Summary
Signaling via the receptor tyrosine kinase (RTK) /Ras pathway promotes tissue growth during organismal development and is increased in many cancers. The precise way in which this pathway activates cell growth is still not well understood. Here we show that the HMG box protein Capicua (Cic) has a key role in growth regulation by the RTK/Ras pathway. Cic restricts cell growth in Drosophila imaginal discs and its levels are, in turn, downregulated by Ras signaling. Unlike normal cells, the growth of cic mutant cells is undiminished in the complete absence of a Ras signal. In addition to a general role in growth regulation, the importance of cic in regulating cell fate determination downstream of Ras appears to vary from tissue to tissue. In the developing eye, the analysis of cic mutants shows that the functions of Ras in regulating growth and cell fate determination are separable. Thus, the DNA-binding protein Cic is a key downstream component in the pathway by which Ras regulates growth in imaginal discs.
Results and Discussion
Signaling via receptor tyrosine kinases (RTKs) and Ras promotes cell growth and proliferation in a number of different organisms. The importance of its proper regulation is underscored by the observation that mutations in many human cancers result in increased signaling via this pathway [1]. A key aspect of RTK/Ras signaling that is still not well understood is the precise mechanism by which this pathway promotes cell growth (mass accumulation). Moreover, in addition to regulating tissue growth, the RTK/Ras pathway also functions in cell survival, cell fate specification, terminal differentiation and progression through mitosis. An important question is how the same canonical pathway can elicit strikingly different responses in different cell types [2-6]. Also, most of the functions of the Ras pathway appear to be mediated by the same downstream effectors [5, 7]. Thus how the same pathway elicits different responses in different cell types is still unresolved. Here we show that the HMG-box protein Capicua functions as an important downstream component of Ras in regulating cell growth in Drosophila imaginal discs.
Inactivation of capicua results in increased growth but does not affect cell fate determination in the developing eye
We performed a genetic screen, using mitotic recombination in the developing eye, for mutations that allow homozygous mutant cells to outgrow their wild-type neighbors [8]. In addition to mutations in genes that encode negative regulators of growth such as Tsc1, Tsc2, Pten, salvador, warts and hippo (reviewed in [9]) that result in grossly enlarged eyes, we identified mutations where the only observable abnormality was an overrepresentation of mutant over wild-type tissue. Four such mutations belonged to a single lethal complementation group. Eyes containing mutant clones showed an increased relative representation of mutant tissue over wild-type tissue (Fig. 1C-E) when compared to the parent chromosome used in the screen (Fig. 1B). Eyes containing mutant clones also consistently contained more ommatidia (mean=763 ommatidia; n=6) and were thus slightly larger than eyes containing clones that were homozygous for the parent chromosome (mean=703 ommatidia; n=6, p=0.0037). Otherwise, the eyes were normal in appearance.
Figure 1.
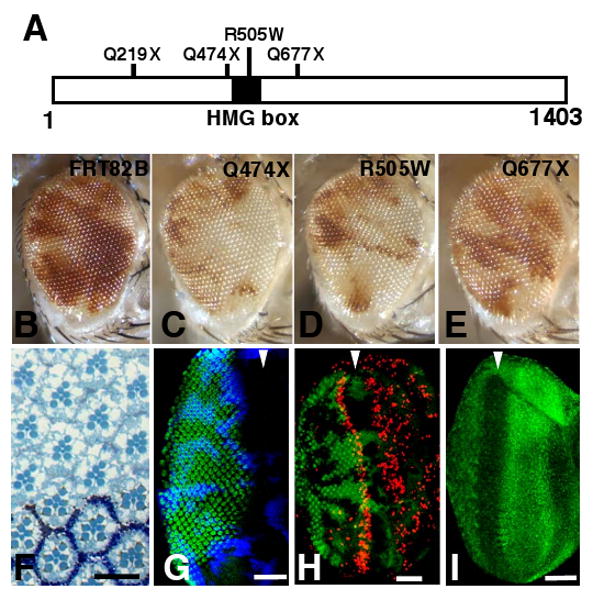
