Abstract
The possible molecular basis for the previously described antagonistic interactions between adenosine A1 receptors (A1R) and dopamine D1 receptors (D1R) in the brain have been studied in mouse fibroblast Ltk− cells cotransfected with human A1R and D1R cDNAs or with human A1R and dopamine D2 receptor (long-form) (D2R) cDNAs and in cortical neurons in culture. A1R and D1R, but not A1R and D2R, were found to coimmunoprecipitate in cotransfected fibroblasts. This selective A1R/D1R heteromerization disappeared after pretreatment with the D1R agonist, but not after combined pretreatment with D1R and A1R agonists. A high degree of A1R and D1R colocalization, demonstrated in double immunofluorescence experiments with confocal laser microscopy, was found in both cotransfected fibroblast cells and cortical neurons in culture. On the other hand, a low degree of A1R and D2R colocalization was observed in cotransfected fibroblasts. Pretreatment with the A1R agonist caused coclustering (coaggregation) of A1R and D1R, which was blocked by combined pretreatment with the D1R and A1R agonists in both fibroblast cells and in cortical neurons in culture. Combined pretreatment with D1R and A1R agonists, but not with either one alone, substantially reduced the D1R agonist-induced accumulation of cAMP. The A1R/D1R heteromerization may be one molecular basis for the demonstrated antagonistic modulation of A1R of D1R receptor signaling in the brain. The persistence of A1R/D1R heteromerization seems to be essential for the blockade of A1R agonist-induced A1R/D1R coclustering and for the desensitization of the D1R agonist-induced cAMP accumulation seen on combined pretreatment with D1R and A1R agonists, which indicates a potential role of A1R/D1R heteromers also in desensitization mechanisms and receptor trafficking.
During the 1980s, indications for the existence of intramembrane interactions between different G protein-coupled receptors, mainly between neuropeptide and monoamine receptors, were obtained in several brain areas (1, 2). It was later proposed that a possible molecular mechanism for this phenomenon was receptor heteromerization (3) and direct evidence for homo- and heteromerization of G protein-coupled receptors has been obtained by several groups. It was first shown that serotonin 5-HT-1B receptors exist as monomers and dimers (4). This was followed by demonstration of dimers and oligomers of dopamine D1 and D2 receptors (D1 and D2R) in transfected Sf cells (5–7) and of adenosine A1 receptors (A1Rs) in a natural cell line and in mammalian brain (8). It has recently been reported that a fully functional γ-aminobutyric acid (GABA) type B receptor demands the heterodimerization of GABABR1 and GABABR2 receptors (9–12). Moreover, two functional opioid receptors, the κ and δ subtypes, can undergo heteromerization, which changes the pharmacology of the individual receptors and potentiates signal transduction (13). Finally, D2R and somatostatin receptor subtype 5 have been shown to physically interact by forming heterooligomers with enhanced functional activity (14). Direct protein–protein coupling can also exist between G protein-coupled anion channel receptors, as recently shown for dopamine D5 receptor and GABAA receptor, making possible bilateral inhibitory interactions between these receptors (15).
Antagonistic adenosine/dopamine interactions have been widely reported in the central nervous system in behavioral and biochemical studies. Furthermore, in animal models, adenosine agonists and antagonists are potent atypical neuroleptics and antiparkinsonian drugs, respectively (16–18). Thus, adenosine agonists inhibit and adenosine antagonists, such as caffeine, potentiate the behavioral effects induced by dopamine agonists. The evidence suggests that this antagonism is at least in part caused by an intramembrane interaction between specific subtypes of dopamine and adenosine receptors, namely, between A1Rs and D1Rs and between adenosine A2A receptors (A2ARs) and D2Rs (16). This antagonism is evident in crude membrane preparations from cell lines expressing the two receptors and from rat striatum in which, for instance, activation of A1Rs reduces the proportion of D1Rs in the high-affinity state without changing the dissociation constants of the high- and the low-affinity binding sites (19, 20). In the present paper, indications have been obtained that the postulated intramembrane interactions between A1Rs and D1Rs may involve the formation of heteromeric complexes regulated by A1R and D1R agonists.
Methods
Cell Cultures.
Previously characterized mouse fibroblast Ltk− cells transfected with human D1R cDNA (D1 cells) and with both human D1R and human A1R cDNAs (A1/D1 cells) were used (20). For control experiments, Ltk− cells cotransfected with human D2R (long-form) and human A1R cDNAs (A1/D2 cells) were obtained with the calcium phosphate precipitation method (20). Ltk− cells were grown as described (20). Primary cultures of neurons were obtained from 17- to 18-day-old Sprague–Dawley rat embryos as described (21).
