Abstract
Acne is a human disease of the sebaceous hair follicle. Unlike humans, most animals produce little or no triglycerides in hair follicles to harbor Propionibacterium acnes a fact that has encumbered the development of novel treatments for acne lesions. Although genetic mutant mice with acne-like skins have been used for screening anti-acne drugs, the mice generally have deficits in immune system that turns out to be inappropriate to generate antibodies for developing acne vaccines. Here, we employed a bioengineering approach using a tissue chamber integrated with a dermis-based cell-trapped system (DBCTS) to mimic the in vivo microenvironment of acne lesions. Human sebocyte cell lines were grown in DBCTS as a scaffold and inserted into a perforated tissue chamber. After implantation of a tissue chamber bearing human sebocytes into ICR mice, P. acnes or PBS was injected into a tissue chamber to induce host immune response. Infiltrated cells such as neutrophils and macrophages were detectable in tissue chamber fluids. In addition, a proinflammatory cytokine macrophage-inflammatory protein-2 (MIP-2) was elevated after P. acnes injection. In tissue chamber fluids, 13 proteins including secreted proteins and cell matrix derived from mouse, human cells or P. acnes were identified by proteomics using isotope-coded protein label (ICPL) coupled to nano-LC-MS analysis. After P. acnes infection, four proteins including fibrinogen, α polypeptide, fibrinogen β chain, S100A9, and serine protease inhibitor A3K showed altered concentrations in the mimicked acne microenvironment. The bioengineered acne model thus provides an in vivo microenvironment to study the interaction of host with P. acnes and offers a unique set-up for screening novel anti-acne drugs and vaccines.
Keywords: Acne, Bioengineering, Dermis-based cell-trapped system, Microenvironment, Propionibacterium acnes
1 Introduction
Acne vulgaris is a common skin condition caused by inflammation of the hair follicles and sebaceous glands of the skin that afflicts more than 50 million people in the USA alone [1]. There are rationales in support of developing acne animal models for evaluation of novel drugs or vaccines against acne vulgaris. First, Propionibacterium acnes is highly associated with acne vulgaris. Second, new therapeutic treatments including drugs and vaccines for acne are urgently needed. At this time, most of available topical treatments for acne lesions are palliative and effective only while treatment is maintained. When treatments are discontinued, increased acne inevitably results. Systemic antibiotic therapy nonspecifically kills the majority of skin bacteria, which impacts the homeostasis of skin-resident microflora [2]. Furthermore, antibiotic-resistant P. acnes may be created after long-term treatment with antibiotics [3]. For severe acne, isotretinoin, a vitamin A-derived retinoid, effectively smother the progression of acne lesions, but the medicine is associated with a high risk of depression and an increased rate of birth defects [1, 4]. Therefore, its use is now tightly regulated by the Food and Drug Administration.
Most animals do not produce sufficient triglycerides to harbor P. acnes, a fact that has encumbered the development of anti-acne drugs and vaccines targeting P. acnes infection [5]. It has been reported that dogs have no detectable triglycerides in sebaceous glands, while mice, rabbits, and hamsters only have low concentrations [5]. Only the sebaceous regions of guinea pigs harbor a significant P. acnes population [5]. Rabbit ears and Rhino mice have been commonly utilized to determine compound comedogenicity of acne lesions [6]. However, the rabbit ear model has a lack of bacterial colonization and inflammation [7]. In addition, the use of rabbits may be inconvenient for vast drug screening and vaccinations. Rhino mice with utricles that create larger follicles have been utilized to determine compound comedogenicity [6]. However, the Rhino mouse with an immune defect is a hairless mutant that carries the rh gene, a recessive allele of the hairless gene [8]. The mice cannot produce antibodies against thymus-dependent antigens that may not be an appropriate model for vaccination. As a result, the genetic approaches may not sufficiently provide a means to create acne animal models for evaluation of acne vaccines.