cic mutant cells have a proliferative advantage over wild-type cells but undergo normal cell fate determination. (A) Cic protein showing the location of the HMG box (black) and the positions of the mutations. In three instances (cicQ219X, cicQ474X, and cicQ677X) the mutations change a CAG (Gln) codon to a TAG (stop) resulting in the generation of a truncated protein. The fourth allele, cicR505W, changes an arginine residue within the HMG box to a tryptophan. (B-E) Adult mosaic eyes containing tissue homozygous for the parent chromosome bearing the FRT82B element (B), the cicQ474X (C), the cicR505W (D), and the cicQ677X (E) alleles. Mutant tissue is white and wild-type twin spot tissue is red. The cicQ677X mutation, which is predicted to make a truncated protein that still has an intact HMG-box, has a slightly weaker phenotype than the other alleles (E) indicating that the that CicQ677X protein may retain some of its function. (F) Retinal section of an adult eye containing cicQ474X mutant clones. Mutant ommatidia do not have the white pigment but are otherwise indistinguishable from wild-type ommatidia. (G) Larval eye imaginal disc showing normal photoreceptor differentiation marked by anti-Elav staining (green) of cic mutant tissue as compared to wild-type tissue. Mutant clones fail to stain with anti β-galactosidase (β-gal) (blue). The approximate position of the morphogenetic furrow is indicated by the arrowhead. (H) BrdU incorporation (red) showing S phases in the larval third instar eye disc. Mutant clones fail to express GFP (green). (I) Expression of the Cic protein in the third instar eye disc as shown by anti-Cic staining (green). Anterior is to the right in all panels. Scale bars: (F) 10 μm; (G-I) 50 μm. (For details of methods, see Supplemental Experimental Procedures).
All four alleles failed to complement the lethality of two alleles of capicua (cic), cicfetU6 and cicfetE11 [10]. Mutations in the cic locus (also known as fettucine and bullwinkle) have previously been isolated in screens for mutations that disrupt either embryonic patterning or patterning of the eggshell [10-12], but the role of cic as a negative regulator of growth has not been described previously. cic encodes a protein with a single high-mobility group (HMG)-box that localizes to the nucleus and likely binds DNA via its HMG-box motif. Each of the four mutant chromosomes isolated in our screen, has a mutation in the coding region of the cic gene (Fig. 1A).
An antibody that recognizes the C-terminal portion of Cic stains nuclei throughout the eye imaginal disc. There is a stripe of increased expression immediately anterior to the morphogenetic furrow and reduced expression in the morphogenetic furrow itself (Fig.1I). Staining is not detected in clones of cicQ474X cells (Supplementary Figure S1), thus confirming that the antibody recognizes the C-terminal portion of the Cic protein.
In the eye imaginal disc, loss-of-function mutations in cic appear to increase tissue growth but do not seem to perturb cell fate specification or differentiation. cic mutant ommatidia were indistinguishable from wild-type ommatidia in terms of the size, number and arrangement of photoreceptor cells in the adult retina (Fig. 1F) and appear to develop normally at earlier stages (Fig. 1G). Discs containing cic clones also showed normal patterns of BrdU incorporation throughout the eye imaginal disc (Fig. 1H). However, cic clones anterior to the morphogenetic furrow contained a 2-3 fold higher density of Cyclin E-positive cells per unit of pixel area than wild-type clones (n=15, p<0.0001) (Figure 2A and B), consistent with the increased rate of cell proliferation in mutant clones (see below). As in wild-type discs, no BrdU incorporation was observed in cic mutant discs posterior to the second mitotic wave (Figure 1H) and ectopic Cyclin E protein was not observed in cic clones posterior to the second mitotic wave (data not shown). The patterns of mitosis as assessed by staining with anti-phospho-histone H3 [13], were also unchanged (data not shown). Thus cic cells maintain a relatively normal pattern of S-phases and mitoses in the eye disc and are still able to exit from the cell cycle in a timely manner. In mature pupal eye discs, occasional extra interommatidial cells are observed in mutant clones, suggesting that cic cells may have a subtle defect in apoptosis (data not shown).
Figure 2.

