Abstract
MicroRNAs are small noncoding RNA species, some of which are playing important roles in cell differentiation. However, the level of participations of microRNAs in epithelial cell differentiation is largely unknown. Here, utilizing an epithelial differentiation model with T84 cells, we demonstrate that miR-338-3p and miR-451 contribute to the formation of epithelial basolateral polarity by facilitating translocalization of β1 integrin to the basolateral membrane. Among 250 microRNAs screened in this study, the expression levels of four microRNAs (miR-33a, 210, 338-3p and 451) were significantly elevated in the differentiated stage of T84 cells, when epithelial cell polarity was established. To investigate the involvement of these microRNAs in terms of epithelial cell polarity, we executed loss-of- and gain-of-function analyses of these microRNAs. The blockade of endogenous miR-338-3p or miR-451 via each microRNA-specific antisense oligonucleotides inhibited the translocalization of β1 integrin to the basolateral membrane, whereas inhibition of miR-210 or miR-33a had no effect on it. On the other hand, simultaneous transfection of synthetic miR-338-3p and miR-451 accelerated the translocalization of β1 integrin to the basolateral membrane, although the introduction of individual synthetic microRNAs exhibited no effect. Therefore, we concluded that both miR-338-3p and miR-451 are necessary for the development of epithelial cell polarity.
INTRODUCTION
Epithelial cells carry out key directional functions such as absorption, secretion and protection against the surrounding environment for host multicellular organisms, and these functions depend upon tight junctions (TJs) and the polarized distribution of plasma membrane molecules. The TJs, which are rigid paracellular permeability barriers between the outside and the inside of an organism, divide the plasma membrane of epithelial cells into two domains: an apical domain facing the external environment and a basolateral domain in contact with the internal environment. These plasma membrane domains have different protein and lipid compositions (1–6).
Recent studies suggested that three major polarization complexes contribute to the formation of TJs, apical domains and basolateral domains, respectively (7,8). The differentiation process of epithelial cells is initiated in response to cell–cell and cell–matrix adhesion. These adhesion stimulations form a complex of partition-defective (PAR) 3, PAR6 and atypical protein kinase C (aPKC), and this complex subsequently stabilizes belt-like adherens junctions (AJs), cortical actin bundles and TJs. Thereafter, the PAR3/PAR6/aPKC complex and a Crb/Pals1/PATJ complex (Crumbs, proteins associated with Lin seven and protein-associated with tight protein 102) contribute to formation of the apical domain. On the other hand, the formation of the basolateral domain was accelerated by PAR1b and an Lgl/Scrib/Dlg complex (Lethal giant larvae, Scribble and Discs large). Additionally, membrane trafficking of specific molecules to apical or basolateral membranes play key roles in maturation of the polarized membrane (9). N-/O-glycans, glycosylphosphatidylinositol-anchored proteins (GPI-APs) and lipid rafts are known to be involved in the transport to the apical domain, whereas clathrin, adaptor protein 1B (AP1B), and CD98 play important roles in the trafficking to the basolateral membrane (1,9–11). Basolateral sorting signals of basolateral membrane-specific proteins are mainly found in the cytoplasmic domain, consisting of tyrosine or leucine, while apical sorting signals of apical proteins have not been discovered yet (9). Currently, mechanisms underlying construction and maintenance of the TJs and epithelial cell polarity are not fully understood.
Recently, it has been shown that expression of some microRNAs (miRNAs) was altered during the differentiation process of epithelial cells, although roles of their miRNAs in the differentiation process remained unknown (12). MiRNAs are evolutionarily conserved small noncoding RNAs (20–23 nt), which regulate gene expression by translational inhibition or cleavage of target mRNAs. The miRNAs play important roles in the development, differentiation and function of various cell types, and in the pathogenesis of various human diseases, e.g., cancer (13,14). Currently, over 800 human miRNAs have been identified and registered in the miRNA database, miRBase (15). Strikingly, about 30% of protein-coding human transcripts are predicted to be regulated by these miRNAs (16,17). Here, we investigated the potential roles of miRNAs in the epithelial cell differentiation.
