SUMMARY
Lyme borreliosis serves as a model to understand strategies used by pathogens to migrate from mammals to arthropods. We show that a tick protein, Salp25D, plays a critical role - in the mammalian host - for acquisition of Borrelia burgdorferi by Ixodes scapularis. RNA interference-mediated silencing of salp25D in tick salivary glands impaired spirochete acquisition by ticks engorging on B. burgdorferi-infected mice. Immunization of mice with Salp25D also decreased Borrelia acquisition by I. scapularis. Salp25D detoxified reactive oxygen species at the vector-pathogen-host interface, thereby providing a survival advantage to B. burgdorferi at the tick feeding site in mice. These data demonstrate that a pathogen can exploit an arthropod molecule to defuse mammalian responses in order to successfully enter the vector from the reservoir host.
INTRODUCTION
Ixodes scapularis are efficient vectors of microbes, including the Lyme disease agent Borrelia burgdorferi (Barbour and Fish, 1993), Anaplasma phagocytophilum (Telford et al., 1996), Babesia microti (Spielman, 1976) and a tick-borne encephalitis-like virus (Telford et al., 1997). Ticks engorge for several days on vertebrates, penetrating the skin, damaging vessels and obtaining blood from the ensuing hematoma (Sauer and Hair, 1986). In response, neutrophils and other inflammatory cells are recruited to the bite site to disrupt tick feeding (Nuttall, 1998; Wikel and Alarcon-Chaidez, 2001). Ticks counteract these forces with an assortment of immunomodulators, anticoagulants, and other biologically active proteins in saliva (Ribeiro et al., 2006; Ribeiro and Francischetti, 2003). Pathogens acquired by the vector from the infected host traffic through this complex feeding site and may potentially benefit from proteins injected by the arthropod into the mammalian host (Nuttall and Labuda, 2004).
Salp25D is expressed by I. scapularis (Das et al., 2001) and has homology to peroxiredoxins (Barr and Gedamu, 2001, 2003), thiol-specific antioxidants that detoxify reactive by-products of molecular oxygen formed during the respiratory bursts of neutrophils (Flohe et al., 2003; Rhee et al., 2001). We now examine whether B. burgdorferi needs the I. scapularis protein, Salp25D, to modulate responses at the vector-pathogen-host interface, in order for Borrelia to be acquired by ticks engorging on spirochete-infected mice.
RESULTS
SALP25D FACILITATES ACQUISITION OF BORRELIA BY I. SCAPULARIS
Tissue-specific silencing of salp25D
salp25D mRNA and protein are expressed in the tick midgut and salivary glands (Fig 1). Administering salp25D dsRNA into the body or anal pore of I. scapularis preferentially abrogated the expression of salp25D in the salivary glands or midguts respectively, thereby providing a method of assessing the role of salivary gland or midgut Salp25D in Borrelia acquisition. This preferential silencing, represented in Fig 1, was consistently observed in all experiments and therefore not shown for each subsequent study.
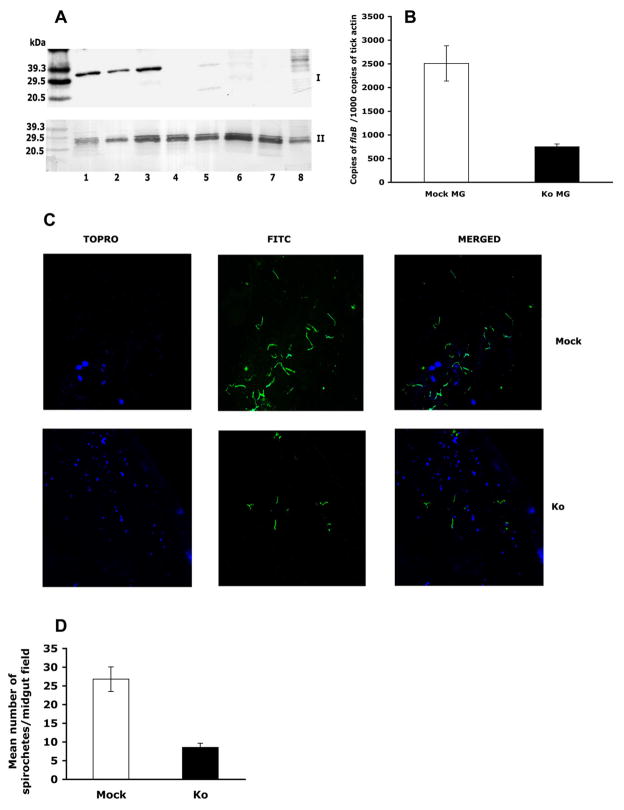
Fig 1. Tissue-selective silencing of salp25D.
A. Immunoblots of salivary gland (SG) and midgut (MG) extracts probed with Salp25D antisera. B. Confocal microscopy of SG and MG tissues probed with Salp25D antisera (Red). Nuclei are stained with TOPRO (Blue). Maltose-binding protein (MBP) antisera served as a control. Magnification × 63. C. Quantitative RT-PCR showed that ds salp25D RNA injected into the tick body reduced salivary gland salp25D (Ko SG) compared to the Mock group (Mock SG; P = 0.0018). salp25D levels not significantly altered in the midguts of both groups (Mock MG and Ko MG). D. Quantitative RT-PCR showed that ds salp25D RNA injected into the tick anal pore decreased salp25D levels in the midguts (Ko MG) compared to the Mock group (Mock MG; P = 0.01) without significantly altering the levels in the salivary glands of both groups (Mock SG and Ko SG). Results are the mean ± SEM of three experiments.
Salivary gland salp25D is important for Borrelia acquisition
salp25D dsRNA was injected into the body of ticks, which were then placed on B. burgdorferi-infected mice. After 66 h of feeding the tick weights in the Mock (n = 27; 4.5 mg ± 0.35 SEM) and ds salp25D RNA-injected (n = 32; 4.3 mg ± 0.44 SEM) groups were comparable, demonstrating that the dsRNA did not influence engorgement. Injecting salp25D dsRNA into the body of I. scapularis abrogated the expression of salp25D in the salivary glands but not the midgut. Western blot confirmed the decrease in Salp25D protein in the salivary glands (Fig 2A). The spirochete burden in the midguts of the salp25D dsRNA-injected ticks was markedly reduced when compared to controls, as determined by quantitative RT-PCR (Fig 2B; P = 0.00016) and confocal microscopy (Fig 2C and 2D; P = 0.007). A correlation coefficient of 0.71 (n = 32) was observed at 66 h of feeding further indicating that diminished salp25D levels resulted in decreased Borrelia acquisition.
Fig 2. Salivary gland salp25D is crucial for Borrelia acquisition.
ds salp25D RNA or buffer was injected into the body of nymphs. A. Immunoblot of salivary gland extracts from Mock (lanes 1–3) and salp25D dsRNA-injected (lanes 4–8) nymphs probed with Salp25D (Panel I) or control Salp14 (Panel II) antisera. B. Quantitative RT-PCR of flaB levels in midguts from Mock and ds salp25D RNA (Ko-MG) groups. Results are the mean ± SEM of a representative out of three experiments. C. Confocal microscopy of midguts from Mock and salp25D dsRNA (Ko) nymphs. Midgut nuclei and spirochetes stained with TOPRO (blue) or FITC-conjugated Borrelia antisera (green), respectively. Magnification × 63. D. The number of spirochetes/field in Mock and salp25D dsRNA-injected (Ko) midgut. Results are the mean ± SEM of a representative of three experiments.