P. acnes-induced ear swelling has been used for testing anti-acne drugs in rabbits, dogs, and mice [7, 9]. Compared to larger animals, mice have several advantages, including small size, low cost, and they are available in large enough numbers for statistical evaluation. However, animal ears contain a cell type composition that is not equivalent to the situation of human hair follicles. To circumvent the hurdles mentioned above, we employed a bioengineering approach to create a humanized acne microenvironment by implanting a tissue chamber. The implantation of a tissue chamber was first described and extensively characterized in the guinea pig [10] and then adapted to the mouse [11] for mimicking bacterial infections in vivo. To imitate the sebaceous glands, we inserted a human sebocyte cell-trapped dermis into a tissue chamber. This humanized acne microenvironment model enabled us to grow bacteria in vivo and detect the lipid production from human sebocytes. Most importantly, proteomics integrated with this bioengineering approach allowed us to investigate the in vivo interactions among P. acnes, human sebocytes and host immune cells.
2 Materials and methods
2.1 Implantation of tissue chamber
ICR mice were anesthetized by administrating 10 mg of ketamine and 1.5 mg of xylazine per 100 g of body weight. The tissue chamber was fabricated based on a protocol as described [12]. Briefly, a tissue chamber (id and od, 1.5 and 3.0 mm, respectively; length, 1 cm) was made of a closed PTFE Teflon cylinder with 12 spaced 0.1 mm holes [13]. The tissue chamber was sterilized by soaking in 70% ethanol overnight. The sterile tissue chamber was then implanted subcutaneously in the abdominal skins for 7 days to ensure the chamber was fully wrapped by mouse tissues in subcutaneous environment. After that, P. acnes (20 µL in PBS; 107 colony forming unit (CFU/mL) or PBS (20 µL) was injected into tissue chambers. Three days after bacterial injection, tissue chamber fluids were drawn by percutaneous aspiration for determining the CFUs of P. acnes and the level of macrophage-inflammatory protein MIP-2. All experiments using mice were conducted according to institutional guidelines. CFU was enumerated by plating serial dilutions (102–108) of tissue chamber fluids containing bacteria on Brucella broth agar plates (Section 2.2) after incubation for 3 days under anaerobic conditions at 37°C. Tissue chamber fluids were diluted by PBS for counting CFU. The concentrations of MIP-2 were determined with sandwich ELISA kits (R&D Systems, Minneapolis, MN) according to the provided protocols. Data collected from three mice are presented as mean ± standard error (SE). The Student’s t-test was used to assess the significance of independent experiments. The criterion p<,0.05 was used to determine statistical significance.
2.2 Culture of P. acnes
P. acnes (ATCC® 6919) was grown on Brucella broth agar (BD, Sparks, MD), containing 5% v/v defibrinated sheep blood (Lampire Biological Laboratories, Pipersville, PA), vitamin K (5 µg/ml, Remel, Lenexa, KS), and hemin (50 µg/mL, Remel, Lenexa, KS), under an anaerobic condition using GasPak (BD, Sparks, MD) at 37°C. Single colony was inoculated in Reinforced Clostridium Medium (Oxford, Hampshire, England) and cultured at 37°C until reaching OD600 = 1.0–3.0 (logarithmic growth phase) under the anaerobic condition. Bacteria were centrifuged at 50006 × g for 10 min, washed with PBS three times, and suspended to appropriate amount of PBS for the experiments.
2.3 Cell staining
Fluids in the tissue chamber were drawn by percutaneous aspiration before and after P. acnes injection. After centrifugation at 5000 × g for 5 min, infiltrated cells including macrophages and neutrophils in the pellet were detected by incubation of cells with biotinylated CD11b and Gr-1 (BD Biosciences, San Diego, CA), respectively followed by adding the tetramethylrhodamine isothiocyanate (TRITC)-conjugated streptavidin (Invitrogen, Carlsbad, CA). All cells were counterstained with nucleus dye Hoechst 33258. The fluorescence was viewed on a CX41 fluorescent microscope (Olympus, Miami, FL).