Radioligand-Binding Experiments.
Membrane preparations from Ltk− cells were obtained as described (20). Saturation experiments with the D2R antagonist [3H]raclopride (79.3 Ci/mmol; NEN; 1 Ci = 37 GBq) and the 3H-labeled A1R antagonist 1,3-dipropyl-8-cyclopentylxanthine ([3H]DPCPX; 120 Ci/mmol; NEN) and competition experiments of [3H]raclopride versus dopamine (in the presence and absence of the selective A1R agonist N6-cyclopentyladenosine (CPA; 10 nM) were performed as described (20, 22). Data from saturation experiments were analyzed by nonlinear regression analysis (graphpad) for the determination of dissociation constants (Kd) and the total number of receptors (Bmax). Data from competition experiments were also analyzed by nonlinear regression analysis, and the dissociation constants for the high-affinity (KH) and low-affinity (KL) binding sites and the proportion of binding sites in the high-affinity state (RH) were determined. The amount of nonspecific binding was calculated by extrapolation of the displacement curve. Protein determinations were performed by using BSA as a standard. The Mann–Whitney U test was used to analyze differences in RH, KH, and KL values.
cAMP Determination.
Treatments were performed for 30, 60, and 120 min with 10 μM (±)-SKF-38393 (Research Biochemicals, Natick, MA) and/or 100 nM (R)-(−)N6-(2-phenylisopropyl)adenosine (R-PIA, Research Biochemicals). After two washes at 4°C with culture medium containing the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (Sigma), cAMP accumulation was induced by stimulating D1Rs for 15 min with 10 μM SKF-38393. The reaction was stopped by adding HCl (0.1 M final concentration) and cAMP was extracted from cells and quantified according to Nordstedt and Fredholm (23). Absolute values were used in the statistical analysis by means of repeated measures ANOVA with post hoc Scheffe's test.
Double-Immunolabeling Experiments.
For immunofluorescence staining, cells (A1/D1 or A1/D2 cells, or primary cultures of cortical neurons) growing on glass coverslips were incubated in the absence or presence of 100 nM R-PIA, 10 μM SKF-38393, or 100 nM R-PIA plus 10 μM SKF-38393 in serum-free medium for 1 h at 37°C. They were then rinsed in PBS, fixed in 4% paraformaldehyde in PBS for 15 min, and washed in PBS containing 20 mM glycine. Cells were permeabilized for 7 min with 0.01% saponin (A1/D1 and A1/D2 cells) or 0.2% Triton X-100 (cortical neurons) in PBS and subsequently treated with PBS/20 mM glycine/1% BSA for 30 min at room temperature. Double immunostaining was performed with fluorescein-conjugated anti-A1R antibody (PC21-FITC, 20 μg/ml for transfected cells or 50 μg/ml for cortical neurons) (8, 24) and Texas red-conjugated anti-D1R (D1–356-446-Tx, 5 μg/ml for transfected cells or 10 μg/ml for cortical neurons) (25) or Texas red-conjugated anti-D2R (D2–246-316-Tx) (25) for 1 h at 37°C. The coverslips were rinsed for 40 min in the same buffer and mounted with medium for immunofluorescence (ICN). Confocal microscopic observations were made with a Leica TCS 4D (Leica Lasertechnik, Heidelberg, Germany) confocal scanning laser equipment adapted to an inverted Leitz DMIRBE microscope. The extent of colocalization of the two labelings was assessed by means of computerized image analysis (KS300, Kontron, Zurich). A couple of images of the same field stained with the two labelings were analyzed at each time. In each image, the specific staining was discriminated from the nonspecific background by means of the threshold function and the discriminated images of the two labelings were superimposed and subtracted by means of the and Boolean operator function. By using this function, a new image is created containing only pixels that are positive in both original discriminated images. The percent coexistence is obtained by expressing the number of positive pixels in the new image in percent of the number of positive pixels in each of the original discriminated images.
Immunoprecipitation of A1R, D1R, and D2R.