To exclude the possibility that the impairment of B. burgdorferi acquisition was due to a dsRNA-mediated off-target effect, observed in some instances with RNAi (Jackson et al., 2003; Persengiev et al., 2004), we used 2 separate small interfering (si) RNAs specific to 2 different regions of salp25D (si-61 and si-181). siRNA specific to salp9pac, a tick salivary gland gene unrelated to salp25D (Narasimhan et al., 2004; Valenzuela et al., 2002) served as a control. Quantitative RT-PCR from salivary glands showed a significant (P < 0.005) decrease in the level of salp25D in the si-salp25D-injected groups when compared to the si-salp9pac group (Suppl Fig 1A). Consistent with the observations made using salp25D dsRNA, the si-salp25D-mediated decrease in salp25D reduced the efficiency of Borrelia acquisition (Suppl Fig 1B; P < 0.001). si-salp9pac did not impair the acquisition of Borrelia from the murine host (Suppl Fig 1B). si-salp9pac did reduce the expression of salp9pac (Suppl Fig 1C) and neither si-salp9pac nor si-salp25D altered the expression of other selected salivary gland genes (Suppl Fig 1D).
Midgut salp25D is not crucial for Borrelia acquisition
dsRNA was microinjected into the anal pore of I. scapularis, which were then placed on Borrelia-infected mice. Quantitative RT-PCR showed that salp25D expression in the midgut was preferentially reduced and western blot analysis confirmed the decrease of Salp25D in the midguts in the dsRNA-injected group (data not shown). The decrease in salp25D expression in the midgut of salp25D dsRNA-injected ticks did not have a significant impact on Borrelia acquisition as demonstrated by quantitative RT-PCR (Suppl Fig 2B; P = 0.137), or confocal microscopy (Suppl Fig 2C and D; P = 0.1).
Salivary gland salp25D is needed for the initial entry of viable spirochetes into the tick midgut
To determine whether Borrelia acquisition was impaired during, or following, entry into the midgut, salp25D dsRNA was injected into the body of I. scapularis nymphs. Ticks were then placed on Borrelia-infected mice and removed 24 or 48 h after attachment. salp25D was predominantly silenced in the salivary glands, not in the midguts of the dsRNA-injected ticks (Fig 3A). The midguts of experimental ticks showed a significant (P < 0.005) decrease in the B. burgdorferi burden at 24 and 48 of tick feeding when compared to control ticks (Fig 3B). Overall, correlation coefficients of 0.91 (n = 15) and 0.67 (n = 28) were observed at 24 and 48 hr respectively. The observation that spirochete levels were reduced as early as 24 h of feeding suggested that salivary gland Salp25D facilitates the entry of viable B. burgdorferi into the midgut.
Fig 3. Silencing salivary gland salp25D alters the entry of viable spirochetes into the midgut.
ds salp25D RNA or buffer was injected into the body of nymphs and analyzed. A. Quantitative RT-PCR of salivary glands and midguts from buffer-injected (Mock SG, Mock MG) and dsRNA-injected (Ko SG, Ko MG) groups at 24 and 48 h of feeding. Results are mean ± SEM of two experiments. B. flaB levels in the midguts of Mock (Mock MG) and salp25D dsRNA-injected group (Ko MG) groups assessed by quantitative RT-PCR at 24 and 48 h of feeding. Results are expressed as mean ± SEM of two experiments.
Immunization with Salp25D impairs Borrelia acquisition by nymphs and larvae
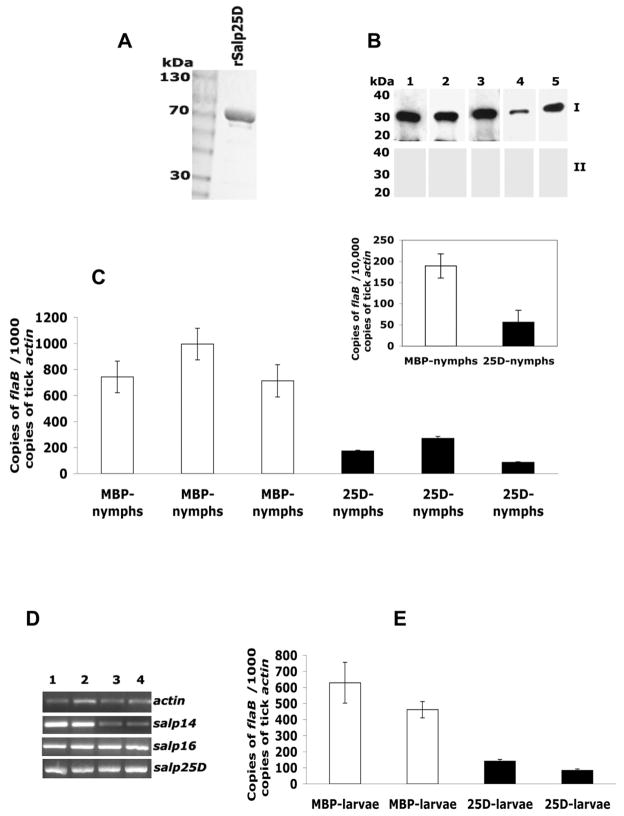
We then determined whether immunization of mice with Salp25D could also influence B. burgdorferi acquisition. Recombinant Salp25D (rSalp25D, Fig 4A) or maltose binding protein (MBP, control antigen) was used to immunize groups of 5 C3H/HeN mice. Western blots of salivary gland extracts showed that the antisera from all the rSalp25D-immunized mice, but not from control mice, reacted with the native protein (Fig 4B). The nymphal engorgement weights were comparable in both control (n= 26; 4.3 mg ± 1.19 SEM) and experimental (n = 24; 4.64 mg ± 0.62 SEM) groups. Salp25D immunization reproduced the RNAi phenotype as evidenced by a significant (P < 0.005) decrease in the acquisition of spirochetes by nymphs that fed on rSalp25D-immunized mice compared to MBP-immunized mice (Fig 4C). This was also confirmed by genomic DNA-PCR to rule out the possibility of transcriptional bias (Fig 4C, inset; P = 0.02).
Fig 4. Host immunity to Salp25D decreases Borrelia acquisition by nymphs and larvae.
A. Coomassie-stained SDS-PAGE of Salp25D fusion protein. B. Immunoblots of salivary gland extracts probed with sera from 5 mice immunized with Salp25D (Panel I, lanes 1–5) or MBP (Panel II, lanes 1–5). C. Quantitative RT-PCR of flaB levels in midguts from nymphs fed on MBP-immunized (MBP-nymphs) or Salp25D-immunized (25D-nymphs) Borrelia-infected mice. Results expressed as mean ± SEM of one representative experiment. C. Inset. DNA-PCR estimation of flaB levels in the midguts of individual nymphs fed on MBP-immunized (MBP-nymphs) or Salp25D-immunized (25D-nymphs) Borrelia-infected mice. D. RT-PCR of larval RNA (4 separate pools of 10 larvae) shows the expression of salp25D, salp16 and salp14. Tick actin serves as control gene. E. Quantitative RT-PCR of flaB levels in larvae fed on MBP-immunized (MBP-larvae) or Salp25D-immunized (25D-larvae) Borrelia-infected mice. Results are presented as mean ± SEM of one representative experiment.