2.4 Human sebocyte culture
The immortalized human sebocyte line SZ95 was cultured in Sebomed Basal medium (Biochrom, Berlin, Germany), supplemented with 5 ng/mL human recombinant epidermal growth factor (Sigma, St. Louis, MO), 10% v/v heat-inactivated FBS, at 37°C under atmosphere of 5% v/v CO2 in air.
2.5 A dermis-based cell-trapped system (DBCTS)
The dead dermis was prepared based on the protocols as described [12]. Briefly, a dorsal skin of ICR mouse was excised and incubated with 10 × PBS for one week. After removal of epidermis and fat by forceps, de-epidermized dead dermis was obtained by continuously freezing and thawing in liquid nitrogen for 100 min (10 min for 10 times) to deplete all living cells in dermis. Subsequently, the base membrane on the top of dermis was disintegrated by a razor blade. After that, a deepidermized dead dermis was placed with dermal side facing up in a polycarbonate filter chamber (3.0 µm Millicell-pc, 12 mm diameter; Millipore Corporation, Bedford, MA) in six-well culture dishes. The surface of de-epidermized dead dermis then was seeded with 106 of human SZ95 sebocytes. The cells, visualized by staining with hematoxylin and eosin (H&E) (Sigma diagnostics) [14] and nucleus dye Hoechst 33258, grew and formed a layer upon the top of dermis 5 days after seeding. Some cells migrated into the dermis. The sebocyte cell-trapped de-epidermized dermis then was inserted into a tissue chamber. The tissue chamber bearing a sebocyte cell-trapped dermis was subcutaneously implanted into the abdominal skin of an ICR mouse. Seven days after implantation, 20 µL of P. acnes (107 CFU/mL) or PBS was injected into a tissue chamber. One day after injection, tissue chamber fluids were drawn by percutaneous aspiration for MS analysis (Sections 2.6 and 2.7) and sebocyte cell-trapped dermis was pulled out for oil red O staining. For oil red O staining [15], cell-trapped dermis was fixed for 60 min at room temperature in 4% formaldehyde and washed with 70% ethanol. After that, dermis was stained with 2% (w/v) oil red O (Sigma) for 5 min at room temperature. Excess staining was removed by washing in 70% ethanol, followed by rinsing in several changes of distilled water.
2.6 Isotope-coded protein label (ICPL)
Isotopic labeling of proteins using the ICPL-kit (SERVA Electrophoresis, Heidelberg, Germany) was conducted as described previously [16, 17]. Tissue chamber fluids were pooled from the same group of mice (n = 4), lyophilized and reconstituted in 20 µL of 6 M guanidine HCl, 0.1 M HEPES, pH 8.5 to obtain a total protein concentration of 3 µg/µL. Equal amounts (50 µg) of proteins obtained from fluids in PBS- and P. acnes-injected tissue chambers were labeled with light (12C6) and heavy (13C6) forms of N-nicotinoyloxy-succinimid (Nic-NHS), respectively at room temperature for 2 h. The protein concentration was measured by using a BCA Protein Assay Kit (Pierce, Rockford, IL). The disulfide bonds were reduced by tris-2-carboxyethyl phosphine and alkylated by iodoacetamide. After removal of excess reagents with 6 µM hydroxylamine, equal aliquots of both samples were mixed together. The labeled proteins were then purified using acetone precipitation. Subsequently, the labeled proteins were enzymatically digested with trypsin (0.2 µg, 25 mM Tris, 4 M urea, pH 7.8) at 37°C overnight. Tryptic peptide digests were analyzed by nano-LC-MS [18]. Samples from three separate experiments were used for ICPL labeling and quantitation. One representative of nano-LC-MS spectra was illustrated.