Changes in Cic levels do not alter cell cycle phasing or cell size but affect growth and cell proliferation. In all panels, profiles of control populations are shown in green; cic mutant populations are shown in black. (A) Larval eye discs containing either clones of the FRT82B parent chromosome (GFP-negative) and wild-type sister clones (GFP-positive) - top panels, or cicQ474X (GFP-negative) and wild-type sister clones (GFP-positive) – bottom panels, stained with anti-Cyclin E (red). Arrowheads indicate the approximate position of the morphogenetic furrow. Anterior is to the right. (B) Quantification of number of Cyclin E-positive cells. Clone area was measured using the histogram function of Adobe Photoshop. cic mutant clones contain 2.3-fold more Cyclin E-positive cells per 1,000 pixel area when compared to wild-type clones (n=15, p<0.0001). In contrast, control FRT82B clones contain similar numbers of CycE-positive cells as compared to wild-type clones (n=10, p=0.69). (C-D) Analysis of DNA content (C) and cell size (D) by flow cytometry of dissociated cells from wing imaginal discs. The forward scatter (FSC) ratio of cic mutant population to control population is shown (0.97). (E) Areas of individual cic mutant clones and their corresponding wild-type twin spots calculated using the histogram function of Adobe Photoshop. (F) Number of cells in individual cic clones and their corresponding wild-type sister clones arranged in order of increasing number. Both the observed increase in clonal area (E) and cell numbers (F) in mutant clones are significant as measured by a paired student's t-test (p<0.0001). (For details of methods, see Supplemental Experimental Procedures).
To examine the growth characteristics of cic cells at greater resolution, cells from the eye and wing discs of early third instar larvae (120 hr AED) were dissociated and analyzed by flow cytometry. The distribution of mutant cells in the different phases of the cell cycle as assessed by their DNA content was very similar to that of wild-type cells, as was cell size as assessed by forward scatter in cells of the eye disc (data not shown) or the wing disc (Fig. 2C-D). As in the adult eye and the eye-imaginal disc, the area occupied by mutant clones in the wing disc was larger than the corresponding wild-type twin-spots, suggesting that the mutant cells collectively grow (accumulate mass) faster than their wild-type neighbors (Fig. 2E). Also, mutant clones typically contained more cells than their wild-type twin spots (Fig. 2F). The inferred population doubling time calculated from the median clone size was 10.3 hr in mutant clones compared to 12.3 hr in the wild-type twin spots. The simplest interpretation of all of these observations is that cic cells have an increased rate of growth (mass accumulation) compared to wild-type cells but maintain a normal size due to a commensurate acceleration of the cell cycle. These findings indicate that a normal function of cic is to restrict cell growth in both the eye and wing imaginal discs.
Cic levels are regulated by Ras signaling in the eye disc
Previous work has shown that the levels of Cic protein are responsive to the level of signaling via RTKs and Ras. In the embryo, the level of Cic protein in the terminal regions is decreased upon signaling via the Tor RTK [12]. Activation of Ras in the cells of the wing imaginal disc also reduces Cic levels in those cells [14]. In eye discs, loss-of-function clones of Egfr (Fig. 3A-C) or Ras (Fig. 3D-I), though small, had clearly elevated levels of Cic protein. Conversely, clones of cells expressing the activated form of Ras, Ras (Val12), had reduced levels of Cic (Fig. 3J-L). Thus, as in other tissues, increased signaling via the Egfr/Ras pathway reduces Cic protein levels in the eye disc. Furthermore, studies with mutations in the effector domain of Ras suggest that Ras regulates Cic primarily via the Raf/MAPK pathway (Supplementary Figure S2). This is consistent with a recent study that has shown a direct interaction between Cic and MAPK [15].
Figure 3.

Cic expression can be modulated by changes in Ras or Egfr signaling. (A-L) Third instar eye imaginal discs. Anterior is to the right. The morphogenetic furrow is indicated by an arrowhead. (A, D, G, J) anti-Cic staining (red). Clones of Egfr[k05115] (A-C) or RasΔC40b (D-I) grow poorly and thus are very small. Levels of Cic are increased in Egfr or Ras mutant clones (A, D, G) but are lowered in cells overexpressing RasV12 (J). (B, E, H) β-galactosidase staining to mark wild-type cells in the disc (green). Mutant cells fail to stain for β-gal. (K) Cells overexpressing RasV12 express GFP (green). (C, F, I, L) Merged images of Cic and β-gal (or GFP) stainings. Scale bars: (C, I, L) 10μm; (F) 50 μm. (For details of methods, see Supplemental Experimental Procedures).