We also used the immunization strategy to extend our observations to larvae, the first stage of I. scapularis that can acquire B. burgdorferi in nature. RT-PCR of RNA isolated from 4 different pools of 10 fed larvae showed that salp25D, in addition to several other genes, are expressed by larvae (Fig 4D) and provided further rationale to examine the role of Salp25D in larval acquisition of Borrelia. Immunization with rSalp25D resulted in a 4-fold decrease (P < 0.05) in the ability of larvae to acquire Borrelia (Fig 4E).
Silencing the salivary gland salp25D does not impair Borrelia transmission
The observation that salivary gland Salp25D was vital for Borrelia acquisition prompted us to examine the role of salivary gland Salp25D in Borrelia transmission. salp25D dsRNA or buffer was microinjected into the body of B. burgdorferi-infected nymphs and placed on pathogen-free C3H mice. Salivary gland salp25D levels were significantly decreased (P = 0.003) in the dsRNA-injected group (2,500 ± 750 copies of salp25D/1000 copies of actin) compared to that in the Mock group (32,000 ± 4,000 copies of salp25D/1000 copies of actin). This did not affect the spirochete load in the tick salivary glands or midguts as seen by comparable levels of flaB transcripts in both mock and dsRNA-injected groups (Fig 5, Inset). Transcript levels were normalized to tick actin for the salivary gland samples. Thirty days after tick detachment, the mice were sacrificed and skin punch biopsies (distant from, and at the attachment site), joints, heart, spleen and bladder collected for DNA isolation. The absence of salp25D in the salivary glands did not affect the transmission of Borrelia as judged by the comparable levels of flaB amplicons in skin (P = 0.1), heart (P = 0.1), bladder (P = 0.5), joints (P = 0.8), and spleen (P = 0.4) of control and dsRNA-injected group (Fig 5)
Fig 5. Salivary gland Salp25D is not essential for B. burgdorferi transmission to the murine host.
Quantitative RT-PCR estimation of flaB levels in the salivary glands and midguts of Mock (Mock SG, Mock MG) and ds RNA-injected (Ko SG, Ko MG) nymphs (Inset), and quantitative DNA PCR estimation of flaB levels in the skin, heart, bladder, joints and spleen of mice that were engorged upon by ticks from these groups (Mock and Ko). Results are expressed as mean ± SEM of one representative experiment.
SALP25D -- FUNCTION AND PROTECTION OF B. BURGDORFERI
rSalp25D protects Borrelia from •OH-mediated damage in vitro
Peroxiredoxins detoxify •OH radicals, the reactive products of O2− and H2O2, which cause lethal damage by peroxidation of cellular components (Barr and Gedamu, 2001; Jackson et al., 1989; Jackson et al., 1987). We therefore determined whether rSalp25D like other peroxiredoxins (Barr and Gedamu, 2001) could enzymatically detoxify •OH radicals in vitro. We used the thiol-mixed function oxidation (MFO) system (Lim et al., 1993), which generates •OH radicals in vitro, to examine the ability of rSalp25D to protect supercoiled DNA from •OH-mediated nicking (Suppl Fig 3A). In the presence of the thiol-MFO, the supercoiled plasmid DNA was nicked. Upon incubation with increasing amounts of rSalp25D (2.8 – 400 nM), nicking diminished and was abolished at concentrations greater than 14 nM. rMBP at comparable and higher concentrations (40 and 400 nM) did not protect the plasmid DNA.
•OH radicals generated by the thiol-MFO system (Lim et al., 1993) were also detrimental to in vitro grown B. burgdorferi and incubation with rSalp25D, but not rMBP, provided significant protection from OH radical-mediated damage as observed by the Live-Dead assay (Suppl Fig 3B; P < 0.05) and by culture (Suppl Fig 3C; P < 0.005). These data showed that hydroxyl radicals are detrimental to B. burgdorferi, and that rSalp25D protected Borrelia from •OH-mediated damage.
Salp25D contributes to the ability of tick saliva to quench extracellular superoxide released activated neutrophils and protect Borrelia
Adult ticks were used in these studies because they produce large amounts of saliva. Consistent with earlier observations (Ribeiro et al., 1990), adult tick saliva efficiently quenches extracellular O2− released by phorbol myristyl acetate (PMA)-activated neutrophils (Fig 6A); the efficiency was higher at 700 ng (10 μl) than at 70 ng (1μl). 700 ng of saliva was therefore used in subsequent analyses. The incubation of B. burgdorferi (5×103) with resting neutrophils did not result in the release of extracellular O2− (Fig 6A); and B. burgdorferi incubated with PMA-activated neutrophils could not quench the extracellular O2− (Fig 6A). However, when B. burgdorferi were incubated with PMA-activated neutrophils in the presence of I. scapularis saliva, saliva quenched the extracellular O2− (Fig 6A). We now show by RNAi that Salp25D contributes to this process. Buffer or salp25D dsRNA was microinjected into the body of adult ticks and ticks allowed to feed to repletion. As observed with nymphs, the engorgement weights of control (n = 23; 121 mg ± 20 SEM) and experimental ticks (n = 25; 72 mg ± 15 SEM) were not different (P = 0.06). Western blot analysis of salivary glands demonstrated the ablation of Salp25D protein in the salp25D dsRNA group (Fig 6B). Saliva from mock-injected adults quenched extracellular O2− efficiently as observed by a decrease in luminescence comparable to saliva from untreated ticks (Fig 6C). In contrast, the ability of saliva from dsRNA-injected ticks to quench O2− was decreased (Fig 6C; P < 0.05). Normal adult saliva quenched extracellular superoxide radicals even when saliva was added 10 min after neutrophil activation with PMA (Fig 6D). In contrast, saliva from salp25D dsRNA-injected ticks was less efficient at quenching the extracellular superoxide radicals (Fig 6D; P = 0.004).
Fig 6. Silencing salp25D reduces the ability of tick saliva to quench extracellular superoxide.