MATERIALS AND METHODS
Cell culture
T84 cells were cultured in Dulbecco's modified Eagle's Medium (DMEM)/Ham's F-12 mixture (Invitrogen, San Diego, CA) containing 10% heat-inactivated (56°C for 30 min) fetal bovine serum at 37°C in a fully humidified 5% CO2 atmosphere. To differentiate into polarized epithelial cells, T84 cells were seeded onto polycarbonate filters with 24-mm diameter and 0.4 µm pores in transwell chambers (Coster, Cambridge, MA) at a density of 5 × 105 cells per well, and incubated for 7 days. Cultured cell media were changed into fresh medium on alternate days.
RNA extraction and qRT-PCR
T84 cells were harvested at the indicated periods after plating in transwell chambers, and total RNA was extracted by the acid guanidinium thiocyanate-phenol–chloroform method (18), followed by qRT-PCR. For quantification of intestinal alkaline phosphatase (ALPI) mRNA expression levels, total RNA was transcribed to cDNA using random primer and SuperScript II (Invitrogen), and quantitative PCR was performed in 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) using Power SYBR Green PCR Master Mix (Applied Biosystems). The gene expression was quantified using standard curves generated by serially dilluted reference samples, and normalized by the expression level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The specificity of the PCR products was confirmed by gel electrophoresis and a dissociation curve analysis. Primer sequences were shown as follow (ALPI forward: 5′-tcagctcatctccaacatgg-3′, reverse: 5′-tgagatgggtcacagactgg-3′; GAPDH forward: 5′-gaaggtgaaggtcggagtc-3′, reverse: 5′-ggaagatggtgatgggatttc-3′). For quantification of miRNAs and RNA U6 small nuclear 2 (RNU6-2), we use TaqMan MicroRNA Assays (Applied Biosystems) following the manufacturer's protocol, which detects specifically mature miRNAs (19,20). The miRNA expression was normalized by the expression level of RNU6-2.
Transfection and immunostaining
Just after plating 5 × 105 cells on a polycarbonate filter, cells were transfected with 15 pmol of oligonucleotides for miR-210, miR-338-3p, miR-33a, miR-451 or RNA which sequence has minimal homology with human miRNAs (denoted as ‘NC’ negative control) (Pre-miR miRNA Precursor Molecule; Ambion, Austin, TX) using Hiperfect (Qiagen, Valencia, CA) for overexpression. For inhibition of miRNAs function, 150 pmol of specific microRNA Hairpin Inhibitors or NC (cel-miR-239b; minimal sequence identity with miRNAs in human, mouse and rat) (Dharmacon, Chicago, IL) was transfected. Filters with T84 cells were harvested at 5 or 7 days after transfection, and were fixed in 4% formaldehyde. For ZO1 and β1 integrin staining, filters were incubated in can get signal (Toyobo, Osaka, Japan) with a rabbit anti-ZO1 antibody (1:100) (Zymed Laboratories, South San Francisco, CA) and a mouse anti-β1 integrin antibody (1:100) (BD Bioscience, Bedford, MA), and then were incubated with a Alexa 488-conjugated goat anti-mouse IgG and a Alexa 594-conjugated donkey anti-rabbit IgG (1:1000) (Molecular Probes, Leiden, Netherlands). No significant staining was observed when cells were incubated without primary antibody. All experiments were repeated at least three times. F-actin was stained by Alexa 647-conjugated phalloidin to investigate formation of cortical actin bundles. The signal area was calculated with ImageJ software.