A1/D1 cotransfected cells were incubated in the absence or presence of 100 nM R-PIA or 10 μM SKF-38393 in serum-free medium for 1 h at 37°C. Cell membranes were obtained by centrifugation (105,000 × g for 45 min at 4°C) after disruption of cells with a Polytron homogenizer (Kinematica, PTA 20TS rotor, setting 4; Brinkmann) for three 5-s periods in 50 mM Tris⋅HCl, pH 7.4. Membranes were separated at 105,000 × g (45 min at 4°C). Pretreated or control membranes were solubilized in ice-cold lysis buffer (PBS, pH 7.4/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS) for 1 h on ice and then centrifuged at 80,000 × g for 90 min. The supernatants (1 mg of protein per ml) were precleared by incubation (6 h) with staphylococcal protein A-Sepharose beads. After centrifugation at 10,000 × g for 15 s, the supernatants were transferred to a tube containing affinity-purified anti-A1R antibody (PC11) (8) or a control rabbit IgG, both antibodies covalently coupled to protein A-Sepharose (24). Nonspecific immunoprecipitation was performed by incubating the same amount of protein A-Sepharose coupled to anti-A1R antibody with membrane extracts (obtained as described above) from D1 cells, which do not express A1R. Immunoprecipitates were washed twice in ice-cold lysis buffer containing 0.1% Nonidet P-40/0.05% sodium deoxycholate/0.01% SDS and once in ice-cold PBS, pH 7.4. After centrifugation and isolation of the beads, 60 μl of SDS/PAGE sample buffer was added to the beads. Immune complexes treated at 37°C for 15 min were resolved by SDS/PAGE in 12.5% gels. Proteins were transferred to poly(vinylidene difluoride) (PVDF) membranes (Immobilon-P, Millipore) for 1 h by using a wet transfer system in Towbin buffer (25 mM Tris/192 mM glycine/20% methanol, pH 8.3). Nonspecific protein binding sites on the PVDF membranes were blocked by incubation overnight at 4°C by using 10% (wt/vol) dehydrated milk in PBS. After blocking, PVDF membranes were washed three times (10 min per wash) in 10 mM Tris⋅HCl buffer containing 500 mM NaCl and 0.5% Tween-20 (TBS-TII; pH 7.4) and incubated for 2 h with the purified anti-A1R antibody (PC11; 10 μg/ml), purified anti-D1R antibody (D1–356-446; 10 μg/ml), or purified anti-D2R antibody (D2–246-316; 10 μg/ml) in TBS-TII, including 0.02% NaN3. Immunoreactive bands were detected with a donkey anti-rabbit IgG antibody conjugated to horseradish peroxidase (1/10,000 Promega W401 B 8846301), followed by development with a chemiluminescence detection system (Pierce SuperSignal). A similar protocol was used to study possible coimmunoprecipitation of A1R and D2R, by using an anti-D2R antibody (25) for immunoprecipitation and immunoblotting in membranes from A1/D2 cells.
Results
Studies on A1R- and D1R-Containing Fibroblast Cells.
Immunoprecipitation experiments.
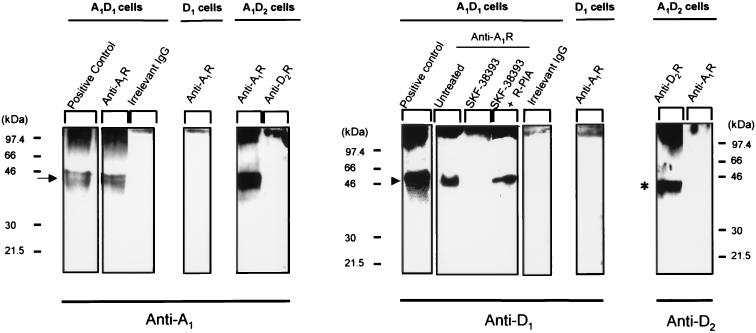
As seen in Fig. 1, the A1R antibody immunoprecipitated a band in A1/D1 cells with a molecular mass of 46 kDa, which was detected by a specific antibody for human D1R. This band did not appear when an irrelevant antibody was used or when D1 cells (transfected with D1R cDNA alone) were analyzed (Fig. 1). Immunoblottings of cell lysates were used as positive controls. Treatment of the A1/D1 cells with 10 μM of the D1R agonist SKF-38393 for 1 h reduced the intensity of the 46-kDa band detected by the anti-D1R antibody. This action of SKF-39393 was no longer seen after combined treatment with SKF-38393 (10 μM; 1 h) and the A1R agonist R-PIA (100 nM, 1 h) (Fig. 1).
Figure 1.