A. Extracellular superoxide radicals released by phorbol myristyl acetate (PMA)-activated neutrophils (1×106 cells/ml) in the presence of 70–700 ng/1–10 μl of untreated tick saliva; or Borrelia (5×103); or Borrelia + saliva. Neutrophils not activated with PMA served as baseline. Luminescence recorded as Relative Light Units (RLU). B. Immunoblot of tick salivary gland extracts from body-injected Mock (lanes 1–4) and salp25D dsRNA (lanes 5–8) groups probed with rSalp25D (Panel I) or control rSalp14 antisera (Panel II). C. Extracellular superoxide radicals released by PMA-activated neutrophils (1×106/ml) in the presence of saliva (700 ng) from body-injected Mock or salp25D dsRNA ticks. Resting neutrophils served as baseline. D. Extracellular superoxide in the reaction, 10 min after PMA-activation of neutrophils in the presence of: 700 ng of saliva from untreated, Mock-injected, ds salp25D RNA-injected ticks. Luminescence plotted as mean ± SEM of 2 experiments. E. Live dead assay to assess viability of spirochetes incubated with: resting neutrophils (2); PMA-activated neutrophils (3); PMA-activated neutrophils + normal saliva (700 ng; 4); PMA-activated neutrophils + rSalp25D (200 nM; 5); and PMA-activated neutrophils + rMBP (200 nM; 6). Dead spirochetes stained red and viable spirochetes stained green. Spirochetes not exposed to neutrophils (1) served as control. F. Ratio of number of viable/dead spirochetes with no treatment (1); upon incubation with neutrophils, either resting (2); or PMA activated (3); PMA activated with saliva (4) or with rSalp25D (5) or with rMBP (6).
We then examined the impact of extracellular superoxide radicals on spirochete viability. Spirochetes incubated with naïve neutrophils or not exposed to neutrophils were predominantly live (Fig 6E). While Borrelia incubated with activated neutrophils in the absence of saliva or in the presence of rMBP appeared, for the most part, dead, incubation with saliva or rSalp25D provided protection as seen by a significant increase in the number of live spirochetes (Fig 6E and F; P < 0.005). The treated spirochetes were also cultured in BSK medium for 5 days and counted. The viability of B. burgdorferi incubated with PMA-activated neutrophils was decreased 5-fold (9.0 × 105 ± 1.4 × 105 SEM; P < 0.005) when compared to spirochetes incubated with PMA-activated neutrophils in the presence of saliva (3.6 × 106 ± 1.0 × 105 SEM) or rSalp25D (2.8 × 106 ± 4.5 × 105 SEM). The viability of spirochetes not incubated with neutrophils (3.0 × 106 ± 9.3 × 104 SEM) or incubated with resting neutrophils (2.5 × 106 ± 1.9 × 105 SEM) was comparable to spirochetes incubated with activated neutrophils in presence of rSalp25D or saliva. These data suggest that reactive oxygen species generated and released extracellularly by activated neutrophils compromise the viability of Borrelia, and in such a milieu, tick saliva provides a survival advantage to B. burgdorferi by neutralizing the released reactive oxygen species. These observations support the conclusion that Salp25D plays a role in protecting B. burgdorferi from reactive oxygen species-mediated damage.
DISCUSSION
Ticks acquire Borrelia as larvae or nymphs while engorging on B. burgdorferi-infected hosts such as white-footed mice, and infected nymphs and adults can transmit Borrelia while engorging on vertebrate hosts (Barbour and Fish, 1993). As a paradigm for understanding how a pathogen may require vector molecules - in the mammalian host - for successful migration to and from the mouse, we define the interplay between a specific host reaction to tick feeding, the vector’s concomitant anti-inflammatory response, and Borrelia acquisition and transmission. Tissue-selective gene silencing was achieved by our microinjection (Fig 1) techniques due, perhaps, to the barrier posed by the midgut epithelium to the efficient entry of dsRNA from the hemocoel to the midgut, and vice versa and allowed us to address the functional significance of salivary gland and midgut Salp25D in pathogen acquisition. Salivary gland, but not midgut, Salp25D was more critical for spirochete acquisition (Fig 2 and Suppl Fig 2), indicating that Salp25D is required at the vector-host interface for B. burgdorferi. The observation that Borrelia entry into ticks was impaired as early as 24 h of feeding underscored the importance of salivary gland Salp25D early in the acquisition process (Fig 3).
Larval ticks, are important for enzootic spirochete acquisition, but tolerated the microinjection procedure poorly (recovery rates at about 10 %) due to their small size. Further, RNAi mediated decrease of target gene expression was also achieved in only 50 % of the microinjected larvae. We therefore used nymphs throughout the RNAi studies. However, a batch of larval ticks was also examined by RNAi to corroborate the detailed observations made with nymphs. At least 250 I. scapularis larvae were microinjected with ds salp25D RNA. Recovered larvae (10 %) with decreased expression of salp25D (Mean 500 ± 200 copies of salp25D/1000 copies of tick actin) when compared to ds Mock-injected larvae (Mean 70,000 ± 30,000 copies of salp25D/1000 copies of tick actin) also had significantly decreased (P = 0.0260) Borrelia burden (Mean 5,000 ± 2,000 copies of flaB/1000 copies of tick actin) when compared to Mock-injected larvae (Mean 13,000 ± 2,000 copies of flaB/1000 copies of tick actin). Salp25D immunization studies conducted with both nymphs (Fig 4C) and larvae (Fig 4E) additionally complemented the RNAi efforts (Fig 2). Moreover, the immunization studies suggest the theoretical possibility that vaccination of the reservoir host with Salp25D could interrupt the B. burgdorferi life cycle and reduce the spirochete population within ticks in areas endemic for Lyme disease. Overall, these data demonstrate that Salp25D facilitates Borrelia acquisition by both larval and nymphal ticks.
Borrelia is exposed to reactive oxygen intermediates generated by neutrophils at the vector-host interface as it traffics through the feeding lesion during acquisition and transmission. Spirochete superoxide dismutase (Nichols et al., 2000; Whitehouse et al., 1997) and CoA disulphide reductase (Boylan et al., 2006) possibly enable the removal of O2− generated intracellularly in spirochetes. Our findings indicate that Borrelia may utilize Salp25D to quench extracellular reactive oxygen species (Fig 6). Tick saliva readily quenches extracellular O2− produced by neutrophils (Fig 6A) and when tick saliva lacks Salp25D, its ability to detoxify O2− is compromised (Fig 6C–D). Reactive oxygen species generated by PMA-activated neutrophils are detrimental to Borrelia and both rSalp25D and tick saliva offer protection for spirochetes from these toxic compounds (Fig 6E). We infer that when tick saliva lacks Salp25D or it is neutralized, spirochete viability decreases, and Borrelia cannot readily enter the tick midgut (Figs 2–4). That Salp25D has the ability to quench reactive oxygen species and provide a survival advantage to Borrelia is also evidenced by in vitro studies with rsalp25D (Suppl Fig 3).
Salp25D did not influence Borrelia transmission (Fig 5B), demonstrating the difference between entering into, and exiting from, the vector. Within ticks Borrelia has the capacity to alter I. scapularis gene expression, increasing the expression of salivary genes such as salp15 during transmission (Ramamoorthi et al., 2005). Simultaneously, in preparation for exit from the tick, the expression of several Borrelia proteins including outer surface protein C (OspC) is increased (Schwan et al., 1995). Our earlier study showed that Salp15 binds to OspC on spirochetes during exit from the tick and offers the spirochete a protective advantage against host immune responses (Ramamoorthi et al., 2005). Specific interactions between B. burgdorferi and tick salivary proteins may thus enable the spirochete to escape reactive oxygen-mediated damage during transmission. Lending credence to this hypothesis is the observation that the numbers of viable Borrelia were significantly higher (P = 0.0045) when spirochetes were pre-incubated with tick salivary gland extracts and then exposed to PMA-activated neutrophils (Suppl Fig 4) compared to the numbers of viable spirochetes in the control group wherein spirochetes were not pre-incubated with tick salivary gland extracts. In addition, presumably, as spirochetes exit the tick, they are in the process of rapidly moving away from the feeding site and may escape the full brunt of the toxic environment.