Microarray analysis
Total RNA of T84 cells was labeled and prepared for hybridization to GeneChip Human Genome U133 Plus 2.0 arrays (Affymetrix, Santa Clara, CA) using standard methods. The GEO database accession code of this microarray data is GSE15385. We used the robust multi-array average (RMA) expression measure for log transformation (log2) and normalization of the GeneChip data (21). The RMA measures were computed using the R package program, which is freely available on the web site (http://www.bioconductor.org).
Statistics
Results are expressed as mean ± SE. Student's t-test or Welch test was used to compare data between two groups. P-values < 0.05 were considered as statistically significant. Individual experiments were performed in triplicate, and each experiment was independently performed three times.
RESULTS
A differentiation model of epithelial cells
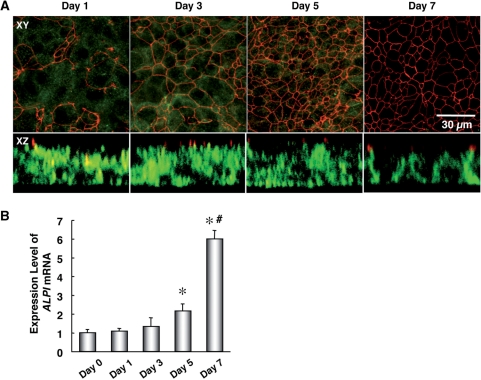
To investigate a role of miRNAs in the epithelial cell differentiation, we utilized an epithelial cell differentiation model using T84 cells, a human colon cancer cell line. T84 cells can transform into a differentiated state with TJs and cell polarity, and are frequently used in the research of epithelial cells (2). To confirm the degree of epithelial cell differentiation from Day 1 to Day 7 after plating undifferentiated T84 cells (day 0), localization of ZO1 and β1 integrin, a TJ marker and a basolateral membrane marker, respectively, was assessed by immunostaining and a confocal microscopy (2–4). In horizontal sections at a relatively higher level of T84 cells, ZO1 was gradually localized in a chicken wire pattern from Day 1 to Day 5, which was consistent with the distribution of TJs (Figure 1A). On the other hand, signals of β1 integrin were gradually disappeared in horizontal sections at the height where the ZO1 protein localized. Additionally, in vertical sections, β1 integrin stayed in intracellular regions (presumably Golgi apparatus and granules) until Day 5, and β1 integrin was translocalized into the basolateral membrane at Day 7 (Figure 1A). Accordingly, the expression level of ALPI mRNA, a marker of epithelial cell differentiation and apical membrane (22,23), was significantly increased by Day 7 (Figure 1B). These findings demonstrated that T84 cells successfully gained morphological characteristics of differentiated epithelial cells during the 7-day culture. To be more exact, T84 cells formed TJs by Day 5, and showed the apical and basolateral polarization from Day 5 to Day 7.
Figure 1.
Time course of cellular distribution of ZO1, β1 integrin and ALPI mRNA accumulation in differentiation-induced T84 cells. T84 cells were cultured onto polycarbonate filters in transwell chambers at confluence for the indicated periods. (A) Confocal sections of T84 cells were immunolabeled for ZO1 (red), β1 integrin (green) to detect endogenous proteins by immunofluorescence. Upper panels show horizontal (XY-crossed) sections of T84 cells. Scale bars, 30 µm. Lower panels show vertical (XZ-crossed) sections. (B) The amounts of ALPI mRNA were analyzed by qRT-PCR as described in the ‘Materials and Methods’ section. T84 cells before plating in transwell chamber were used for the value at Day 0, and the values are shown as the fold of values obtained from the sample at Day 0 (Student's t-test: *P < 0.01 for cells plated in transwell chamber versus cells at Day 0; #P < 0.01 for Day 7 versus Day 5), and are represented as mean ± SE (n = 3). The data are a representative of three independent experiments.