Inactivation of cic enables cells to grow without Ras function
In the eye-imaginal disc, clones of RasΔC40b [16], a null allele of Ras, were much smaller than their wild-type twin spots. Strikingly, clones of cells that were mutant for both cic and RasΔC40b were indistinguishable from cic clones in that they were typically larger than their twin-spots (Fig. 4A-D and Supplementary Figure S3). Thus, the loss of cic function completely bypasses the requirement for Ras in promoting cell growth. In contrast to the result obtained with cic, clones that were doubly mutant for Ras as well as a different negative regulator of growth, Tsc1 [9], were no larger than Ras clones (Fig. 4Q-S). Hence the ability of cic to suppress the growth defect of Ras clones is specific and not a general property of negative regulators of growth. Also, cic mutations did not suppress the growth defect of mutations in the Insulin Receptor (InR) [17], Akt [18] and Rheb [19, 20] (Supplementary Figure S4). Thus, cic mutations appear capable of rendering cell growth independent of Ras-mediated signaling but not independent of InR/PI3K- or Tor-mediated signaling. Taken together, these findings support the notion that the ability of Cic to restrict cell growth is specific to its function as a downstream component of the Ras pathway.
Figure 4.

Loss of cic suppresses the poor growth phenotype of Ras mutant clones but does not rescue the differentiation defect. (A-L) Third instar eye imaginal discs containing clones of the FRT82B parent chromosome (A, E, I), cicQ474X (B, F, J), RasΔC40b (C, G, K) and RasΔC40b, cicQ474X (D, H, L) were generated by eyFLP-induced mitotic recombination. Wild-type cells are marked by β-galactosidase expression (red) in the discs (A-D). (E-H) Mutant clones were examined for photoreceptor differentiation using anti-Elav staining (green). (I-L) Merged images of anti-Elav and anti-β-gal stainings. The inset in L shows regularly spaced single Elav-positive nuclei in the mutant clone. (M-P) Adult fly eyes containing clones of FRT82B parent chromosome (M), cicQ474X (N), RasΔC40b (O), and RasΔC40b, cicQ474X (P). Mutant tissue is white and wild-type tissue is red. A greater relative representation of mutant tissue is seen in discs and adult eyes containing cic mutant clones (B, N) than FRT82B control clones (A, M). Ras mutant clones are poorly represented in the disc (C) and are not visible in the adult eye (O). Discs with clones doubly mutant for Ras and cic (D) resemble discs with cic clones alone (B) with respect to clone size. In the adult eye, while tissue deficient in both Ras and cic is observed (P), the mutant tissue lacks the normal ommatidial architecture. Scale bar: 50 μm. Discs containing Ras Tsc1 (double mutant) clones (Q-S) where mutant clones fail to express GFP (green). Mutant clones are small in the imaginal disc, lack normal ommatidia and are absent in the adult eye (T). (For details of methods, see Supplemental Experimental Procedures).
Cic mutations do not affect cell fate determination in the developing eye
In addition to promoting tissue growth, the recruitment of photoreceptor cell precursors to the developing ommatidia occurs via reiterated use of the EGFR/Ras pathway [4]. Clones of cells that are mutant for RasΔC40b do not contain clusters of cells expressing the neural marker Elav [4, 6] but instead contain regularly spaced single Elav-positive nuclei that belong to the R8 photoreceptor cells. While clones doubly mutant for cic and RasΔC40b are of normal size, they, like Ras clones, contain single nuclei that stain with anti-Elav (Fig. 4E-L) and express the R8-specific marker Senseless (not shown). Thus, loss of cic function does not bypass the requirement for Ras function in the specification of photoreceptor cells R1-7. Mutations in Tsc1 neither suppress the requirement for Ras in growth nor in photoreceptor differentiation (Fig 5Q -T). Thus, adult eyes containing clones doubly mutant for cic and RasΔC40b have large patches of tissue lacking any recognizable ommatidia (Fig. 4M-P). In retinal sections, there are no photoreceptor cells in the cic Ras double-mutant clones and all the photoreceptor cells at the borders of the clone are wild-type for Ras (not shown). Thus, although they exhibit impaired photoreceptor differentiation, cic Ras double-mutant clones are not impaired in their growth and unlike Ras clones, are not outcompeted by neighboring cells. Indeed, the phenotype of cells doubly mutant for Ras and cic is extremely similar to that of large Ras clones that are generated in a Minute background [5], suggesting that cic mutations primarily rescue the growth disadvantage of Ras clones.