Coimmunoprecipitation of A1R and D1R. Cell membranes from A1/D1, A1/D2, or D1 cells were obtained and processed for immunoprecipitation (see Methods) by using the purified anti-A1R antibody PC11, the anti-D2R antibody, or an irrelevant goat IgG; all were covalently coupled to protein A-Sepharose. Immunoblottings of cell lysates (positive control) and immunoprecipitates were performed to detect A1R with anti-A1R antibody, D1R with anti-D1R antibody, or D2R with anti-D2R antibody. When indicated, A1/D1-cotransfected cells were incubated for 1 h with 10 μM SKF-38393 in the absence or presence of 100 nM R-PIA. The arrow indicates the band for A1R, the arrowhead the band corresponding to D1R, and the asterisk the band for D2R.
Double-immunolabeling experiments.
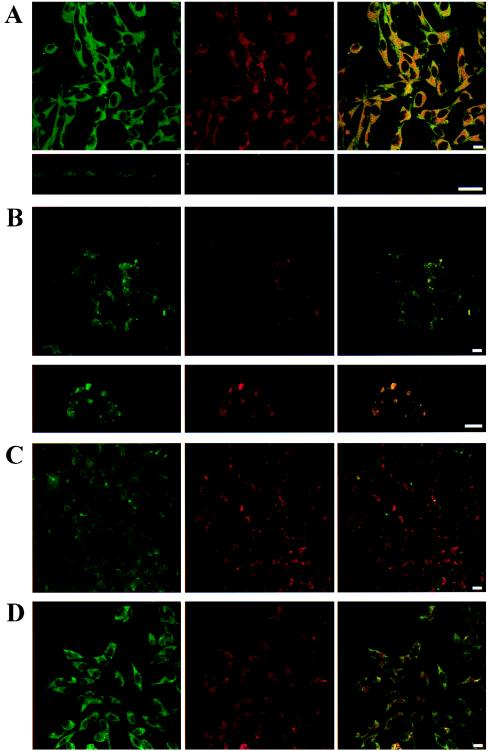
The degree of D1R immunoreactivity was similar in D1 cells and A1/D1 cells. The antibody against the A1R labeled A1/D1 cells but not D1 cells. With the confocal laser microscopy, it was possible to see a homogenous distribution of A1Rs and D1Rs on the cell surface of A1D1 cells. The analysis of these cells showed a marked overlap in the distribution of the two receptor proteins. The percentage of colocalization was 71% for the A1R immunoreactive area and 77% for the D1 immunoreactive area in the absence of agonists. The vertical optical sections demonstrated that the colocalization of A1R and D1R exists both in the cell membrane and in the cytoplasm (Fig. 2A). When the cells were treated for 1 h with the A1R agonist R-PIA, a redistribution of A1Rs and D1Rs was observed (Fig. 2B). Thus, R-PIA induced the aggregation of both proteins in clusters seen as punctate fluorescence, where colocalizations between adenosine and dopamine receptors approach 100% (see the intensity of yellow in Fig. 2B). In contrast, the D1R agonist SKF-38393 clustered D1Rs but not A1Rs (Fig. 2C). The R-PIA- or SKF-38393-induced clusters had a similar appearance; the clusters were very variable in size with no preferential localization within the cell. Furthermore, combined treatment with SKF-38393 and R-PIA as above did not result in any clustering either of the D1R or the A1R (Fig. 2D).
Figure 2.
Distribution of A1R and D1R in A1/D1-cotransfected fibroblast cells. Cells were incubated for 1 h with medium in the absence (A) or presence of 100 nM R-PIA (B), 10 μM SKF-38393 (C), or 100 nM R-PIA plus 10 μM SKF-38393 (D) and were processed for immunostaining (see Methods) by using fluorescein (green)-conjugated rabbit anti-A1R antibody and a Texas red-conjugated rabbit anti-D1R antibody. The cells were analyzed by confocal laser microscopy. Superimposition of images (Right images in each panel) reveals the colocalization of A1R and D1R in yellow. (A Lower) A vertical section of representative cells is also shown. (B Lower) A magnification of a representative cell is also given. (Scale bars: 10 μm.)
cAMP determination.
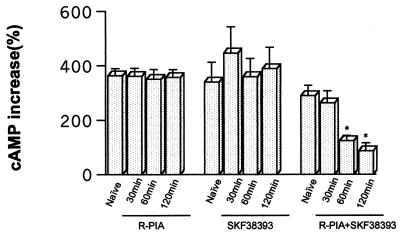
In A1/D1 fibroblast cells, pretreatment with the D1R agonist SKF-38393 (10 μM) for 30–120 min did not alter the SKF-38393-induced increase in cAMP accumulation (Fig. 3). The same was also true after pretreatment with 100 nM of R-PIA for 30–120 min. In contrast, a significant reduction of the SKF-38393-induced cAMP accumulation was found after combined pretreatment with SKF-38393 (10 μM) and R-PIA (100 nM) for 60 and 120 min (Fig. 3).
