]