2.7 Nano-LC-MS
The automated nano-LC-MS setup consisted of an Agilent 1200 Nano-2D LC system, a switch valve, a C18 trap column (Agilent, Santa Clara, CA), and a capillary RP column (10 cm in length, 75 µm id) packed with 5 µm, C18 AQUASIL resin with an integral spray tip (Picofrit, 15 µm tip, New Objective, Woburn, MA). A RP LC directly linked to a Bruker Daltonics HCTultra PTM system mass spectrometer (Bruker Daltoniks, Bremen, Germany) was performed using linear gradient elution from buffer A (H2O plus 0.1% formic acid) to 50% buffer A plus 50% buffer B (ACN plus 0.1% formic acid) in 100 min. The set-up was operated in the data-dependent mode. Four strongest ions above an intensity of 2 × 105 was accumulated with dynamic exclusion enabled and the collision energy set at 35%. For protein ratio normalization, two standard proteins were spiked into the sample in the following amount: 1 and 0.25 µg of chicken ovalbumin and 0.5 and 1 µg carbonic bovine anhydrase II.
2.8 Spectrum analysis and database searching
Large-scale MS/MS spectra were extracted using default value by BioTools 3.1 (Bruker Daltoniks). All MS/MS spectra were analyzed using in-house MASCOT 2.1 server (Matrix Science, London, UK) for protein identification. WarpLC 1.1 software (Bruker Daltoniks) was utilized for calculating the peak area of individual peptide peak and calculating the ratios of related peaks. MASCOT was established to search the target-decoy mouse, human and P. acnes databases (downloaded from NCBI website http://www.ncbi.nlm.nih.gov/) containing protein sequences in both forward and reverse orientations using trypsin as a digestion enzyme with the allowance of up to five missed cleavages. The false positive rates were determined by doubling the ratio between the number of decoy hits and the total number of hits [19]. The mass tolerances of a fragment ion and a parent ion were set as 0.5 and 1.0 Da, respectively. A molecular mass of 57 Da was added to all cysteines to justify carboxyamidomethylation. Differential search includes methonine +16 Da for oxidation; lysine and protein N-terminal +105 Da (light) or +111 Da (heavy) to reflect the mass difference between labeled and unlabeled peptides. The relative abundance of each identified protein in the two groups of samples was calculated by comparing the areas under the curves in the elution profiles for each of the two peptides which have identical sequences but different masses (6.0 Da absolute mass difference for each labeled lysine or N-termini) using WarpLC 1.1. Student’s t-test was used for statistical analysis. The threshold of MASCOT score set for each filtered peptide was 20. The proteins with ratios greater than 1.5-fold or less than 0.6-fold were preferred as a set of proteins that expressed differentially in two groups of samples.