Vector-borne pathogens interact with both arthropod and mammalian defenses. At a unique point in the infectious agent’s life cycle, e.g., when the microbe is transferred from a mammal to a vector, or from an arthropod to a mammal, a complex vector-host-pathogen interface occurs, with many competing elements. Our data show that a tick molecule that has a function at the vector-host interface facilitates the successful migration of B. burgdorferi from mice to I. scapularis. To date, the control and prevention of many arthropod-borne diseases has been difficult, with the emphasis primarily on targeting pathogen antigens. Focus is now shifting towards the arthropod, in the hope that vector molecules crucial for pathogen transmission to the host, or acquisition by the vector, may be manipulated to influence a microbe’s life cycle (Aksoy et al., 2001; de la Fuente and Kocan, 2006; Trimnell et al., 2002; Willadsen, 1997). It is likely that vector antigens that pathogens require for successful migration from host to arthropod can serve as additional targets for strategies to reduce the prevalence and spread of vector-borne diseases.
EXPERIMENTAL PROCEDURES
I. scapularis ticks
I. scapularis nymphs and larvae were obtained from a tick colony at the Connecticut Agricultural Experiment Station (New Haven, CT). Nymphs were fed to repletion on pathogen-free C3H/HeN mice and allowed to molt to adults. Feeding experiments with adult ticks involved placing 10–15 adult female I. scapularis on each ear of naive New Zealand White rabbits. Ears were secured with cotton socks, and a restraining collar was placed around the neck of each rabbit. Adult I. scapularis males were placed with females at a 1:1 ratio to ensure mating and feeding.
B. burgdorferi-infected mice and nymphs
A low-passage-number clonal isolate of B. burgdorferi N40 that is infectious to mice (Thomas et al., 2001) was used to inoculate C3H mice. Approximately, 100 μl of 1×105 N40 spirochetes/ml was injected subcutaneously. Skin punch biopsies were collected from each mouse 2 weeks after inoculation and DNA isolated using the DNeasy kit (QIAGEN, Valencia, CA) and tested by PCR for the presence of spirochetes as described below. I. scapularis larvae were placed on B. burgdorferi-infected C3H mice and fed larvae molted to generate B. burgdorferi-infected nymphs.
Double stranded RNA (dsRNA) synthesis and injection
Fed-nymph salivary gland cDNA was prepared as described (Narasimhan et al., 2002) and used as template to amplify DNA encoding a fragment of salp25D (600 bp; GenBank accession number: AF209911). Gene-specific primers containing BglII and KpnI restriction sites were used in the PCRs (5′agatctccacgaatggctcggc-3′ and 5′ggtaccggaacagcttgagaatc-3′). The resultant amplicons were purified and cloned into the BglII–KpnI sites of the L4440 double T7 Script II vector (Fire et al., 1998). Ds RNA complementary to the salp25D sequence were synthesized using the Megascript RNAi kit (Ambion Inc, Austin, TX) as described in the user manual. In acquisition experiments, 3–5 nl of salp25D-dsRNA (1×1012 molecules/μl) was microinjected into the body or anal pore of pathogen-free I. scapularis nymphs using glass capillary needles as described earlier (Narasimhan et al., 2004). In experiments to test the impact of Salp25D on larval ticks, 2 nl of salp25D-dsRNA (1×1012 molecules/μl) was injected into the body of I. scapularis larvae as described above for nymphs. Ticks in the control group were injected with the same volume of ds RNA elution buffer provided in the Megascript RNAi Kit (Ambion Inc, TX). In transmission experiments, the same amounts of salp25D-dsRNA as above was microinjected into the body of Borrelia-infected I. scapularis nymphs using glass capillary needles. In experiments to test the role of Salp25D on adult tick feeding, 0.5 μl of salp25D dsRNA (1×1012 molecules/μl) was microinjected into the body of pathogen-free adult female ticks.
siRNA construction and synthesis
siRNA target sites were chosen based on the guidelines described by Elbashir et al (Elbashir et al., 2001) and target sites selected using the siRNA design guidelines available online (www.ambion.com/techlib/misc/siRNA_design.html). The general strategy was to ensure that the 21-mer oligonucleotide had TT overhangs; GC content cutoff was less than 50 %; and tracts of 4 or more Gs and Cs in a row were avoided. The selected sequences were then compared to both mouse and I. scapularis database using the BLAST program (www.ncbi.nlm.nih.gov/BLAST) to eliminate sequences that had more than 16–17 base pair of homology to unrelated coding sequences. The sequences of the two sets of siRNAs for salp25D were si-181salp25D antisense:5′AAGAAGGGTGTCAAGCTCATC 3′ and sense:5′AAGATGAGCTTGACACCCTTC -3′ (corresponding to positions 184–205 of salp25D) and si-61salp25D antisense 5′AAGATCGACTTCCACGAATGG -3′ and sense 5′AACCATTCGTGGAAGTCGATC -3′ (corresponding to positions 61–82 of salp25D). The sequence for si-salp9pac was as follows: and si-salp9pac antisense 5′AAACCAGGCTTAATAAAGATACCTGTCTC -3′ and 5′-AATATCTTTATTAAGCCTGGTCCTGTCTC -3′ (corresponding to positions 412–433 of salp9pac). Eight-nucleotide sequence complementary to the T7 promoter primer was added to the 3′ ends of both the sense and antisense sequences during oligonucleotide synthesis at the W.M. Keck Oligonucleotide synthesis facility at Yale University. siRNAs were enzymatically synthesized by in vitro transcription using the Silencer siRNA construction kit (Ambion, TX). The siRNAs (~ 4 nl of 20 μM) were microinjected into the body of the tick as described for dsRNAs.
B. burgdorferi acquisition
In experiments to address Borrelia acquisition, at least 20 pathogen-free I. scapularis nymphs (Mock or salp25D dsRNA injected into the body or the analpore as described above) were placed on each B. burgdorferi-infected mice and allowed to feed for 66h. Ticks were then gently removed or collected for analysis. At least 3 mice were used in each experiment and 10–12 ticks were recovered from each mouse. Salivary glands were analyzed in pools of 3, and midguts analyzed in pools of 2. Results of a representative experiment out of 3–4 experiments are shown. In experiments to determine whether Salp25D affected Borrelia acquisition during or after entry into the midgut, at least 20 pathogen-free I. scapularis nymphs (Mock or ds salp25D RNA-injected into the body of the ticks) were placed on each B. burgdorferi-infected mice and ticks removed at 24 and 48 h after attachment. At least 3 mice were used in each group for each time point and at least 25 ticks were analyzed per group and RNA isolated from pools of 3 salivary glands or 2 midguts.
