Abstract
Background
Enfuvirtide is a potent inhibitor of systemic HIV-1 replication, but its penetration into the human central nervous system (CNS) has not been analyzed. Here, we define cerebrospinal fluid (CSF) enfuvirtide pharmacokinetics and present a case illustrating the use of enfuvirtide as a probe to trace the origins of CSF HIV-1 quasispecies.
Methods
Enfuvirtide CSF PK was assessed in 18 CSF and plasma sample pairs from 4 HIV-1-infected subjects. Enfuvirtide levels were measured by liquid chromatography tandem mass spectrometry using known standards and controls that including spiked CSF samples from untreated, HIV-negative subjects. A segment of the gp41-coding region encompassing the heptad repeat (HR)-1 and HR-2 domains was amplified from selected CSF and plasma samples, and independent clones sequenced to assess resistance-associated mutations.
Results
CSF and plasma samples obtained between 2 and 20 hrs after enfuvirtide injection showed plasma concentrations similar to previous reports (mean 3.687 +/−1.828 µg/ml SD) with prolonged decay. By contrast, enfuvirtide in all CSF samples was below the assay detection limit of 0.025 µg/ml. In one subject, who developed a transient increase in CSF HIV-1 RNA, 7 of 7 CSF and plasma clones had identical enfuvirtide resistance-associated V38A mutation, suggesting that the CSF quasispecies derived from that of blood.
Conclusions
Enfuvirtide CSF penetration into CSF is negligible, and thus in clinical settings where direct CNS drug exposure is critical, this drug will likely not directly contribute to the local therapeutic effect. Enfuvirtide can be used as a tool to dissect the origin of the CNS virus.
Keywords: HIV-1, enfuvirtide (T-20), cerebrospinal fluid (CSF), central nervous system (CNS), AIDS dementia complex (ADC), treatment, resistance
INTRODUCTION
Enfuvirtide (T-20) is a 36-amino acid synthetic peptide that interferes with HIV-1 infection by inhibiting virion fusion and subsequent entry of the viral genome into the target cell [1]. It mimics the heptad repeat 1 (HR-1) region of the viral gp41 and, through interaction with the HR-2 region, prevents formation of the hairpin configuration essential to the fusion process [2]. Enfuvirtide differs from other classes of anti-HIV-1 drugs in several important properties, including: 1. extracellular site of action, 2. absence of cross-resistance with other approved drugs, and 3. minimal pharmacological interaction with other antiviral drugs [3]. Its principal disadvantage is that it must be given by injection to avoid degradation by proteases. Indeed, this is perhaps the major reason that the drug is now used almost exclusively by patients with multidrug HIV-1 resistance. While HIV-1 may also develop resistance to enfuvirtide, this is related principally to mutations in the highly conserved region of the N terminus of HR-1 of gp41 [4–7] and unrelated to resistance to other licensed antiretroviral drugs.
While its chemical structure and results of preclinical animal studies suggests that enfuvirtide would not reach effective antiviral concentrations in the brain, there have not been any human studies assessing central nervous system (CNS) pharmacokinetics and effects. We therefore undertook the current study to measure whether enfuvirtide reached effective concentrations in the cerebrospinal fluid (CSF). Additionally, we describe a case example illustrating how the properties of enfuvirtide can be used to examine some broader issues of CNS infection and antiviral treatment.
METHODS
LP and CSF analysis
CSF and blood samples for this study were obtained in the context of a longitudinal Sentinel Neurological Cohort (SNC) study evaluating the effects of HIV-1 infection on CSF; this study was approved by the University of California, San Francisco (UCSF) Committee on Human Research. In all cases CSF was obtained for study purposes rather than for clinical diagnosis and was processed in standardized fashion as previously described [8]. Archived matched CSF and plasma samples that had been aliquoted and stored at −80° C were chosen for these analyses; CSF samples were centrifuged to remove cells before supernatants were stored. The time of drug administration, phlebotomy, and CSF collection were all recorded; the mid-point of the CSF collection, which took 10 – 12 minutes, was used as the CSF sample time point for calculations. Plasma samples were obtained within one hour before or after this time point. We selected all subjects who had been treated with enfuvirtide while participating in the SNC study. This initially designated six possible subjects for inclusion. All medications, including enfuvirtide, were prescribed by the subjects’ caregivers and not determined by our observational CSF studies. None of the CSF samples had >10 red blood cells per µL.