Screening of miRNAs related with differentiation
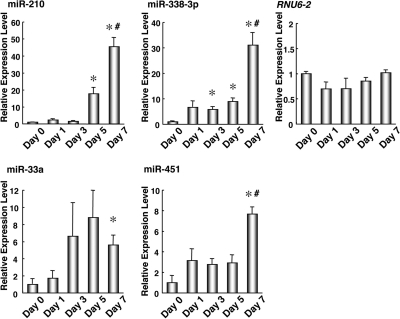
In order to identify miRNAs involved in epithelial cell differentiation, we assumed that these miRNAs were differentially expressed between undifferentiated and differentiated cells. Firstly, utilizing qRT-PCR, we measured expression levels of 250 miRNAs on Day 0, Day 1 and Day 7 in the T84 cells (Supplementary Table 1). To screen the differentially expressed miRNAs, we set a criterion that cycle threshold (Ct) values (in Day 0 or 7 samples) <32, and Ct value difference >2.5, where Ct value is defined as the number of cycles required for accumulation of the fluorescent signal to cross the threshold. Thus, we selected four candidate miRNAs (miR-210, miR-338-3p, miR-33a and miR-451) for further study. These miRNAs were upregulated in T84 cells on Day 7, compared to Day 0 and/or 1. On the other hand, among examined 250 miRNAs, we could not find any downregulated miRNAs that met the criterion. We confirmed that there was no significant change in the expression level of RNU6-2 as a negative control.
Next, we performed more detailed time course analysis on the expression of these four miRNAs (Figure 2). The expression levels of three miRNAs, miRNA-210, miRNA-338-3p and miRNA-451, were significantly elevated between Day 5 and Day 7 (P-values < 0.05). RNU6-2 was not changed in the detailed time course of epithelial differentiation. Thus, the elevation of these miRNA expression levels were closely linked with translocalization of β1 integrin (Figure 1A) and the elevation of ALPI mRNA levels (Figure 1B). Therefore, this finding suggested that these three miRNAs would be correlated to the cellular polarization after the TJ formation.
Figure 2.
Increased expression levels of miR-210, miR-338-3p, miR-33a and miR-451 along with the epithelial cell differentiation of T84 cells. The expression levels of miR-210, miR-338-3p, miR-33a and miR-451 in T84 cells cultured in transwell chambers for the indicated periods were determined by the qRT-PCR. The changes in expression levels are shown as fold of the value for Day 0. The values are shown as mean ± SE (n = 3). The data are a representative of three independent experiments. *P < 0.01 is regard as significant by the Welch test (versus Day 0), #P < 0.05 is regard as significant by the Welch test (Day 7 versus Day 5).
Furthermore, we validated this finding using a different model. The expression levels of these three miRNAs were also significantly upregulated in differentiated Caco-2 cells (Supplementary Figure 1), which is a human colon cancer cell line and was applied to an epithelial cell differentiation model.
Roles of miRNAs in basolateral polarity
The marked elevation of miRNA-210, miRNA-338-3p and miRNA-451 expression between Day 5 and Day 7 strongly suggested that these miRNAs would be involved in the establishment of epithelial cell polarity, such as apical and basolateral domains, after the formation of TJs, because kinetics of these miRNA expressions matched those of ALPI mRNA expression levels and polarized localization of β1 integrin (Figures 1 and 2). Thus, we performed gain- and loss-of-function analyses for these miRNAs to determine whether these miRNAs are related with phenotypes of the epithelial polarity.
We initially assessed transfection efficiency and stability of exogenous RNA oligonucleotides in T84 cells. According to the observation by confocal microscopy, cy3-labeled synthetic control miRNA appeared to be transfected in almost all cells (Supplementary Figure 2A). Additionally, sequential quantification of transfected synthetic miR-210, miR-338-3p, miR-33a and miR-451 by qRT-PCR indicated that amount of these four miRNAs introduced into T84 cells was maintained more than endogenous expression levels of each miRNA for 7 days, although the introduced amount of miR-33a was less than those of other three miRNAs (Supplementary Figure 2B–F). There was little difference in the stability of four synthetic miRNA oligonucleotides. These results suggested that this miRNA transfection protocol is sufficient to evaluate the functions of miRNAs.