Thus, in the eye disc, there may be a branching of the Egfr/Ras pathway. One branch, functioning via Cic, appears important for growth regulation while the other branch, acting via Pnt, appears to mediate many of the other outcomes of Ras-mediated signaling, most notably that of photoreceptor cell fate specification. In contrast to Ras clones, clones of pnt in the eye-imaginal disc do not show a marked growth defect (Supplementary Figure S5), suggesting that pnt has a minor role in regulating tissue growth in the eye disc.
In mammalian cells, several extracellular growth factors that act via RTKs increase the activity of Cyclin D/Cdk4 or Cyclin D/Cdk6 complexes [21] that can phosphorylate and inactivate the retinoblastoma protein (pRb) and thus promote S-phase entry. However, it is still unclear how inactivation of pRb can cause cell growth (mass accumulation). At least in Drosophila, the role of Cic appears distinct from Cyclin D because Cyclin D protein levels are not elevated in cic clones (not shown) nor is the growth advantage of cic cells over wild-type cells compromised in flies that completely lack Cdk4/6 function (Supplementary Figure S6). Other studies suggest that Ras can promote cell growth by stabilizing Myc protein via MAPK-mediated phosphorylation [22, 23]. This mode of Ras function also appears to be dispensable under conditions where cic function is inactivated but may still be relevant at physiological levels of Ras signaling.
Notably, our data also show that Cic also functions as a negative regulator of tissue growth in the wing disc. However, in this tissue, Cic has a role in specifying cell fates as well, since others have shown that cic mutations result in the formation of ectopic vein tissue [14]. Thus, while the role of Cic as a regulator of growth in imaginal discs appears to be general, the importance of Cic in pathways that regulate cell fate determination may vary from one tissue to another.
The human and mouse genome each appear to have a single cic ortholog [12, 24] whose function in the regulation of growth has not been addressed to date. However, a recent study that determined the DNA sequence of 13,023 genes from 11 breast and 11 colorectal cancers found missense mutations in the human cic ortholog in 3 of the breast cancers [25]. Although the functional consequences of these mutations have not been evaluated, these data suggest that Cic may indeed function to restrict cell growth in human cells.
Supplementary Material
Acknowledgments
We thank Donald Morisato for providing the cicfetU6 and cicfet-E11 alleles prior to publication and for helpful discussions, Terry Orr-Weaver for the anti-Cyclin E antibody, JoAnn Yetz-Aldape for help with flow cytometry, Nick Baker, Bruce Edgar, Jordi Casanova, Ernst Hafen and Sean Oldham for fly stocks and Nick Dyson for discussions. This work was funded by NIH grant RO1 GM61672 to IH. HK was supported in part by a fellowship from the Japan Society for the Promotion of Science.
Footnotes
SUPPLEMENTAL DATA: Experimental Procedures and Six Figures
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zwick E, Bange J, Ullrich A. Receptor tyrosine kinases as targets for anticancer drugs. Trends Mol Med. 2002;8:17–23. doi: 10.1016/s1471-4914(01)02217-1. [DOI] [PubMed] [Google Scholar]
- 2.Baker NE, Yu SY. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell. 2001;104:699–708. doi: 10.1016/s0092-8674(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez M, Wasserman JD, Freeman M. Multiple functions of the EGF receptor in Drosophila eye development. Curr Biol. 1998;8:1039–1048. doi: 10.1016/s0960-9822(98)70441-5. [DOI] [PubMed] [Google Scholar]
- 4.Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell. 1996;87:651–660. doi: 10.1016/s0092-8674(00)81385-9. [DOI] [PubMed] [Google Scholar]
- 5.Halfar K, Rommel C, Stocker H, Hafen E. Ras controls growth, survival and differentiation in the Drosophila eye by different thresholds of MAP kinase activity. Development. 2001;128:1687–1696. doi: 10.1242/dev.128.9.1687. [DOI] [PubMed] [Google Scholar]
