Abstract
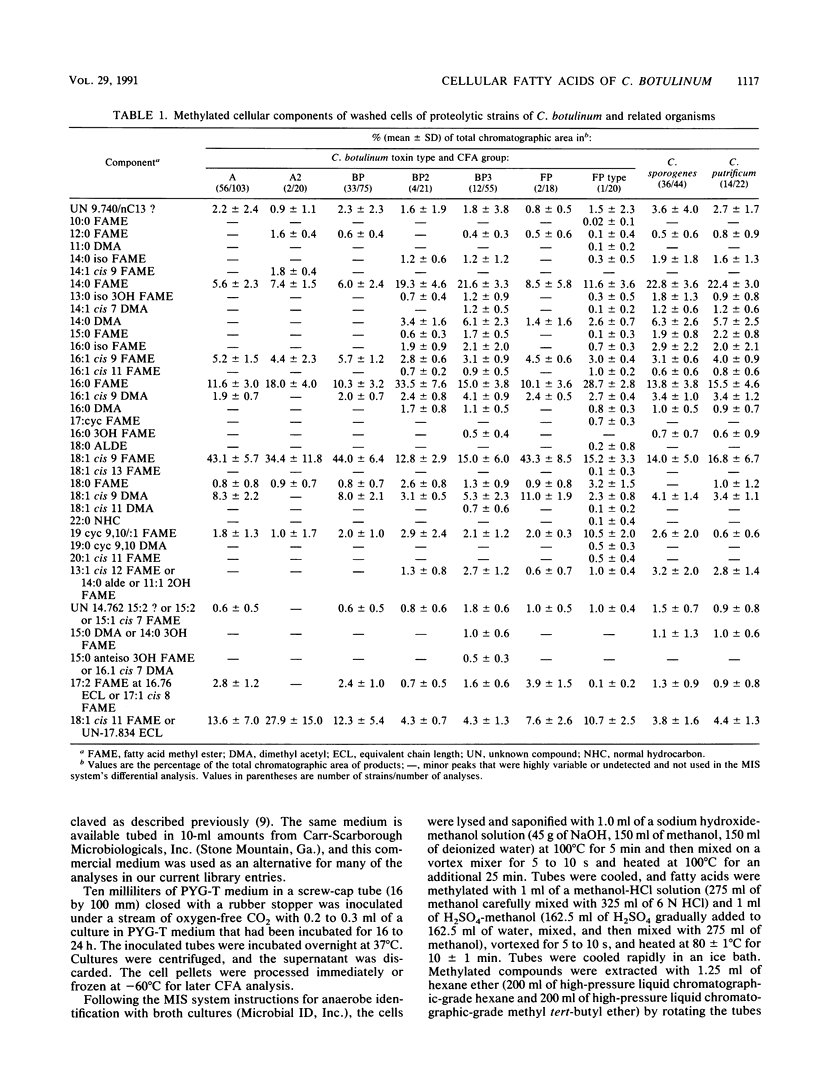
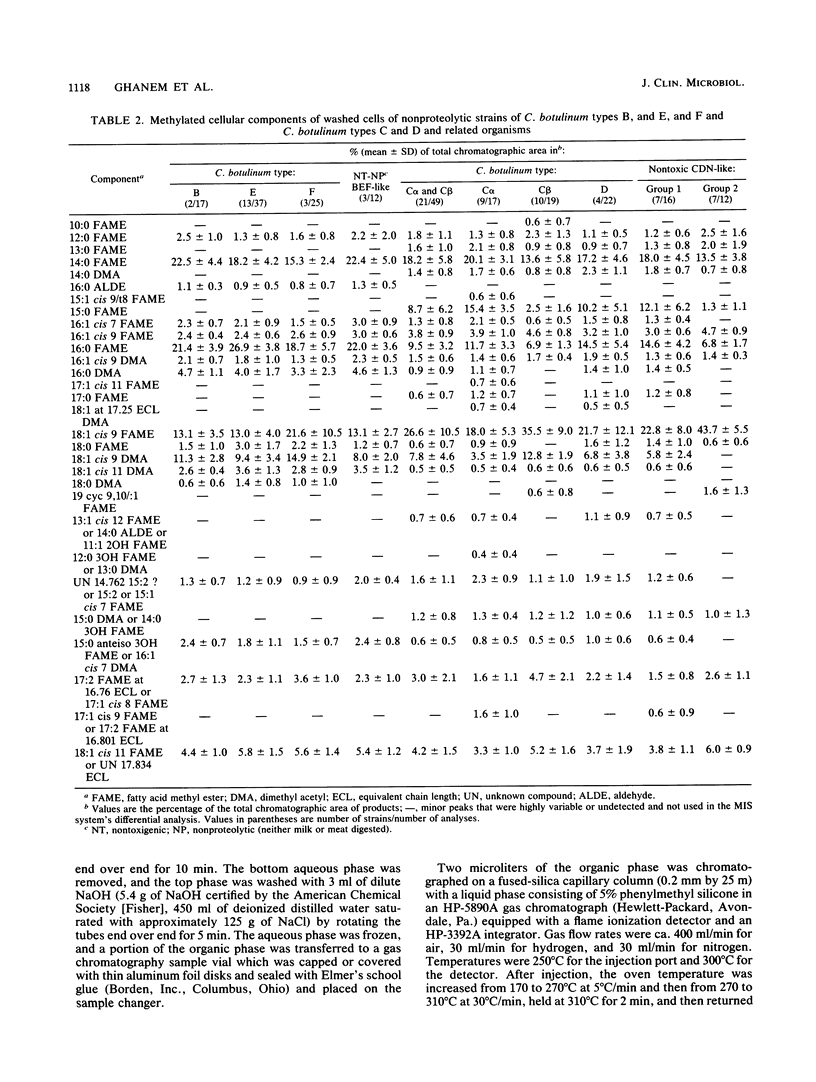
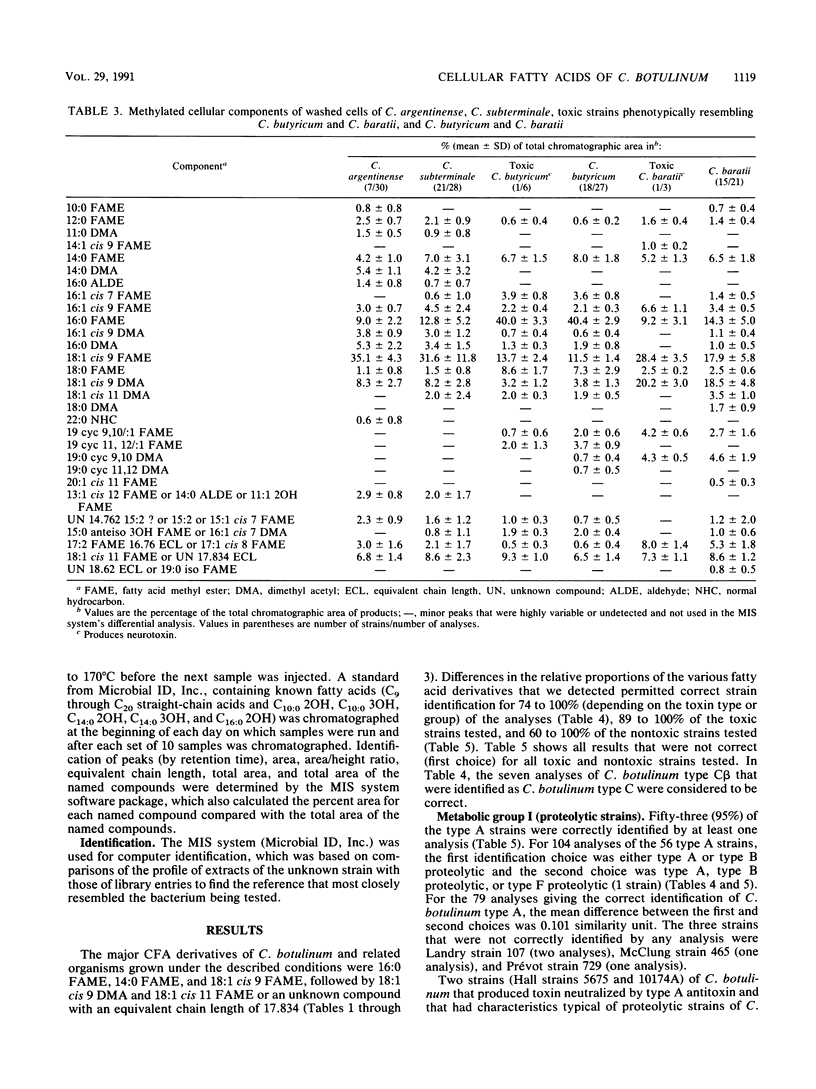
On the basis of 686 analyses of 285 strains of Clostridium botulinum, Clostridium argentinense (formerly C. botulinum type G), and phenotypically related organisms, 14 cellular fatty acid (CFA) groups of toxic organisms and 6 CFA groups of nontoxic organisms were delineated. The CFA groups of toxic strains included two of type A, three of proteolytic strains of type B, two of proteolytic strains of type F, one each of nonproteolytic strains of types B, E, and F, and one each of types C alpha, C beta, and D and C. argentinense. The groups of phenotypically similar nontoxic strains included Clostridium sporogenes, Clostridium putrificum, nontoxic strains with phenotypic characteristics similar to those of nonproteolytic strains of C. botulinum types B, E, and F (BEF-like), two groups of nontoxigenic organisms with phenotypic characteristics similar to those of C. botulinum types C and D and Clostridium novyi (CDN-like), and Clostridium subterminale, which has phenotypic characteristics similar to those of C. argentinense. Within the toxin types, 89 to 100% of the strains were correctly identified by CFA analysis, and 74 to 100% of the analyses were correct. Of 36 strains of C. sporogenes, 30 (83%) were correctly identified; 17% of the strains of C. sporogenes were incorrectly identified as C. botulinum type A or B. All analyses of C. putrificum and C. subterminale