As a loss-of-function analysis, 2′-O-methylated antisense RNA oligonucleotides of each miRNA were transfected individually into T84 cells to inhibit each endogenous miRNA. The blockage of either miR-338-3p or miR-451 inhibited translocalization of β1 integrin in basolateral membrane, whereas inhibition of either miR-210 or miR-33a did not alter the translocalization of β1 integrin to the basolateral membrane (Figure 3). Moreover, the inhibition of these four miRNAs did not affect formation of TJs with a dense chicken wire pattern (data not shown), values of transepithelial electrical resistance (TER) as a function of TJs in Caco-2 cells at Day 7 (when TER value reaches a plateau) (Supplementary Figure 3A and B) and the ALPI mRNA expression level (Supplementary Figure 4A). Taken together, these findings suggested that miR-338-3p or miR-451 could regulate basolateral polarity of the T84 cells, but not the formation of TJ and apical polarity.
Figure 3.

Effects of functional inhibition of endogenous miR-210, miR-338-3p, miR-33a and miR-451 on the localization of β1 integrin to the basolateral domain. Fluorescence images of T84 cells transfected with microRNA Hairpin Inhibitors of miR-210, miR-338-3p, miR-33a, miR-451 or NC were stained for ZO1 (red) and β1 integrin (green). For immunostaining studies, T84 cells at the Day 7 were fixed, permeabilized and incubated with the specific antibodies against ZO1 and β1 integrin. The data are a representative of three independent experiments.
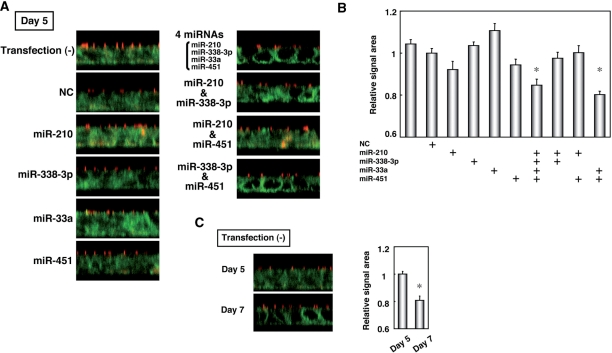
As a gain-of-function analysis, at first, we individually transfected miR-210, miR-338-3p, miR-33a or miR-451 into T84 cells at Day 0. Each transfected miRNA did not alter the pattern of β1 integrin localization in the basolateral membrane as compared with cells transfected with none or NC (Figure 4A). However, when these four miRNAs were transfected together, translocalization of β1 integrin was observed in the basolateral membrane at Day 5. As a result of the quantification of areas with a signal of β1 integrin, the extent of the relocation of β1 integrin was significantly altered when four miRNAs were transfected simultaneously (Figure 4B). The degree of this change was nearly equaled to that in nontransfected T84 cells at Day 7 to 5 (Figure 4B and C). On the other hand, transfection of individual miRNAs or even these four miRNAs together did not affect the formation (data not shown) and function (Supplementary Figure 3C) of TJs, and the expression levels of ALPI mRNA (Supplementary Figure 4B). Lastly, we investigated whether both miR-338-3p and miR-451 are sufficient for promotional formation of basolateral polarity (Figure 4). When both miR-338-3p and miR-451 were transfected, translocalization of β1 integrin as well as at Day 7 of nontransfected T84 cells was observed in the basolateral membrane at Day 5 (Figure 4). Transfection of miR-210 together with either miR-338-3p or miR-451 had no effect on the translocalization of β1 integrin in the basolateral membrane. Accordingly, our experiments showed that both of miR-338-3p and miR-451 are required for acceleration of basolateral polarity formation, and that miR-210 and miR-33a are not necessary for it.
Figure 4.