Enfuvirtide measurements
Enfuvirtide was measured in both plasma and CSF by liquid chromatography tandem mass spectrometry (LC–MS/MS) using an adaptation of a previously described method [9]. The CSF assay was performed using calibration standards and quality control samples prepared in fluid collected from HIV-negative volunteers spiked with known concentrations of enfuvirtide. The performance of the LC-MSMS method was characterized for plasma; however characterization studies were not performed for CSF because of insufficient control matrix. Nonetheless, the same method was used for analysis of both matrices, and acceptable data for quality control samples assayed with patients’ samples indicated data should be reliable for CSF. We examined freeze/thaw stability, and noted an approximate 20 percent loss of enfuvirtide in CSF at low concentrations upon freezing and thawing the spiked samples, perhaps related to a limited amount of sticking of enfuvirtide to the tubes. Whatever the cause, CSF results may underestimate the actual concentrations to this degree. The lower limit of quantitation of enfuvirtide in CSF and plasma was 0.025 µg/mL.
Virological and general laboratory methods
HIV RNA was measured in cell-free CSF and plasma by the Roche Amplicor Monitor assay (versions 1.0 and 1.5, Roche Diagnostic Systems, Inc., Branchburg, N.J) using the standard and Ultrasensitive extraction methods. Concurrent paired CSF and plasma samples were treated identically and run at the same time. Background determinations of CSF cell counts, differential counts, protein, and albumin along with blood CD4+ and CD8+ T cell counts, and blood albumin were performed in the San Francisco General Clinical Laboratories using routine methods. The CSF:blood albumin concentration ratio (CSF albumin/serum albumin × 10−3) was used as an index of blood-brain barrier disruption with normal value of 4.6 +/− 1.3 (SD) [10].
Enfuvirtide resistance
Enfuvirtide resistance mutations were evaluated by sequence analysis of amplified cloned segments an approximately 400 nucleotide segment of the gp41-coding region encompassing the HR-1 and HR-2 domains derived from samples of CSF and plasma, using techniques previously described [6].
Statistical analysis
Statistics were performed using Prism 5.0 (GraphPad Software Inc, San Diego, CA).
RESULTS
Study subjects
A total of 6 subjects were identified who had been prescribed enfuvirtide while participating in CSF cohort studies. In two of these subjects, enfuvirtide was not detected in the blood (or CSF), and they were therefore omitted from the pharmacokinetic analysis because they were presumed to be nonadherent to their enfuvirtide. One of these two subjects was viremic, and gp41 sequence analysis showed no resistance mutations, supporting the presumption of nonadherence (not shown), while the other individual subsequently admitted to not taking T20. For the other four subjects, 18 pairs of CSF and blood samples were available for analysis, and were used to define CSF enfuvirtide levels. All subjects were male between 45 and 54 years old at the first sampling interval (Table 1). They exhibited a broad range of plasma and CSF HIV-1 RNA concentrations and blood CD4+ T cell counts. In general, CSF white blood cell counts (WBCs) were low, except for an episode in one of the subjects (7044) described in more detail below. Likewise, CSF:blood albumin ratios were normal except for one mildly elevated level (8.27) in this same patient.
Table 1. Subject information.