Figure 3.
cAMP accumulation induced by incubation with 10 μM SKF-38393 (15 min) after pretreatment of A1/D1-cotransfected cells with 100 nM R-PIA and/or 10 μM SKF-38393. Control cells (naive) were treated with medium alone for 120 min. Data represent the means ± SEM (n = 6) of the percentage of increase versus basal values. The basal values of cAMP for the groups treated with R-PIA, SKF-38393, and R-PIA plus SKF-38393 were (means ± SEM, in pmol/mg of protein; n = 4) 11,020 ± 1,345; 24,060 ± 2,279; and 14,030 ± 1,375; respectively. Repeated measures ANOVA with post hoc Scheffé's test: *, P < 0.01 with respect to control cells (not significantly different from basal values).
Studies on A1R- and D2R-Containing Fibroblast Cells.
Radioligand-binding experiments.
The clone chosen for subsequent studies had a high density of A1R labeled with 1,3-dipropyl-8-cyclopentylxanthine ([3H]DPCPX) (means ± SEM; Bmax, 4.4 ± 0. 1 pmol/mg of protein; Kd, 4.6 ± 0.4 nM; n = 4) and D2R labeled with [3H]raclopride (means ± SEM; Bmax, 0.9 ± 0.06 pmol/mg of protein; Kd, 5.7 ± 0.9 nM; n = 4). In competition experiments with [3H]raclopride versus dopamine, KH, KL, and RH values (medians, and in parentheses, interquartile ranges) were 0.07 (0.1) μM, 1.9 (0.5) μM, and 28.3 (15.3)% (n = 4), respectively. The A1 agonist N6-cyclopentyladenosine (10 nM) failed to significantly influence these values, being 0.09 (0.2) μM, 2.5 (1.4) μM, and 27.5 (16.8)% (n = 4), respectively.
Immunoprecipitation experiments.
As seen in Fig. 1, A1R antibodies could not detect immunocomplexes precipitated by D2R antibodies and D2R antibodies could not detect immunocomplexes precipitated by A1R antibodies.
Double-immunolabelling experiments.
D2R immunoreactivity and A1R immunoreactivity was demonstrated in the A1/D2 cells but a low colocalization could be seen in the absence (Fig. 4A) or presence (Fig. 4B) of R-PIA.
Figure 4.
Distribution of A1R and D2R in A1/D2-cotransfected fibroblast cells. Cells were processed for immunostaining (see Methods) by using fluorescein (green)-conjugated rabbit anti-A1R antibody and a Texas red-conjugated rabbit anti-D2R antibody. The cells were analyzed by confocal laser microscopy. (B) Cells were treated with 100 nM R-PIA. Superimposition of images reveals the lack colocalization of A1R and D2R. (Scale bars: 10 μm.)
Studies on Primary Rat Cortical Cultures.
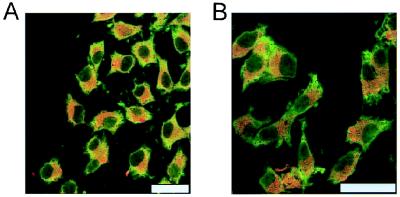
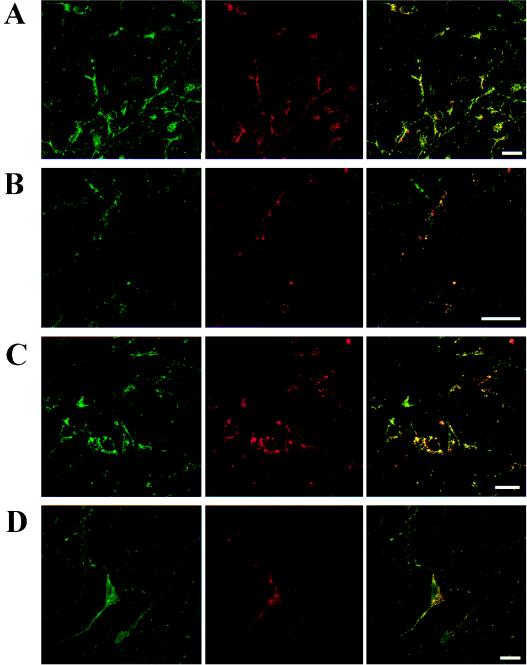
As seen in Fig. 5, the cultured neurons showed A1R and D1R immunoreactivity. The location of both receptors was diffuse in the soma and dendrites with a high degree of colocalization (Fig. 5A). The degree of colocalization between A1R and D1R immunoreactivity was similar to that found in cotransfected fibroblast cells. The A1R agonist R-PIA reproduced the clustering effect on D1Rs and A1Rs observed in the cotransfected fibroblast cells (Fig. 5B). In these cultures, also SKF-38393 (10 μM, 1 h) produced clustering with a high degree of colocalization of D1Rs and A1Rs (Fig. 5C). The simultaneous treatment with SKF-38393 (10 μM) and R-PIA (100 nM) for 1 h instead blocked the formation of the A1R/D1R clusters seen with either of the agonists alone (Fig. 5D).