Effects of overexpression of miR-210, miR-338-3p, miR-33a and miR-451 on the distribution of β1 integrin in the basolateral membrane. (A) T84 cells were transfected with NC or combination of miR-210, miR-338-3p, miR-33a and miR-451 and T84 cells at the Day 5 were stained with the specific antibodies against ZO1 (red) and β1 integrin (green). (B) Quantification of areas with signal intensity of β1 integrin in (A). The values are shown as the fold of values obtained from the sample transfected with NC (Student's t-test: *P < 0.01 versus NC-tansfected cells), and are represented as mean ± SE. (C) Representative fluorescence images of ZO1 (red) and β1 integrin (green) in T84 cells at Day 5 and 7, and quantification of areas with signal intensity of β1 integrin. The values are shown as the fold of values obtained from the sample at Day 5 (Student's t-test: *P < 0.01 Day 7 versus Day 5), and are represented as mean ± SE. The data are a representative of three independent experiments.
Alternation of gene expression patterns by miR-338-3p and miR-451
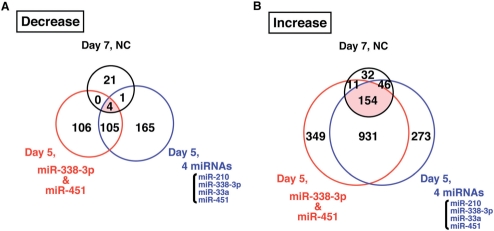
These results suggested that both miR-338-3p and miR-451 were essential for the acquisition of basolateral polarity, although neither miRNA was sufficient on its own (Figures 3 and 4). To understand the role of these miRNAs and their mechanism of action in the formation of basolateral polarity, we performed a comprehensive analysis of gene expression. NC-transfected T84 cells (‘NC’) at Day 5 were used as undifferentiated cells without basolateral polarity. Samples of (i) ‘NC’ at Day 7, (ii) T84 cells transfected with the four miRNAs (‘4 miRNAs’) at Day 5 and (iii) T84 cells transfected with both miR-338-3p and miR-451 (‘miR-338-3p and miR-451’) at Day 5 were assayed as differentiated cells. Total RNA from these cells was hybridized to a microarray.
Candidate target genes of miR-338-3p and miR-451 may show similar expression patterns in the three classes of differentiated T84 cells. Hence, we selected genes whose expression was decreased or increased by more than 2-fold in the three types of differentiated T84 cells compared with ‘NC’ at Day 5, and Venn diagrams of these downregulated and upregulated genes are shown (Figure 5A and B, respectively). Four genes (CLDN2, EEF1DP3, PRKAR2B and SMOC2) were downregulated in all three classes, and none of them contained candidate target sites for either miR-338-3p or miR-451 within their 3′UTRs. In contrast, 154 genes were upregulated in all three classes of differentiated cells, and these included genes such as RAB11a, which accelerates the formation of epithelial cell polarity (Supplementary Table 2) (24).
Figure 5.
Overlapping sets of more than 2-fold decreased or increased genes in ‘NC’ at Day 7, ‘4 miRNAs’ at Day 5 and ‘miR-338-3p and miR-451’ at Day 5 compared with ‘NC’ at Day 5. The number in the overlapping region of the Venn diagram represents shared genes. Genes were selected if the ratio of the relative expression level compared with ‘NC’ at Day 5 was smaller than 0.5 (A) or larger than 2.0 (B).
DISCUSSION
This study demonstrated for the first time that miRNAs contribute to the formation of basolateral polarity in epithelial cells. First, we found that the expression of four miRNAs (miR-210, miR-338-3p, miR-33a and miR-451) was upregulated significantly during the process of epithelial cell differentiation. Among these four miRNAs, we found that miR-338-3p and miR-451 play a fundamental role in the formation of epithelial cell polarity. In an effort to identify the target gene(s) for these miRNAs, we performed a series of microarray and functional studies, which showed that the gene expression profile was altered drastically when T84 cells were transfected with these mRNAs. However, we could not identify a direct link between the affected genes and the miRNAs. Hence, the molecular mechanism by which the two miRNAs induce the formation of epithelial cell polarity remains unclear. However, the results of this study highlight a potentially important role for miRNAs in the process of epithelial cell differentiation.