Subjects in PK study.
| Range of Values |
||||||||
|---|---|---|---|---|---|---|---|---|
| Subject | Age (years) |
Number Sample Pairs |
Timespan of Samples |
Plasma HIV | CSF HIV | Blood CD4 | CSF WBCs | CSF:Blood Albumin Ratio |
| (log10 copies per mL) |
(cells per µL) |
|||||||
| 7044 | 45.7 | 7 | 2.5 years | <20 – 145 | <20 – 4370 | 256 – 549 | 0 – 643 | 3.74 – 8.27 |
| 7071 | 47.2 | 5 | 2 years | <20 | <20 – 69 | 105 – 189 | 0 – 3 | 4.02 – 6.43 |
| 7102 | 54.1 | 4 | 1.5 years | <20 – 30100 | <20 | 71– 89 | 0 – 4 | 4.08 – 6.05 |
| 7105 | 48.6 | 2 | 6 months | 88700, 269000 | 435, 1340 | 7, 44 | 1, 1 | 4.58, 6.21 |
Pharmacokinetics
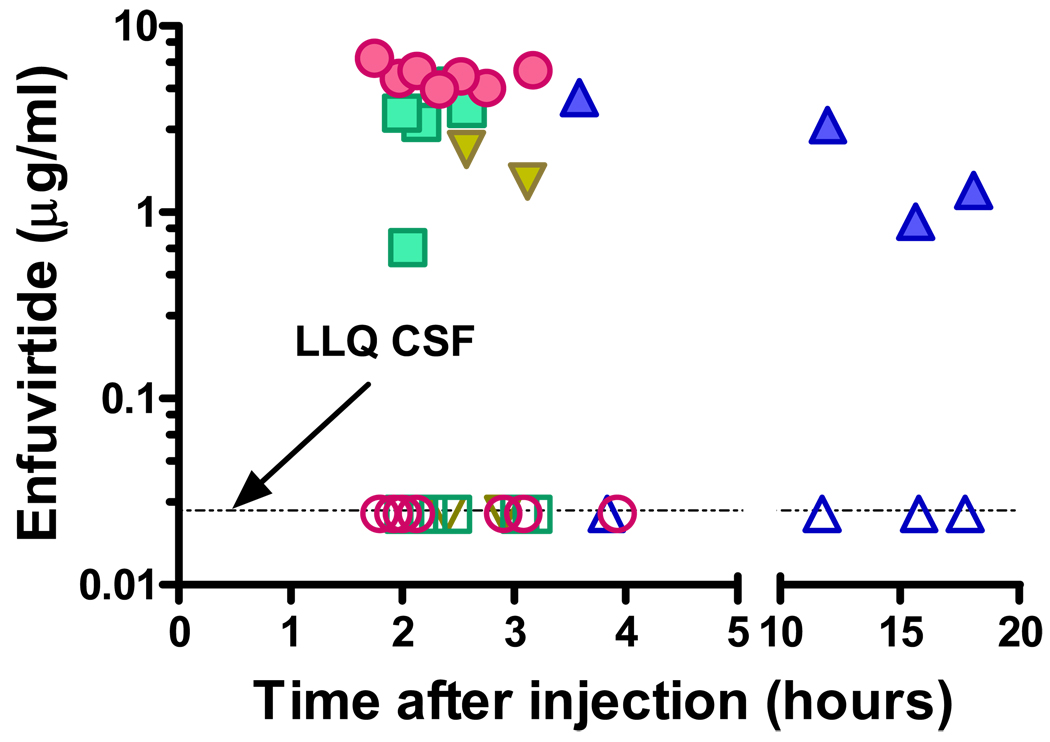
Figure 1 shows the concentrations of enfuvirtide in CSF and plasma from the 18 paired samples. The overall mean plasma enfuvirtide concentration for all intervals was 3.69 µg/mL (SD 1.828 µg/mL), a level above the ED50 of wild-type HIV-1, and in line with previous observations [3, 7, 11]. While most of the samples clustered within the first four hours after enfuvirtide injection, more delayed sampling in one subject showed levels above 1.0 µg/mL, consistent with enfuvirtide’s long half-life and previous observations [3, 11]. In contrast to plasma, CSF concentrations of enfuvirtide were below the level of quantitation in all samples, indicating negligible, subtherapeutic drug penetration into this compartment. The CSF concentrations were significantly different from plasma (P = 0.0001, t = 0.81, paired t test).
Figure 1.