Figure 5.
Distribution of A1R and D1R in primary cultures of cortical neurons. Cells were incubated for 1 h with medium in the absence (A) or presence of 100 nM R-PIA (B), 10 μM SKF-38393 (C), or 100 nM R-PIA plus 10 μM SKF-38393 (D) and were processed for immunostaining (see Methods) by using fluorescein (green)-conjugated rabbit anti-A1R antibody and a Texas red-conjugated rabbit anti-D1R antibody. The cells were analyzed by confocal microscopy. Superimposition of images (Right images in each panel) reveals the colocalization of A1R and D1R in yellow. (Scale bars: 10 μm.)
Discussion
The existence of homo- and/or heteromers of G protein-coupled receptors has recently been proposed and experimental evidence for this concept is starting to be obtained (see Introduction). In the present paper, it is shown that D1Rs and A1Rs form heteromeric complexes under basal conditions and that they can coaggregate (cocluster) under some specific agonist-stimulated conditions. The two phenomena appear to be related to each other in a complex way and may have different functional meaning.
The existence of A1R/D1R heteromers was tested in immunoprecipitation experiments by using membranes from rat fibroblast cells cotransfected with the cDNAs for human D1Rs and A1Rs (20). The antibody against A1R was able to coimmunoprecipitate the D1R in A1/D1 cells but not D2R in A1/D2 cells. These results are of importance, because they indicate that after solubilization, the adjacent A1R/D1R do not become associated in a nonspecific way. Accordingly, the A1R agonist N6-cyclopentyladenosine, which modulates D1R binding (19, 20), did not significantly change the binding parameters obtained from competitive inhibition curves of dopamine versus [3H]raclopride in A1/D2 cells. Overall, these results indicate that the A1Rs and D1Rs are physically associated, directly or indirectly via an additional component, in cells coexpressing both receptors and that these heteromeric complexes exist in the absence of receptor activation by exogenous agonists.
In cotransfected Ltk− fibroblast cells, 1 h of exposure to A1R and D1R agonists, alone or in combination, had remarkable effects on hetero- or homomerization, and on the degree of aggregation of A1Rs and D1Rs. Exposure to the A1R agonist R-PIA induced the formation of clusters (aggregations) containing both A1R and D1R immunoreactivities. In contrast, the D1R agonist SKF-38393 decreased the amount of A1R/D1R heteromers, and induced selective clustering of D1Rs. Different from each single treatment, 1-h exposure with both A1R and D1R agonists maintained the heteromeric association of the two receptors but decreased the amount of A1R/D1R aggregations (clusters).
D1R agonist-induced cAMP accumulation, a main index of D1R function, was studied after the same pretreatment conditions as described above in cotransfected Ltk− fibroblast cells. It was found that nonclustered D1R (naive cells), clustered D1R (D1R agonist treatment), or clustered A1R/D1R (A1R agonist treatment) all cause a similar cAMP response to the D1R agonist challenge. Thus, clustering per se is not a prerequisite for D1R coupling to adenylate cyclase, nor does it appear to modulate D1R function. On the other hand, a clear-cut decrease in D1R signaling to adenylate cyclase was found when the A1/D1 cells were pretreated simultaneously with the A1R and D1R agonists. Notably, after A1R/D1R coactivation, the two receptors remain physically associated, but differently from the effect of A1R activation alone, do not form clusters. These data show that A1R activation influences the D1Rs, which are and remain physically associated in a heteromeric complex, and leads to an uncoupling of D1R from adenylyl cyclase only when the D1Rs are simultaneously activated. Thus, the temporal dynamics of receptor activation and heteromerization may play a key role in A1R/D1R interactions. The uncoupling of D1R and Gs occurring in coactivated A1R/D1R heteromers may contribute to adenosine inhibition of D1R function, and complement the known A1R-induced inhibition of D1R function mediated by the activation of Gi (19, 20). Overall, A1R- and D1R-mediated transmission, heteromerization, and clustering are phenomena related to each other in a complex manner. Homogeneous or heterogeneous clustering does not appear to be a necessary consequence of receptor activation but it may be a prerequisite for internalization (26). On the other hand, the mono-, homo-, or heteromeric state of A1R and D1R is not related in an obvious way to receptor clustering. In fact, the role of homo- and heteromerization may be different in different systems (9–12, 27).