Among the four miRNAs, whose expression was found to be well correlated with the process of epithelial cell differentiation, we further determined that both miR-338-3p and miR-451 are essential for the translocalization of β1 integrin to the basolateral membrane, which contributes to the formation of basolateral polarity in epithelial cells. However, neither miR-338-3p nor miR-451 alone was sufficient to induce translocalization. In contrast, the other two miRNAs, miR-210 and miR-33a, were found to have little effect on epithelial cell differentiation, although miR-210 was the more highly expressed of the four miRNAs. To identify the functional roles of the two miRNAs in the formation of basolateral polarity in epithelial cells, we performed a series of gene expression studies in the presence and absence of exogenous miRNAs. The microarray analyses showed that the expression of many genes was altered dramatically during the process of epithelial cell differentiation; however, we could not identify a close link between the differentially regulated genes and miR-338-3p or miR-451. Our analysis of the transcriptome failed to identify candidate targets of these miRNAs, and the functional role of the miRNAs in the formation of basolateral polarity in epithelial cells still remains unclear. However, it is known that miRNAs can regulate the expression of proteins associated with the biological change without producing a detectable change in the corresponding mRNA levels. In fact, very recently two groups reported that miRNAs can repress the production of hundreds of proteins without downregulating their mRNA levels (25,26).
Our observations should provide a deeper insight into the mechanisms that underlie the construction and maintenance of epithelial cell polarity. Given that the disruption of cell junctions and polarity is associated with poor prognosis for carcinomas that are derived from epithelial cells (27–29), this finding might be beneficial for the development of novel cancer therapies. Thus, miRNAs that promote epithelial cell differentiation may provide a novel therapeutic approach. In addition, given that miR-451 has been reported to negatively regulate the expression of multidrug resistance 1 (MDR1) (30,31), miR-451 may play a role in sensitivity toward anticancer drugs. In conclusion, our present study highlights a potentially important role for miRNAs in epithelial cell differentiation, and further suggests a novel regulatory mechanism for this fundamental phenomenon in cellular physiology and pathophysiology.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Intramural grants from Kyoto University and the Grant-in-aid of the Ministry of Education, Science, and Culture of Japan (to S.T.); the Grant-in-aid of the Ministry of Education, Science, and Culture of Japan; National Institute of Biomedical Innovation; the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO) the Japan Health Science Foundation and the Ministry of Human Health and Welfare; Uehara Memorial Foundation (to G.T.). Funding for open access charge: Kyoto University.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ms. K Kodama and Ms. T Murai for technical assistance. We also thank Dr. J.M. Abraham, GI Division, Johns Hopkins University, for manuscript review.