Enfuvirtide concentrations in matching CSF and blood specimens obtained between 2 and 18 hrs after enfuvirtide injection. Each subject’s results are represented by a different symbol: the solid symbols depict plasma values and the open symbols the CSF results. The plasma enfuvirtide concentrations were similar to those reported in other studies (mean 3.687+/−1.828 ng/ml SD) as was the slow plasma decay [ ]. By contrast, enfuvirtide was below the lower limit of quantitation (LLQ) of 0.025 ng/ml in all CSF samples. While the individual CSF values are overlap because of their tight clustering, each plasma value was accompanied by a CSF determination that was below the limit of quantitation, while all of the plasma values were above this level.
Case example with enfuvirtide resistance in CSF and plasma
The following case illustrates how enfuvirtide resistance mutations can be used to explore the ontogeny of the CSF quasispecies and, more speculatively, how limited drug penetration might modify changes in CSF compared to plasma HIV-1 concentrations.
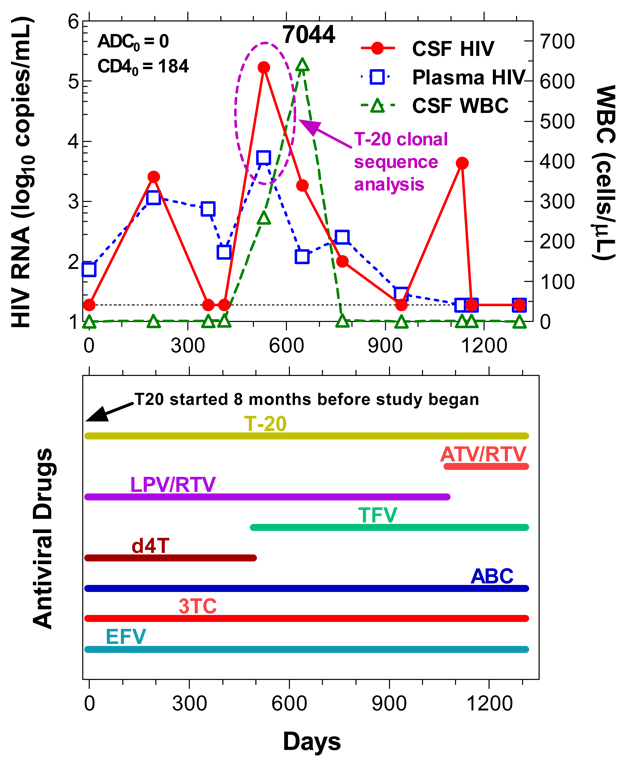
Subject 7044 exhibited three ‘spikes’ in his CSF HIV-1 RNA levels over the course of observation (Figure 2). During one of these episodes (circled in figure), he experienced mild flu-like symptoms, though these were sufficiently mild as to only be elicited in retrospect when the CSF results showed a cell count of 260 WBCs per µL. Despite ongoing treatment with multiple drugs (in addition to enfuvirtide, he was taking lamivudine, abacavir, tenofovir, efavirenz, and lopinavir/ritonavir), his CSF HIV RNA rose to 171,000 copies/mL at this visit, while the plasma HIV RNA levels 5,440 copies/mL. Extensive evaluation by his primary physician failed to identify a cause, and though sustained and even increased at a subsequent visit 4 months later, the CSF cells and virus eventually remitted spontaneously. He reported excellent adherence to his medications during this time. Whether this episode related to an intercurrent viral infection, a change in therapy (from d4T to tenofovir 7 weeks earlier) or other cause, was not established. The two other increases in CSF HIV-1 RNA levels were not accompanied by CSF pleocytosis; the last of these developed 10 weeks after switching from lopinavir/ritonavir to atazanavir/ritonavir, but also resolved without further changes in medications. At the times of the concurrent CSF and plasma HIV-1 RNA elevations during the first three years, he exhibited high-level phenotypic and genotypic resistance to all the other antiretroviral drugs in his regimen, and the same major reverse transcriptase and protease resistance mutations were identified in both CSF and plasma with the exception of preserved tenofovir phenotypic susceptibility (1.4 fold in plasma and 1.5 fold in CSF) in the presence of genotypic resistance in both fluids by PhenoSense assay (Mongram Biosciences, South San Francisco, CA). The subject remains well 5 years after the circled episode.