B. burgdorferi transmission
In experiments to address Borrelia transmission, a group of 6–7 mock-injected and salp25D dsRNA-injected B. burgdorferi (N40)-infected nymphs were placed on each C3H/HeN mouse (a group of at least 5 mice each in the mock and dsRNA groups) and allowed to feed for 66 h. Ticks were gently removed and midguts and salivary glands dissected and processed in pools of 3 salivary glands and 2 midguts for quantitative RT-PCR analysis analyzed by quantitative RT-PCR as described below. A representative experiment is shown. After 30 days, the mice were sacrificed and skin, heart, bladder, spleen and joints aseptically collected and assessed for spirochete burden by DNA-PCR as described below.
Tick RNA isolation and RT-PCR
The microinjected-nymphs or larvae were allowed to feed on naïve, B. burgdorferi-infected C3H/HeN mice. The fed nymphs were dissected and salivary glands (in pools of three pairs) and midguts (in pools of 2) suspended in Trizol and RNA isolated according to the manufacturer’s protocol (Invitrogen, CA). Fed larvae were pooled in groups of three ticks, ground under liquid nitrogen and suspended in Trizol and RNA isolated according to the manufacturer’s protocol (Invitrogen, CA). cDNA was synthesized using the iScript RT-PCR kit (BIORAD, CA) and analyzed by PCR for the expression of the following tick genes: salp25D, tick actin, salp9pac, salp25B, salp16, lrp (encoding for the predicted salivary protein AAY66745) and trx (encoding for the predicted salivary protein AAY66603) genes using the primers listed: 25DF-5′cctttccccaacttcacc3′ and 25DR-5′gtccatggttgttcggag3′; tick actin F-5′ggcgacgtagcag 3′ and tick actin R-5′ggtatcgtgctcgactc 3′; and salp9pacF-5′catggggttgactgag 3′ and salp9pacR 5′tacctttattaag; salp25BF-5′cctgaagaagacc 3′ and salp25BR 5′ gtgttgcaatattc 3′; salp16F-5′ ctgaagttctttattctcttc 3′ and salp16R 5gcagggtccttcttcggg 3′; lrpF- 5′cccctgcacaag 3′ and lrpR-5′ gagatcagggcatcg 3′; and trxpF-5′ gttcggaaagcaag 3′ and trxpR-5′ gttccaacggatgtcg 3′. For amplification of the transcripts corresponding to Borrelia flaB gene, the following PCR primer set was utilized: flaBF-5′ttcaatcaggtaacggcaca 3′ and flaBR-5′gacgcttgagaccctgaaag 3′. Quantitative PCR was performed using the iQ Syber Green Supermix (Biorad, CA) on a MJ cycler (MJ Research, CA). Data were analyzed using Microsoft Excel software and results expressed as mean ± SE. Significance of the differences between the mean values was assessed by a two-tailed Student’s t-test. A P value < 0.05 was scored as a significant difference.
Mouse DNA isolation and PCR
Mouse skin punch biopsies, joints, heart, spleen and bladders were suspended in DNAeasy suspension buffer (QIAGEN, CA) and processed for DNA isolation according to the manufacturer’s protocol. The resultant DNA was analyzed by PCR for the presence of Borrelia using flaB primers and results normalized using the mouse actin primers as shown here: 5′agcgggaaatcgtgcgtg-3′ and 5′ cagggtacatggtggtgcc-3′.
Confocal microscopy
Confocal microscopy of control and experimental tick guts was carried out as described earlier (Pal et al., 2004). Briefly the guts were stained with FITC-conjugated anti-Borrelia antibody to visualize spirochetes and with the nuclear stain TO-PRO-3 iodide (TOPRO) (Invitrogen, MD) to visualize the midgut tissue. Guts of mock-injected and experimental ticks (~ 10 ticks per group, n = 3 experiments) were dissected in PBS, placed on sialylated slides (PGC Scientific, Gaithersburg, MD), washed in PBS, and fixed in acetone (10 min at 4°C) as described (Pal et al., 2004). The midgut samples were blocked in PBS, 5% fetal calf serum (FCS) and 0.5% Tween 20 before incubation with FITC-conjugated anti-Borrelia antibody (Kirkegaard and Perry, Gaithersburg, MD). The guts were counterstained with 10 mM TOPRO for 3 min at room temperature; washed briefly with PBS/0.5% Tween 20, mounted in glycerol, and imaged (magnification of 63X) using a Zeiss LSM 510 confocal microscope with multitracking to prevent bleeding between the two channels. The number of spirochetes were counted in at least 5 different fields for each midgut examined and results presented for each group as mean (±SE) number of spirochetes/midgut field. For localizing Salp25D, fed nymphal salivary glands and midguts were processed as described above for midguts and stained with mouse anti-rSalp25D antibody and bound antibody detected with goat anti-mouse TRITC-conjugated IgG (Sigma-Aldrich, MO). Tissues stained with mouse anti-rMBP (Maltose Binding Protein) antibody served as control.
Neutrophil isolation
PMNs were prepared from heparinized human blood essentially as described (Carlyon et al., 2004) and counted using a haemocytometer.
Superoxide production assay
The ability of tick saliva to quench extracellular O2− released by neutrophils was measured using the Luminescence Enhancement System (National Diagnostics, Inc, GA) as described by Carlyon et al (Carlyon et al., 2004). Suspensions of 100,0000 or 500,000 neutrophils in 400 μl of RPMI 1640 were added to luminometer cuvettes (Promega, Madison, WI.) and incubated at 37°C for 15 minutes with 100 μl of Diogenes (National Diagnostics, Atlanta, GA) that is highly specific for O2−. This was followed by incubation for 5 min with tick saliva from untreated adults at 70 (1 μl), 350 (5 μl) and 700 ng (10 μl) or 700 ng of saliva from mock-injected or salp25D dsRNA-injected ticks. The neutrophils were activated by the addition of 4 μl of phorbol myristyl acetate (PMA) (100 ng/ml; Sigma) and changes in Diogenes chemiluminescence (DCL) were recorded in total relative light units (RLU) using a TD-20/20 luminometer (Turner Designs; Promega, WI). Intensity of light produced by Diogenes in the presence of superoxide is directly proportional to the O2− concentration. Neutrophils that were not incubated with PMA provided the baseline score and served as a control for monitoring neutrophil integrity during the course of the reaction. In experiments to determine the ability of saliva from untreated adult, mock-injected and ds salp25D RNA-injected ticks to quench pre-existing superoxide radicals, neutrophils were activated with PMA for 10 min prior to addition of the above mentioned test samples in the presence of 0.01mM Dithithreitol (DTT) that served as an extraneous electron donor/reducing agent in the peroxiredoxin catalyzed reaction. Two replicate experiments were carried out in duplicate and the observed chemiluminescence plotted as mean ± SD.