- 6.Kumar JP, Tio M, Hsiung F, Akopyan S, Gabay L, Seger R, Shilo BZ, Moses K. Dissecting the roles of the Drosophila EGF receptor in eye development and MAP kinase activation. Development. 1998;125:3875–3885. doi: 10.1242/dev.125.19.3875. [DOI] [PubMed] [Google Scholar]
- 7.Yang L, Baker NE. Cell cycle withdrawal, progression, and cell survival regulation by EGFR and its effectors in the differentiating Drosophila eye. Dev Cell. 2003;4:359–369. doi: 10.1016/s1534-5807(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 8.Tapon N, Ito N, Dickson BJ, Treisman JE, Hariharan IK. The Drosophila tuberous sclerosis complex gene homologs restrict cell growth and cell proliferation. Cell. 2001;105:345–355. doi: 10.1016/s0092-8674(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 9.Hariharan IK, Bilder D. Regulation of Imaginal Disc Growth by Tumor-Suppressor Genes in Drosophila. Annu Rev Genet. 2006 doi: 10.1146/annurev.genet.39.073003.100738. [DOI] [PubMed] [Google Scholar]
- 10.Goff DJ, Nilson LA, Morisato D. Establishment of dorsal-ventral polarity of the Drosophila egg requires capicua action in ovarian follicle cells. Development. 2001;128:4553–4562. doi: 10.1242/dev.128.22.4553. [DOI] [PubMed] [Google Scholar]
- 11.Rittenhouse KR, Berg CA. Mutations in the Drosophila gene bullwinkle cause the formation of abnormal eggshell structures and bicaudal embryos. Development. 1995;121:3023–3033. doi: 10.1242/dev.121.9.3023. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez G, Guichet A, Ephrussi A, Casanova J. Relief of gene repression by torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning. Genes Dev. 2000;14:224–231. [PMC free article] [PubMed] [Google Scholar]
- 13.Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- 14.Roch F, Jimenez G, Casanova J. EGFR signalling inhibits Capicua-dependent repression during specification of Drosophila wing veins. Development. 2002;129:993–1002. doi: 10.1242/dev.129.4.993. [DOI] [PubMed] [Google Scholar]
- 15.Astigarraga S, Grossman R, Diaz-Delfin J, Caelles C, Paroush Z, Jimenez G. A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling. EMBO J. 2007;26:668–677. doi: 10.1038/sj.emboj.7601532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou XS, Chou TB, Melnick MB, Perrimon N. The torso receptor tyrosine kinase can activate Raf in a Ras-independent pathway. Cell. 1995;81:63–71. doi: 10.1016/0092-8674(95)90371-2. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Jack J, Garofalo RS. The Drosophila insulin receptor is required for normal growth. Endocrinology. 1996;137:846–856. doi: 10.1210/endo.137.3.8603594. [DOI] [PubMed] [Google Scholar]
- 18.Verdu J, Buratovich MA, Wilder EL, Birnbaum MJ. Cell-autonomous regulation of cell and organ growth in Drosophila by Akt/PKB. Nat Cell Biol. 1999;1:500–506. doi: 10.1038/70293. [DOI] [PubMed] [Google Scholar]
- 19.Stocker H, Radimerski T, Schindelholz B, Wittwer F, Belawat P, Daram P, Breuer S, Thomas G, Hafen E. Rheb is an essential regulator of S6K in controlling cell growth in Drosophila. Nat Cell Biol. 2003;5:559–565. doi: 10.1038/ncb995. [DOI] [PubMed] [Google Scholar]
- 20.Saucedo LJ, Gao X, Chiarelli DA, Li L, Pan D, Edgar BA. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat Cell Biol. 2003;5:566–571. doi: 10.1038/ncb996. [DOI] [PubMed] [Google Scholar]
- 21.Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 22.Sears R, Leone G, DeGregori J, Nevins JR. Ras enhances Myc protein stability. Mol Cell. 1999;3:169–179. doi: 10.1016/s1097-2765(00)80308-1. [DOI] [PubMed] [Google Scholar]
- 23.Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100:435–446. doi: 10.1016/s0092-8674(00)80679-0. [DOI] [PubMed] [Google Scholar]
- 24.Lee CJ, Chan WI, Cheung M, Cheng YC, Appleby VJ, Orme AT, Scotting PJ. CIC, a member of a novel subfamily of the HMG-box superfamily, is transiently expressed in developing granule neurons. Brain Res Mol Brain Res. 2002;106:151–156. doi: 10.1016/s0169-328x(02)00439-4. [DOI] [PubMed] [Google Scholar]
- 25.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