REFERENCES
- 1.Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 3.Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J. Cell Sci. 1994;107(Pt 3):561–576. [PubMed] [Google Scholar]
- 4.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol. Biol. Cell. 2005;16:5040–5052. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg JP, Marchetto S, Le Bivic A, Ollendorff V, Jaulin-Bastard F, Saito H, Fournier E, Adelaide J, Margolis B, Birnbaum D. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat. Cell Biol. 2000;2:407–414. doi: 10.1038/35017038. [DOI] [PubMed] [Google Scholar]
- 6.van Meer G, Simons K. Lipid polarity and sorting in epithelial cells. J. Cell Biochem. 1988;36:51–58. doi: 10.1002/jcb.240360106. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J. Cell Sci. 2002;115:3565–3573. doi: 10.1242/jcs.00032. [DOI] [PubMed] [Google Scholar]
- 8.Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim. Biophys. Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 10.Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 11.Merlin D, Sitaraman S, Liu X, Eastburn K, Sun J, Kucharzik T, Lewis B, Madara JL. CD98-mediated links between amino acid transport and beta 1 integrin distribution in polarized columnar epithelia. J. Biol. Chem. 2001;276:39282–39289. doi: 10.1074/jbc.M105077200. [DOI] [PubMed] [Google Scholar]
- 12.Hino K, Tsuchiya K, Fukao T, Kiga K, Okamoto R, Kanai T, Watanabe M. Inducible expression of microRNA-194 is regulated by HNF-1alpha during intestinal epithelial cell differentiation. RNA. 2008;14:1433–1442. doi: 10.1261/rna.810208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchiya S, Okuno Y, Tsujimoto G. MicroRNA: biogenetic and functional mechanisms and involvements in cell differentiation and cancer. J. Pharmacol. Sci. 2006;101:267–270. doi: 10.1254/jphs.cpj06013x. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruike Y, Ichimura A, Tsuchiya S, Shimizu K, Kunimoto R, Okuno Y, Tsujimoto G. Global correlation analysis for micro-RNA and mRNA expression profiles in human cell lines. J. Hum. Genet. 2008;53:515–523. doi: 10.1007/s10038-008-0279-x. [DOI] [PubMed] [Google Scholar]
- 21.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaoutzani P, Parkos CA, Delp-Archer C, Madara JL. Isolation of plasma membrane fractions from the intestinal epithelial model T84. Am. J. Physiol. 1993;264:C1327–C1335. doi: 10.1152/ajpcell.1993.264.5.C1327. [DOI] [PubMed] [Google Scholar]
- 23.Gu N, Adachi T, Takeda J, Aoki N, Tsujimoto G, Ishihara A, Tsuda K, Yasuda K. Sucrase-isomaltase gene expression is inhibited by mutant hepatocyte nuclear factor (HNF)-1alpha and mutant HNF-1beta in Caco-2 cells. J. Nutr. Sci. Vitaminol. (Tokyo) 2006;52:105–112. doi: 10.3177/jnsv.52.105. [DOI] [PubMed] [Google Scholar]
- 24.Ducharme NA, Hales CM, Lapierre LA, Ham AJ, Oztan A, Apodaca G, Goldenring JR. MARK2/EMK1/Par-1Balpha phosphorylation of Rab11-family interacting protein 2 is necessary for the timely establishment of polarity in Madin-Darby canine kidney cells. Mol. Biol. Cell. 2006;17:3625–3637. doi: 10.1091/mbc.E05-08-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 26.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dow LE, Elsum IA, King CL, Kinross KM, Richardson HE, Humbert PO. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene. 2008;27:5988–6001. doi: 10.1038/onc.2008.219. [DOI] [PubMed] [Google Scholar]
- 28.Joshi J, Fernandez-Marcos PJ, Galvez A, Amanchy R, Linares JF, Duran A, Pathrose P, Leitges M, Canamero M, Collado M, et al. Par-4 inhibits Akt and suppresses Ras-induced lung tumorigenesis. EMBO J. 2008;27:2181–2193. doi: 10.1038/emboj.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karp CM, Tan TT, Mathew R, Nelson D, Mukherjee C, Degenhardt K, Karantza-Wadsworth V, White E. Role of the polarity determinant crumbs in suppressing mammalian epithelial tumor progression. Cancer Res. 2008;68:4105–4115. doi: 10.1158/0008-5472.CAN-07-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gal H, Pandi G, Kanner AA, Ram Z, Lithwick-Yanai G, Amariglio N, Rechavi G, Givol D. MIR-451 and Imatinib mesylate inhibit tumor growth of Glioblastoma stem cells. Biochem. Biophys. Res. Commun. 2008;376:86–90. doi: 10.1016/j.bbrc.2008.08.107. [DOI] [PubMed] [Google Scholar]
- 31.Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol. Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.