Effect of superoxide radicals on spirochete viability
Human neutrophils were prepared and activated with PMA as described above. Ten minutes after activation, 10 μl of in vitro grown B. burgdorferi (N40) at a spirochete density of 500,000 spirochetes/ml was added to the reaction mixture in the presence or absence of 700 ng of tick saliva, 200 nM of rSalp25D or MBP in the presence of 0.01 mM DTT and reaction allowed to proceed for another 30 min. Subsequently, 5 μl of the suspension was visualized for the presence of viable spirochetes using the Live Dead Bacterial Viability kit™ (Invitrogen, CA) using a Zeiss Axioscope Fluorescence Microscope (Carl Zeiss Inc, NY) at a magnification of 40X. At least 10 fields were examined on each slide. The numbers of spirochetes were counted and results expressed as the mean (±SE) ratio of green (live) spirochetes/dead spirochetes/field in each reaction. To more definitively assess the viability of the spirochetes, the remaining reaction mixture was washed in phosphate buffered saline (PBS) and inoculated into 10 ml of complete Barbour-Stoenner-Kelly (BSK-H) (Sigma Chemical Co, MO) and allowed to grow for 5 days at 30 °C. The spirochetes were then counted using a Petroff-Hausser Counter (Hausser Scientific, Horsham, PA) to assess the total number of viable spirochetes in each sample and results expressed as mean (±SE) number of spirochetes. Spirochete numbers were counted by one to two independent observers to rule out bias.
Coating Borrelia with tick salivary gland extracts
100 μl of in vitro grown B. burgdorferi (N40) at a spirochete density of 50,000 spirochetes/ml were incubated with and without 10 μg of adult I. scapularis salivary gland extracts on a rotary shaker for 3–4 h at 4 °C. The spirochetes were then collected by centrifugation (3000 g for 10 min) and washed 3 times in 500 μl of PBS and re-suspended in 100 μl of PBS. Human neutrophils were prepared and activated with PMA as described above. Ten minutes after activation, 10 μl (~ 500 spirochetes) of the washed spirochetes (pre-incubated with and without salivary gland extracts) was added to the reaction mixture and reaction allowed to proceed for another 20 minutes. 5 μl of the suspension was visualized for the presence of viable spirochetes using the Live Dead Bacterial Viability kit™ (Invitrogen, CA) using a Zeiss Axioscope Fluorescence Microscope (Carl Zeiss Inc, NY) at a magnification of 40X. At least 5 fields were examined on each slide. The numbers of spirochetes were counted by two independent observers and results expressed as the mean (±SE) ratio of number of green (live) spirochetes/dead spirochetes/field in each reaction. As additional controls to demonstrate that Borrelia viability was not compromised in the presence of non-activated neutrophils, resting neutrophils were also separately incubated with 10 μl of spirochetes without salivary gland extracts.
Purification of recombinant Salp25D
The coding region of salp25D cDNA was amplified from I. scapularis nymphs by PCR using the primer pair listed: 5′cagaattcatgggtcccctgaacctcggc-3′ and: 5′ccgaatcagtccatggttgttcgg-3′ and cloned into the EcoRI and HindIII sites of the pMALc2x vector (New England Biolabs, MA). The approximately 68 kDa recombinant MBP (Maltose Binding Protein)-Salp25D (rSalp25D) fusion protein was purified on an amylose resin as described by the manufacturer. The MBP fusion tag (rMBP) was also purified using the pMALc2x vector alone.
Immunization of mice with Salp25D to assess influence of Salp25D antibodies on Borrelia acquisition
Six mice were immunized with 10 μg of rSalp25D or rMBP in complete Freunds’ adjuvant and boosted twice at two-week intervals with 10 μg of rSalp25D or rMBP in incomplete Freund’s adjuvant. Sera were collected 2 weeks after the final boost and evaluated in immunoblots for their ability to react with native Salp25D in tick salivary gland extracts and with rSalp25D. Pre-immune serum from each mouse served as internal controls. The immunized mice were challenged with B. burgdorferi and infection confirmed by DNA-PCR of skin biopsies at 2 weeks. 30 I. scapularis nymphs were then placed on 3 rSalp25D-immunized mice and 3 rMBP-immunized mice and allowed to feed to repletion. 25–28 ticks were recovered from each group. Midguts were dissected and pools of 2 midguts were analyzed by quantitative RT-PCR or DNA-PCR for Borrelia burden. To examine the impact of Salp25D antibodies on Borrelia acquisition by I. scapularis larvae, approximately 100 larvae were placed on each of 2 rSalp25D-immunized mice and 2 rMBP-immunized mice. At least 60 larvae were recovered from each mouse. Whole larvae pooled in groups of 5, were processed for quantitative RT-PCR.
Western blot analysis to confirm gene silencing
Salivary glands or midguts were isolated from individual salp25D dsRNA or mock-injected adult ticks or nymphs. The tissues were suspended in sterile PBS (100 μl PBS per adult salivary gland tissue) and homogenized. Total protein was quantified by the Bradford method. Equal amounts of salivary gland or midgut protein (1–2 μg) from mock-injected and dsRNA-injected ticks were electrophoresed on an SDS 4–20% gradient polyacrylamide gel, transferred to nitrocellulose membranes and processed for immunoblotting. The immunoblots were incubated separately with guinea pig polyclonal antibodies against rSalp14 containing an N-terminal MBP fusion tag (Narasimhan et al., 2002) (as a positive control) or guinea pig polyclonal antibodies against rSalp25D containing an N-terminal MBP fusion tag. Bound antibodies were detected by using horseradish peroxidase-conjugated or alkaline phosphatase-conjugated goat anti-guinea pig secondary antibodies (Sigma–Aldrich, St. Louis, MO). The immunoblots were developed using a Western Lightening chemiluminescence kit (Perkin Elmer Life and Analytical Sciences Inc, Wellesley, MA). When polyclonal mouse anti-rSalp25D or anti-MBP were used, bound antibodies were detected by using horseradish peroxidase-conjugated goat anti-mouse secondary antibodies (Sigma-Aldrich, St. Louis, MO).
Hydroxyl radical-DNA nicking assay
The hydroxyl radical-mediated DNA nicking assay was performed essentially as described by Lim et al (Lim et al., 1993). 3 μM FeCl3, 0.1 mM EDTA and 10 mM DTT in HEPES buffer pH 7.2 was incubated at 37 °C for 1 h to generate hydroxyl radicals. This was followed by the addition of 1 μg of PUC19 supercoiled plasmid (New England Biolabs, MA) and incubation continued for another 4 h in the presence or absence of increasing concentrations of rSalp25D or rMBP. The reaction mixture was then electrophoresed on a 1 % agarose gel, stained with ethidium bromide and visualized under UV light.
Effect of hydroxyl radicals on B. burgdorferi viability
Hydroxyl radicals were generated as described above and 10 μl of in vitro grown B. burgdorferi (N40) at a spirochete density of 500,000 spirochetes/ml was added to the reaction mixture in the presence or absence of 200 nM of rSalp25D or rMBP and incubation continued at 37° C for 2 h. Subsequently, 5 μl of the suspension was visualized) for the presence of viable spirochetes using the Live Dead Bacterial Viability kit™ (Invitrogen, CA) using a Zeiss Axioscope Fluorescence Microscope (Carl Zeiss, Inc, NY) at a magnification of 40X and analyzed as described above in the method to determine the effect of neutrophil ROS on spirochete viability.