were correctly identified. There was no significant level of similarity between strains of C. botulinum and phenotypically similar organisms and 85 other species of clostridia or 407 other taxa of gram-positive and gram-negative bacteria. Additionally, the one strain each of Clostridium baratii and Clostridium butyricum previously reported to produce C. botulinum toxin could be differentiated from C.botulinum types as well as from strains of C. baratii and C. butyricum that did not produce neurotoxin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eklund M. W., Poysky F. T. Interconversion of type C and D strains of Clostridium botulinum by specific bacteriophages. Appl Microbiol. 1974 Jan;27(1):251–258. doi: 10.1128/am.27.1.251-258.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Meyers J. A., Pelroy G. A. Interspecies conversion of Clostridium botulinum type C to Clostridium novyi type A by bacteriophage. Science. 1974 Nov 1;186(4162):456–458. doi: 10.1126/science.186.4162.456. [DOI] [PubMed] [Google Scholar]
- Hall J. D., McCroskey L. M., Pincomb B. J., Hatheway C. L. Isolation of an organism resembling Clostridium barati which produces type F botulinal toxin from an infant with botulism. J Clin Microbiol. 1985 Apr;21(4):654–655. doi: 10.1128/jcm.21.4.654-655.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida H., Oguma K., Inoue K. Phage-conversion of toxigenicity in Clostridium botulinum types C and D. Jpn J Med Sci Biol. 1974 Apr;27(2):101–103. [PubMed] [Google Scholar]