Clonal sequence analysis of the gp41 segment containing the HR-1 and HR-2 coding regions was applied to the CSF and plasma samples obtained at the interval circled in Figure 2. The results of both bulk sequencing of CSF and plasma and analysis of 7 individual CSF and 7 plasma clones all showed the valine to alanine mutation at position 38 of the HRI region of the gp41 coding sequence (V38A), a change known to confer enfuvirtide resistance [4–6], while background sequences within this 125 nucleotide region were also nearly identical.
Figure 2.
Changes in CSF and plasma CSF HIV-1 RNA and WBC count during the period of longitudinal observation. The subject showed varying elevations in CSF (red circles) and plasma (blue squares) during the course of the study. During one of the spikes in both CSF and plasma HIV-1, samples were subjected to clonal analysis (circled interval as indicated). He also developed a CSF pleocytosis which rose further on repeat LP, but then resolved. This event occurred about 2 years after starting enfuvirtide treatment. The lower box shows his medications. Abbreviations: T-20, enfuvirtide, enfuvirtide; ATV/RTV, atazanavir with ritonavir boost; LPV/RTV, lopinavir with ritonavir boost; TFV, tenofovir; d4T, stavudine; ABV, abacavir; 3TC, lamivudine; EFV, efavirenz.
DISCUSSION
The failure to detect enfuvirtide in CSF in the face of therapeutic levels in blood that are more than 100-fold higher than the limit of detection indicates that this therapeutic peptide is excluded from crossing the blood-brain and blood-CSF barriers and does not appreciably enter the CSF. Our reconstruction studies with spiked samples suggest that there may be as much as a 20 percent loss of the compound in CSF with storage, freezing and thawing at low concentrations, and results may also have been influenced by differences in protein binding in blood and CSF, since enfuvirtide is about 90% bound to plasma proteins, chiefly albumin [3], and CSF binding is therefore presumed to be much lower (not directly measured). Even correcting for these factors, the CSF levels were uniformly low and likely cell exposure was below the effective therapeutic range. Our findings are paralleled by those of an earlier report demonstrating failure of enfuvirtide to cross the blood-testis barrier and reach therapeutic concentrations in semen [12].
While for some other antiretroviral drugs there may be additional factors that importantly influence in vivo efficacy compared to findings in cell culture (for example, in the case of some nucleosides differences in intracellular metabolism and substrate competition [13] or in cell type [14] or activation [15] may be important), enfuvirtide is active at the cell surface, and therefore the extracellular concentration determines its antiviral activity. Hence, to the extent that local antiviral effect is important in reducing CSF (and, more broadly, CNS) HIV-1 infection, enfuvirtide is predicted not to contribute directly to viral suppression in this compartment, though it is important to caution that neither drug access to nor infection of the CSF space and brain parenchyma are identical. Likewise, without appreciable concentrations of the drug in the CSF, this compartment is less likely to serve as a site for the selection of resistance mutations, though it is possible that low, but undetectable levels in CSF might have contributed to local selection. Also, while concern about CNS toxicity of antiretroviral drugs has increased, if brain parenchymal penetration is similar to that of the CSF, enfuvirtide likely has little potential for causing neurotoxicity.
Here, we report a case in which virus rebounded to a greater extent in CSF than in the plasma. The virus which emerged during this time exhibited genotypic evidence of resistance to enfuvirtide. Notably, the sequences that showed characteristic resistance mutations were identical in the plasma and CSF, suggesting a common source. Given that the enfuvirtide concentrations were measurable only in plasma, this ‘spike’ in CSF HIV-1 RNA in the setting of CSF pleocytosis likely resulted from temporally proximate (almost certainly some time after starting enfuvirtide, and perhaps close to the time of the spike) seeding (transitory infection) and local amplification by activated CD4 T cells among the CSF cell reaction. This would be consistent with analysis of Harrington and colleagues using the heteroduplex tracking assay that suggested the origin of CSF HIV-1 in short-lived cells [16].