Statistical Analysis
Statistical significance of differences observed in experimental and control groups was analyzed using Microsoft Excel version X (Microsoft Corp, WA) or GraphPad Prism version 4.00 (GraphPad Software, CA). A two-tailed Student’s t-Test was utilized to compare the mean values and P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Dr. Stephen Barr for input on the peroxiredoxin assays, Dr. Karen Anthony for scientific advice, and Philippe Male for technical help with microscopy. This work was supported by grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksoy S, Maudlin I, Dale C, Robinson AS, O’Neill SL. Prospects for control of African trypanosomiasis by tsetse vector manipulation. Trends Parasitol. 2001;17:29–35. doi: 10.1016/s1471-4922(00)01850-x. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Fish D. The biological and social phenomenon of Lyme disease. Science. 1993;260:1610–1616. doi: 10.1126/science.8503006. [DOI] [PubMed] [Google Scholar]
- Barr SD, Gedamu L. Cloning and characterization of three differentially expressed peroxidoxin genes from Leishmania chagasi. Evidence for an enzymatic detoxification of hydroxyl radicals. J Biol Chem. 2001;276:34279–34287. doi: 10.1074/jbc.M104406200. [DOI] [PubMed] [Google Scholar]
- Barr SD, Gedamu L. Role of peroxidoxins in Leishmania chagasi survival. Evidence of an enzymatic defense against nitrosative stress. J Biol Chem. 2003;278:10816–10823. doi: 10.1074/jbc.M212990200. [DOI] [PubMed] [Google Scholar]
- Boylan JA, Hummel CS, Benoit S, Garcia-Lara J, Treglown-Downey J, Crane EJ, 3rd, Gherardini FC. Borrelia burgdorferi bb0728 encodes a coenzyme A disulphide reductase whose function suggests a role in intracellular redox and the oxidative stress response. Mol Microbiol. 2006;59:475–486. doi: 10.1111/j.1365-2958.2005.04963.x. [DOI] [PubMed] [Google Scholar]
- Carlyon JA, Abdel-Latif D, Pypaert M, Lacy P, Fikrig E. Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infect Immun. 2004;72:4772–4783. doi: 10.1128/IAI.72.8.4772-4783.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, Fikrig E. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J Infect Dis. 2001;184:1056–1064. doi: 10.1086/323351. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Kocan KM. Strategies for development of vaccines for control of ixodid tick species. Parasite Immunol. 2006;28:275–283. doi: 10.1111/j.1365-3024.2006.00828.x. [DOI] [PubMed] [Google Scholar]
- Elbashir SM, Martinez J, Patkaniowska A, Lendeckel W, Tuschl T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 2001;20:6877–6888. doi: 10.1093/emboj/20.23.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Flohe L, Budde H, Hofmann B. Peroxiredoxins in antioxidant defense and redox regulation. Biofactors. 2003;19:3–10. doi: 10.1002/biof.5520190102. [DOI] [PubMed] [Google Scholar]
- Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- Jackson JH, Gajewski E, Schraufstatter IU, Hyslop PA, Fuciarelli AF, Cochrane CG, Dizdaroglu M. Damage to the bases in DNA induced by stimulated human neutrophils. J Clin Invest. 1989;84:1644–1649. doi: 10.1172/JCI114342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JH, Schraufstatter IU, Hyslop PA, Vosbeck K, Sauerheber R, Weitzman SA, Cochrane CG. Role of hydroxyl radical in DNA damage. Trans Assoc Am Physicians. 1987;100:147–157. [PubMed] [Google Scholar]
- Lim YS, Cha MK, Kim HK, Uhm TB, Park JW, Kim K, Kim IH. Removals of hydrogen peroxide and hydroxyl radical by thiol-specific antioxidant protein as a possible role in vivo. Biochem Biophys Res Commun. 1993;192:273–280. doi: 10.1006/bbrc.1993.1410. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Koski RA, Beaulieu B, Anderson JF, Ramamoorthi N, Kantor F, Cappello M, Fikrig E. A novel family of anticoagulants from the saliva of Ixodes scapularis. Insect Mol Biol. 2002;11:641–650. doi: 10.1046/j.1365-2583.2002.00375.x. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Montgomery RR, DePonte K, Tschudi C, Marcantonio N, Anderson JF, Sauer JR, Cappello M, Kantor FS, Fikrig E. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc Natl Acad Sci U S A. 2004;101:1141–1146. doi: 10.1073/pnas.0307669100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TL, Whitehouse CA, Austin FE. Transcriptional analysis of a superoxide dismutase gene of Borrelia burgdorferi. FEMS Microbiol Lett. 2000;183:37–42. doi: 10.1111/j.1574-6968.2000.tb08930.x. [DOI] [PubMed] [Google Scholar]
- Nuttall PA. Displaced tick-parasite interactions at the host interface. Parasitology. 1998;116(Suppl):S65–72. doi: 10.1017/s003118200008495x. [DOI] [PubMed] [Google Scholar]
- Nuttall PA, Labuda M. Tick-host interactions: saliva-activated transmission. Parasitology. 2004;129(Suppl):S177–189. doi: 10.1017/s0031182004005633. [DOI] [PubMed] [Google Scholar]
- Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, Desilva AM, Bao F, Yang X, Pypaert M, Pradhan D, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Persengiev SP, Zhu X, Green MR. Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs) RNA. 2004;10:12–18. doi: 10.1261/rna5160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, IM BF, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Weis JJ, Telford SR. Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp Parasitol. 1990;70:382–388. doi: 10.1016/0014-4894(90)90121-r. [DOI] [PubMed] [Google Scholar]
- Sauer JR, Hair JA. Morphology, physiology, and behavioral biology of ticks. Chichester, West Sussex, England New York: E. Horwood;Halsted Press; 1986. [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A. Human babesiosis on Nantucket Island: transmission by nymphalIxodes ticks. Am J Trop Med Hyg. 1976;25:784–787. doi: 10.4269/ajtmh.1976.25.784. [DOI] [PubMed] [Google Scholar]
- Telford SR, Armstrong PM, Katavolos P, Foppa I, Garcia AS, Wilson ML, Spielman A. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis. 1997;3:165–170. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford SR, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V, Anguita J, Barthold SW, Fikrig E. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect Immun. 2001;69:3359–3371. doi: 10.1128/IAI.69.5.3359-3371.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimnell AR, Hails RS, Nuttall PA. Dual action ectoparasite vaccine targeting ‘exposed’ and ‘concealed’ antigens. Vaccine. 2002;20:3560–3568. doi: 10.1016/s0264-410x(02)00334-1. [DOI] [PubMed] [Google Scholar]
- Valenzuela JG, Francischetti IM, Pham VM, Garfield MK, Mather TN, Ribeiro JM. Exploring the sialome of the tick Ixodes scapularis. J Exp Biol. 2002;205:2843–2864. doi: 10.1242/jeb.205.18.2843. [DOI] [PubMed] [Google Scholar]
- Whitehouse CA, Williams LR, Austin FE. Identification of superoxide dismutase activity in Borrelia burgdorferi. Infect Immun. 1997;65:4865–4868. doi: 10.1128/iai.65.11.4865-4868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK, Alarcon-Chaidez FJ. Progress toward molecular characterization of ectoparasite modulation of host immunity. Vet Parasitol. 2001;101:275–287. doi: 10.1016/s0304-4017(01)00556-8. [DOI] [PubMed] [Google Scholar]
- Willadsen P. Novel vaccines for ectoparasites. Vet Parasitol. 1997;71:209–222. doi: 10.1016/s0304-4017(97)00028-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.