3 Results
3.1 Tissue chamber harbored bacteria and host phagocytes in vivo
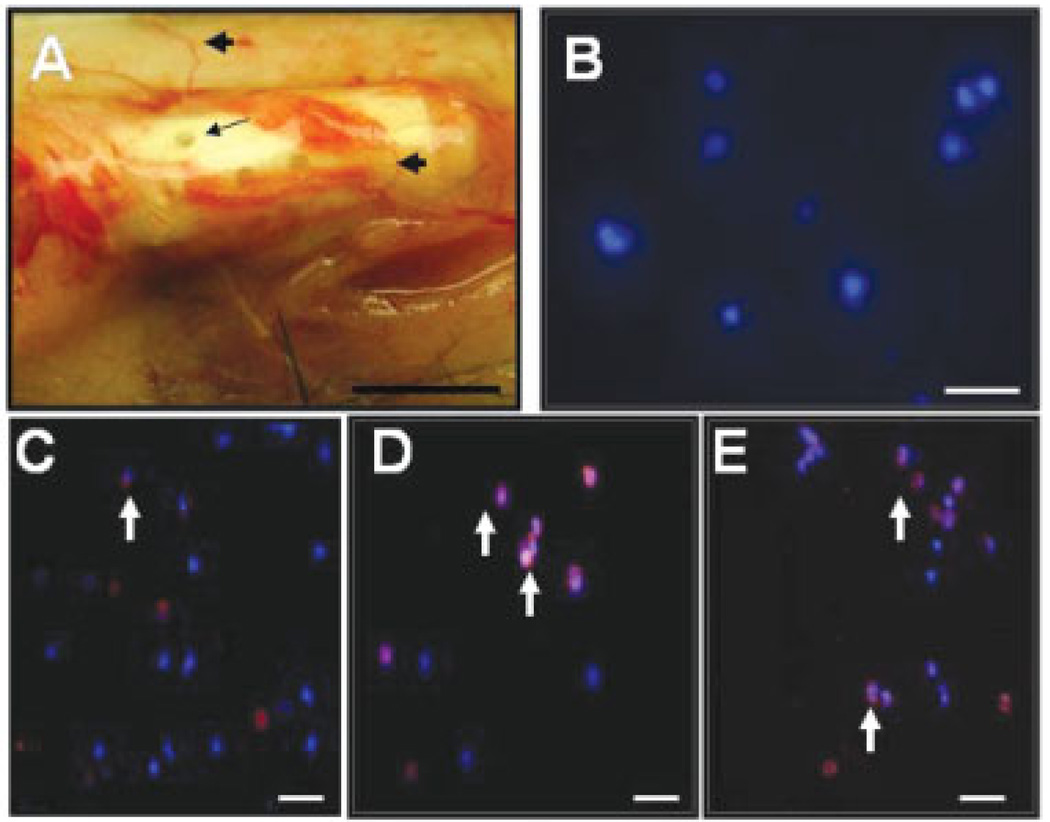
Tissue chambers (cages) have been previously used to grow bacteria in vivo for evaluating various categories of antimicrobial agents [20]. In an attempt to investigate the in vivo interaction of host cells and P. acnes, we fabricated a PTFE Teflon chamber with 0.1 mm holes that allow host cells to infiltrate into a chamber and then interact with injected bacteria. It has been shown that this chamber model accurately mimicked bacterial infections in vivo [20]. A tissue chamber was subcutaneously implanted into the abdominal skin of ICR mice (Fig. 1A). Seven days after implantation, the chamber was fully encapsulated by mouse fibrotic tissues and surrounded by blood vessels. Infiltrated host cells in tissue chamber fluids were drawn by percutaneous aspiration and stained with nucleus dye Hoechst 33258. Positive Hoechst 33258 staining in fluids indicated that the host cells migrated into a tissue chamber (Fig. 1B). We next injected P. acnes (20 µL 107 CFU/mL) into the tissue chamber. Injection with PBS (20 µL) served as a control. The fully encapsulated chamber prevented the leakage of P. acnes from its pores after injection. One day after injection, infiltrated cells were stained with Gr-1 and CD11b, markers for neutrophils and macrophages, respectively. Our data indicated that neutrophils were present in a PBS-injected chamber (Fig. 1C). The populations of neutrophils (Fig. 1D) and macrophages (Fig. 1E) were augmented after P. acnes injection. These results demonstrate the usefulness of a tissue chamber in harboring P. acnes to recruit the infiltration of host phagocytes.
Figure 1.
Infiltration of phagocytes into an implanted tissue chamber. A hollow tissue chamber (A) (length, 1 cm; internal volume, 80 µL) was subcutaneously implanted into abdominal skin and maintained in the ICR mice for 7 days before P. acnes injection. Holes (0.1 mm) (A; arrow) created on the wall of tissue chamber allowed infiltrated cells (B) to penetrate into tissue chamber. The tissue chamber surrounded by blood vessels (A; arrowheads) was completely covered by subcutaneous tissues 7 days after implantation. Then 20 µL of PBS (C) or P. acnes (107 CFU/mL) (D and E) was injected into the tissue chamber. The sealed chamber isolated injected P. acnes and avoided the escape of bacteria to the circulating systems in mice. Before (B) and after PBS (C) or P. acnes (D and E) injection, tissue chamber fluids were percutaneously aspirationed. After centrifugation, infiltrated cells (phagocytes) were stained with markers of neutrophils (C and D; arrows) and macrophages (E; arrows). All cells were counterstained with nucleus dye Hoechst 33258 (blue stains). Bar (A): 0.5 cm. Bars (B–E): 20 µm.