Another of the four subjects (7071) exhibited a similar isolated CSF spike (CSF HIV RNA 8,320 copies per mL, CSF WBC to 33 cells per µL, and plasma HIV RNA levels 26 copies per mL); genotypic resistance testing was not successful in this subject. These two cases, in which virus appeared to replicate better in CSF than plasma while on a stable regimen, were highly unusual in our experience in subjects without neurological disease. In the longitudinal study from which these samples were taken, out of 435 total evaluations (36 with subjects taking enfuvirtide) in 101 subjects on antiretroviral therapy, we found only 13 examples where CSF HIV-1 RNA levels exceeded those of plasma. Seven of these 13 were from subjects 7044 and 7071 included in this report, indicating a high association with enfuvirtide treatment (p <0.0001, χ2 = 37.1), while the others were in subjects starting or stopping therapy during longitudinal follow up, and thus on unstable regimens (unpublished). While this effect also likely relates to the high level of multidrug resistance that led to the selection of these subjects to receive enfuvirtide, the properties of enfuvirtide may also have contributed. One can speculate that enfuvirtide, even in the face of resistance mutations in the illustrated subject, still had a partial effect on systemic HIV-1 replication but none within the CSF in these cases, resulting in a disproportionate rise in this compartment. While resistance to the other drugs may also have contributed, their higher CSF penetration may have given them a lesser role in the increase in CSF HIV-1 RNA. A similar pharmacologic argument has been made by our group for the failure of enfuvirtide to prevent maternal to fetal transmission even as the mother’s plasma HIV-1 RNA levels remained undetectable [17].
On the other hand, the spike of CSF HIV-1, if indeed of recent plasma origin, also may, paradoxically, support an effect of non-CSF penetrating drugs on suppressing CSF HIV-1 infection. To the extent that CSF HIV-1 involves continued seeding by systemic infection, then systemically effective drugs can reduce the viral burden despite poor penetration. Systemic therapy may also reduce immune activation or other factors that modulate infection in the CNS [18, 19]. Thus, the impact of enfuvirtide on CNS infection may vary with the clinical setting and dynamics of seeding and local viral replication.
Without a clear understanding of these dynamics, what can be recommended regarding the use of enfuvirtide in relation to CNS infection in the individual patient? First, enfuvirtide should continue to be used in settings where it can contribute to overall antiviral effect, basing its use on available treatment choices and its effects on systemic HIV-1 infection. Fear that it is not suppressing CNS infection should not deter its use in most settings, particularly since it is now given principally to patients with multidrug resistance as a component of combination treatment that may include more than three drugs. Even in cases where HIV-1 encephalitis is responsible for active CNS injury manifesting as the AIDS dementia complex, it may be used because of its contributions to treating the systemic infection that provides ‘renewal’ of CNS infection. Our findings in CSF may not fully translate into whether enfuvirtide reaches the brain parenchyma, and even if brain penetration is generally similarly restricted, it may be higher in patients with HIV-1 encephalitis due to their characteristic disruption of the blood-brain barrier. Where it is suspected that CNS treatment is less potent than systemic treatment, it may be worthwhile to measure CSF HIV-1 RNA levels and, if there is local escape, test its resistance profile, including resistance to enfuvirtide, and tailor modifications of treatment accordingly.
ACKNOWLEDGEMENTS
Financial support: National Institutes of Health grants: NS-37660, MH-62701, MH-074466, RR-00083, AI-055357, AI-060354 and.RR-016482.
Footnotes
Potential conflicts of interest: SGD has received research support from Roche, and has received honoraria Trimeris and Roche. DK is a consultant to, and has received honoraria and research grant support from Trimeris and Roche.
Presented in preliminary form: 12th Conference on Retroviruses and Opportunistic Infections (CROI), Boston, February 25, 2005, Abstract 402.