The colocalization of D1Rs and A1Rs as well as their clustering in response to A1 and/or D1 agonist treatment as described above was also analyzed in primary cultures of neurons from rat cerebral cortex. A1Rs and D1Rs were highly colocalized and diffusely distributed to the soma and dendrites of the cortical neurons, which is consistent with previous studies that identified D1Rs and A1Rs in cell bodies, dendrites, and spines mainly at extrasynaptic locations (28, 29). The A1R agonist R-PIA reproduced in neurons the effects already observed in cotransfected fibroblast cells, i.e., increases in A1R/D1R colocalization up to 100% and coaggregation in clusters. In contrast to the A1R/D1R-cotransfected fibroblast cells, the effect of SKF-38393 in neurons was similar to that of R-PIA, namely a coclustering of D1Rs and A1Rs. These differential actions may reflect differences in the relative amount of the receptors in the A1R/D1R complexes in the neurons as compared with cotransfected fibroblasts. In addition, the neurons may have membrane components that the A1R/D1R-cotransfected cells do not express, leading to the coclustering of A1R/D1R also after the D1R agonist pretreatment. Nevertheless, the simultaneous pretreatment of neurons with the D1R and the A1R agonists reproduced the results obtained in A1R/D1R-cotransfected cells with failure to cluster either one of the receptors.
Based on immunoprecipitation and double immunolabeling experiments, evidence is presented for the existence of A1R and D1R heteromers, and their presence in membranes of cotransfected fibroblast cells. Functional experiments on the D1R agonist-induced cAMP production suggest that coactivation of A1R and D1R in the heteromeric complex leads to an uncoupling of D1R from Gi. This antagonistic mechanism may contribute to the A1R/D1R functional antagonism found in the brain and offers a basis for the design of novel agents to treat Parkinson's disease and neuropsychiatric disorders, based on the pharmacological properties of the A1/D1 heteromeric complex.
Acknowledgments
This work was supported by the Spanish Commission of Science and Technology (PB97–0984, BIO099–0601-C02–02), a coordinated European BIOMED 2 program (BM4-CT96–0238), an Italian MPI ex60597 grant, the Swedish Medical Research Council, and the Marianne and Marcus Wallenberg Foundation.
Abbreviations
- A1R
adenosine A1 receptor
- D1R
dopamine D1 receptor
- D2R
dopamine D2 receptor
- R-PIA
(R)-(−)N6-(2-phenylisopropyl)adenosine
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.150241097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.150241097
References
- 1.Agnati L F, Fuxe K, Benfenati F, Celani M F, Battistini N, Mutt V, Cavicchioli L, Galli G, Hokfelt T. Neurosci Lett. 1983;35:179–183. doi: 10.1016/0304-3940(83)90547-5. [DOI] [PubMed] [Google Scholar]
- 2.Fuxe K, Agnati L F, editors. Receptor-Receptor Interactions. Wenner-Gren Center Interactional Symposium Series. New York: Macmillan; 1987. [Google Scholar]
- 3.Zoli M, Agnati L F, Hedlund P B, Li X M, Ferré S, Fuxe K. Mol Neurobiol. 1993;7:293–334. doi: 10.1007/BF02769180. [DOI] [PubMed] [Google Scholar]
- 4.Ng G Y, George S R, Zastawny R L, Caron M, Bouvier M, Dennis M, O'Dowd B F. Biochemistry. 1993;32:11727–11733. doi: 10.1021/bi00094a032. [DOI] [PubMed] [Google Scholar]
- 5.Ng G Y, O'Dowd B F, Caron M, Dennis M, Brann M R, George S R. J Neurochem. 1994;63:1589–1595. doi: 10.1046/j.1471-4159.1994.63051589.x. [DOI] [PubMed] [Google Scholar]