3.2 Bacterial growth and production of the proinflammatory MIP-2 cytokine in a tissue chamber
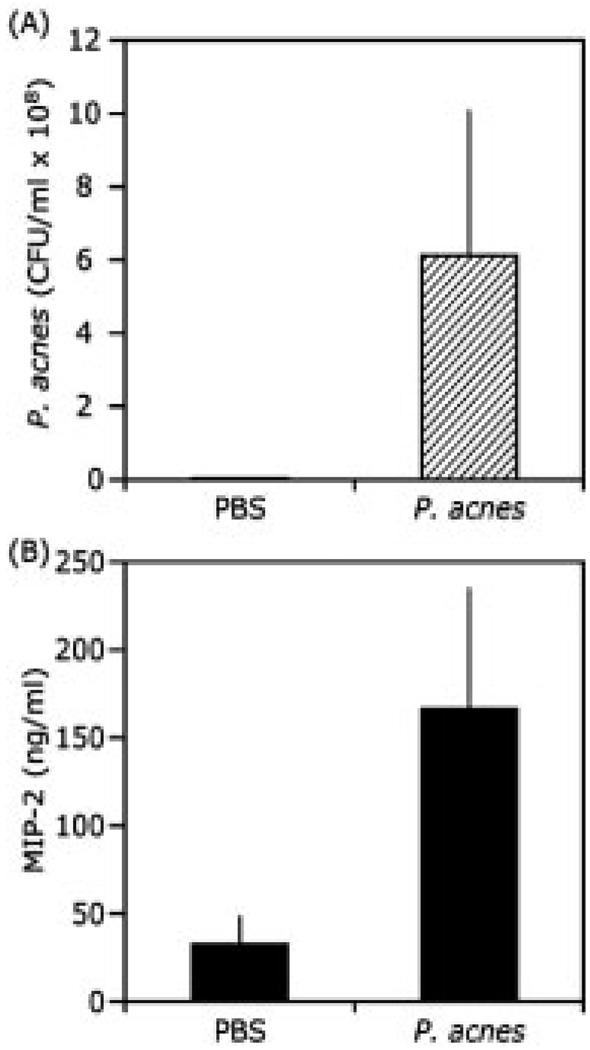
The colonization of P. acnes and induction of proinflammatory responses are two major features of acne vulgaris [21]. Within the implanted tissue chamber, the host response was mediated exclusively by injected P. acnes, infiltrated phagocytes, apoptosis and secretory factors such as cytokines. Three days after injection with PBS or P. acnes, tissue chamber fluids containing bacteria and proinflammatory cytokines were drawn by percutaneous aspiration. The bacterial growth was determining by CFU counting on agar plates. The level of proinflammatory MIP-2 cytokine was measured by ELISA. No bacteria were detected in a PBS-injected chamber, whereas the number of bacteria (6.1 ± 4.2 × 108 CFU/mL) was dramatically increased in a P. acnes-injected chamber (Fig. 2A). The result suggested that the implanted tissue chamber provided a suitable environment for survival and multiplication of P. acnes. Additionally, P. acnes injection induced a remarkable increase in the production of MIP-2 (166.47 ± 69.4 ng/mL) in comparison with that (32.30 ± 16.97 ng/mL) in a chamber injected with PBS (Fig. 2B). The data support the capability of a tissue chamber to harvest proinflammatory cytokines.
Figure 2.
The expression of the proinflammatory cytokine MIP-2 is elevated in the presence of P. acnes. Tissue chamber fluid was drawn by percutaneous aspiration 3 days after injection of PBS (20 µL) or P. acnes (20 µL in PBS; 107 CFU/mL) into a chamber. Bacteria grown within a tissue chamber were determined by counting CFU after plating serial dilutions (102 to 108) of fluids on agar plates (A). Measurement of MIP-2 in the tissue chamber fluid was carried out by sandwich ELISA that used the Quantikine M mouse MIP-2 set (R&D System, Minneapolis, MN). Injection of P. acnes markedly increased the MIP-2 production in tissue chambers (B). Error bars represented mean ± SE (n = 3; *p<0.035 by Student’s t-test).