REFERENCES
- 1.Moore JP, Doms RW. The entry of entry inhibitors: a fusion of science and medicine. Proc Natl Acad Sci U S A. 2003;100:10598–10602. doi: 10.1073/pnas.1932511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci U S A. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel IH, Zhang X, Nieforth K, Salgo M, Buss N. Pharmacokinetics, pharmacodynamics and drug interaction potential of enfuvirtide. Clin Pharmacokinet. 2005;44:175–186. doi: 10.2165/00003088-200544020-00003. [DOI] [PubMed] [Google Scholar]
- 4.Wei X, Decker JM, Liu H, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sista PR, Melby T, Davison D, et al. Characterization of determinants of genotypic and phenotypic to enfuvirtide in baseline and on-treatment HIV-1 isolates. AIDS. 2004;18:1787–1794. doi: 10.1097/00002030-200409030-00007. [DOI] [PubMed] [Google Scholar]
- 6.Lu J, Deeks SG, Hoh R, et al. Rapid emergence of enfuvirtide resistance in HIV-1-infected patients: results of a clonal analysis. J Acquir Immune Defic Syndr. 2006;43:60–64. doi: 10.1097/01.qai.0000234083.34161.55. [DOI] [PubMed] [Google Scholar]
- 7.Stocker H, Kloft C, Plock N, et al. Pharmacokinetics of enfuvirtide in patients treated in typical routine clinical settings. Antimicrob Agents Chemother. 2006;50:667–673. doi: 10.1128/AAC.50.2.667-673.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spudich SS, Nilsson AC, Lollo ND, et al. Cerebrospinal fluid HIV infection and pleocytosis: Relation to systemic infection and antiretroviral treatment. BMC Infect Dis. 2005;5:98. doi: 10.1186/1471-2334-5-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang D, Kolis SJ, Linderholm KH, et al. Bioanalytical method development and validation for a large peptide HIV fusion inhibitor (Enfuvirtide, T-20) and its metabolite in human plasma using LC-MS/MS. J Pharm Biomed Anal. 2005;38:487–496. doi: 10.1016/j.jpba.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Tibbling G, Link H, Ohman S. Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J clin Lab Invest. 1977;37:385–390. doi: 10.1080/00365517709091496. [DOI] [PubMed] [Google Scholar]
- 11.Mould DR, Zhang X, Nieforth K, Salgo M, Buss N, Patel IH. Population pharmacokinetics and exposure-response relationship of enfuvirtide in treatment-experienced human immunodeficiency virus type 1-infected patients. Clin Pharmacol Ther. 2005;77:515–528. doi: 10.1016/j.clpt.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Ghosn J, Chaix ML, Peytavin G, et al. Penetration of enfuvirtide, tenofovir, efavirenz, and protease inhibitors in the genital tract of HIV-1-infected men. AIDS. 2004;18:1958–1961. doi: 10.1097/00002030-200409240-00014. [DOI] [PubMed] [Google Scholar]
- 13.Hoggard PG, Sales SD, Kewn S, et al. Correlation between intracellular pharmacological activation of nucleoside analogues and HIV suppression in vitro. Antivir Chem Chemother. 2000;11:353–358. doi: 10.1177/095632020001100601. [DOI] [PubMed] [Google Scholar]
- 14.Aquaro S, Calio R, Balzarini J, Bellocchi MC, Garaci E, Perno CF. Macrophages and HIV infection: therapeutical approaches toward this strategic virus reservoir. Antiviral Res. 2002;55:209–225. doi: 10.1016/s0166-3542(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 15.Ray AS. Intracellular interactions between nucleos(t)ide inhibitors of HIV reverse transcriptase. AIDS Rev. 2005;7:113–125. [PubMed] [Google Scholar]
- 16.Harrington PR, Haas DW, Ritola K, Swanstrom R. Compartmentalized human immunodeficiency virus type 1 present in cerebrospinal fluid is produced by short-lived cells. J Virol. 2005;79:7959–7966. doi: 10.1128/JVI.79.13.7959-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohan D, Feakins C, Wara D, et al. Perinatal transmission of multidrug-resistant HIV-1 despite viral suppression on an enfuvirtide-based treatment regimen. AIDS. 2005;19:989–990. doi: 10.1097/01.aids.0000171417.84162.af. [DOI] [PubMed] [Google Scholar]
- 18.Spudich S, Lollo N, Liegler T, Deeks SG, Price RW. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006;194:1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair E, Ronquillo R, Lollo N, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0b013e318162754f. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]