- 6.Ng G Y, Mouillac B, George S R, Caron M, Dennis M, Bouvier M, O'Dowd B F. Eur J Pharmacol. 1994;247:7–19. doi: 10.1016/0922-4106(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 7.Ng G Y, O'Dowd B F, Lee S P, Chung H T, Brann S, Seeman P, George S R. Biochim Biophys Res Commun. 1996;227:200–204. doi: 10.1006/bbrc.1996.1489. [DOI] [PubMed] [Google Scholar]
- 8.Ciruela F, Casadó V, Mallol J, Canela E I, Lluis C, Franco R. J Neurosci Res. 1995;42:818–828. doi: 10.1002/jnr.490420610. [DOI] [PubMed] [Google Scholar]
- 9.Jones K A, Borowsky B, Tamm J A, Craig D A, Durkin M M, Dai M, Yao W J, Johnson M, Gunwaldsen C, Huang L Y, et al. Nature (London) 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 10.White J H, Wise A, Main M J, Green A, Fraser N J, Disney G H, Barnes A A, Emson P, Foord S M, Marshall F H. Nature (London) 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 11.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, et al. Nature (London) 1998;396:683–688. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 12.Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau H C. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 13.Jordan B A, Devi L A. Nature (London) 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocheville M, Lange D C, Kumar U, Patel S C, Patel R C, Patel Y C. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 15.Liu F, Wan Q I, Pristupa Z B, Yu X-M, Wang Y T, Niznik H B. Nature (London) 2000;403:274–280. doi: 10.1038/35002014. [DOI] [PubMed] [Google Scholar]
- 16.Ferré S, Fredholm B B, Morelli M, Popoli P, Fuxe K. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 17.Rimondini R, Ferré S, Ogren S O, Fuxe K. Neuropsychopharmacology. 1997;17:82–91. doi: 10.1016/S0893-133X(97)00033-X. [DOI] [PubMed] [Google Scholar]
- 18.Kanda T, Jackson M J, Smith L A, Pearce R K, Nakamura J, Kase H, Kuwana Y, Jenner P. Ann Neurol. 1998;43:507–513. doi: 10.1002/ana.410430415. [DOI] [PubMed] [Google Scholar]
- 19.Ferré S, Popoli P, Giménez-Llort L, Finnman U-B, Martínez E, Scotti de Carolis A, Fuxe K. NeuroReport. 1994;6:73–76. doi: 10.1097/00001756-199412300-00020. [DOI] [PubMed] [Google Scholar]
- 20.Ferré S, Torvinen M, Antoniou K, Irenius E, Civelli O, Arenas E, Fredholm B B, Fuxe K. J Biol Chem. 1998;273:4718–4724. doi: 10.1074/jbc.273.8.4718. [DOI] [PubMed] [Google Scholar]
- 21.Villalba M, Martínez-Serrano A, Gómez-Puertas P, Blanco P, Börner C, Villa A, Casado M, Jiménez E, Pereira R, Bogonez E, et al. J Biol Chem. 1994;244:2468–2476. [PubMed] [Google Scholar]
- 22.Kull B, Ferré S, Arslan G, Svenningsson P, Fuxe K, Owman C, Fredholm B. Biochem Pharmacol. 1999;58:1035–1045. doi: 10.1016/s0006-2952(99)00184-7. [DOI] [PubMed] [Google Scholar]
- 23.Nordstedt C, Fredholm B B. Anal Biochem. 1990;189:231–234. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- 24.Schneider C, Newman R A, Sutherland D R, Asser U, Greaves M F. J Biol Chem. 1982;257:10766–10769. [PubMed] [Google Scholar]
- 25.Bjelke B, Goldstein M, Tinner B, Andersson C, Sesack S R, Steinbusch H W, Lew J Y, He X, Watson S, Tengroth B, et al. J Chem Neuroanat. 1996;12:37–50. doi: 10.1016/s0891-0618(96)00176-7. [DOI] [PubMed] [Google Scholar]
- 26.Saura C A, Mallol J, Canela E I, Lluis C, Franco R. J Biol Chem. 1998;273:17610–17617. doi: 10.1074/jbc.273.28.17610. [DOI] [PubMed] [Google Scholar]
- 27.Hebert T E, Moffett S, Morello J P, Loisel T P, Bichet D G, Barret C, Bouvier M. J Biol Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- 28.Caille I, Dumartin B, Bloch B. Brain Res. 1996;730:17–31. doi: 10.1016/0006-8993(96)00424-6. [DOI] [PubMed] [Google Scholar]
- 29.Rivkees S A, Price S L, Zhou F C. Brain Res. 1995;677:193–203. doi: 10.1016/0006-8993(95)00062-u. [DOI] [PubMed] [Google Scholar]