- Johnson J. L., Francis B. S. Taxonomy of the Clostridia: ribosomal ribonucleic acid homologies among the species. J Gen Microbiol. 1975 Jun;88(2):229–244. doi: 10.1099/00221287-88-2-229. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Riemann H. Correlation of toxic and non-toxic strains of Clostridium botulinum by DNA composition and homology. J Gen Microbiol. 1970 Jan;60(1):117–123. doi: 10.1099/00221287-60-1-117. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Riemann H. The genetic relatedness of proteolytic Clostridium botulinum strains. J Gen Microbiol. 1970 Nov;64(1):85–90. doi: 10.1099/00221287-64-1-85. [DOI] [PubMed] [Google Scholar]
- McCroskey L. M., Hatheway C. L., Fenicia L., Pasolini B., Aureli P. Characterization of an organism that produces type E botulinal toxin but which resembles Clostridium butyricum from the feces of an infant with type E botulism. J Clin Microbiol. 1986 Jan;23(1):201–202. doi: 10.1128/jcm.23.1.201-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Hash D. E., Holdeman L. V., Cato E. P. Polyacrylamide slab gel electrophoresis of soluble proteins for studies of bacterial floras. Appl Environ Microbiol. 1980 Apr;39(4):900–907. doi: 10.1128/aem.39.4.900-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Lewis V. J. Characterization of clostridia by gas chromatography. I. Differentiation of species by cellular fatty acids. Appl Microbiol. 1967 Mar;15(2):390–397. doi: 10.1128/am.15.2.390-397.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