3.3 Establishment of a humanized acne microenvironment using a human sebocytes encapsulated DBCTS
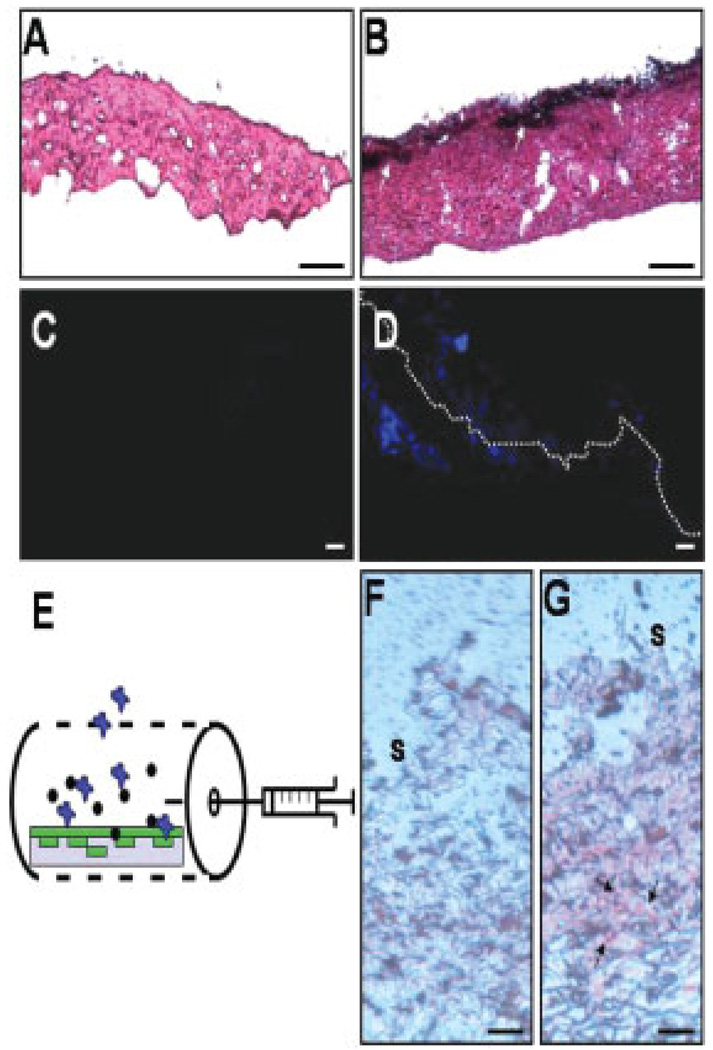
During development of acne lesions, sebocytes within sebaceous glands are major target cells of P. acnes [22, 23]. To investigate the interaction of sebocytes to P. acnes in vivo, we developed a novel technology called DBCTS [12] that provided a biological scaffold to support the growth of human sebocytes in a 3-D manner. After removal of the epidermis, a dead dermis of ICR mouse skin was created by killing living cells with liquid nitrogen. H&E (Fig. 3A) and Hoechst 33258 staining (Fig. 3C) showed that all living cells were eradicated by liquid nitrogen. Prior to seeding cells on the dead dermis, the base membrane on the surface of the dermis was disintegrated for the purpose of aiding the migration and trapping of seeded cells into the dermis. After cell eradication, the major component in the dead dermis was the cell matrix that provided an excellent support for seeding cells. Human SZ95 sebocytes were seeded and cultured on the top of the dermis for 5 days. Data from H&E (Fig. 3B) and Hoechst 33258 staining (Fig. 3D) indicate that human sebocytes are capable of growing on the surface and trafficking into the dead dermis. We then inserted a sebocyte cell-trapped dermis into a tissue chamber and implanted the chamber into an ICR mouse (Fig. 3E). Seven days after implantation, 20 µL of P. acnes (107 CFU/mL) or PBS was injected into the tissue chamber. One day after injection, sebocyte cell-trapped dermis was stained with oil red O, a lysochrome fat soluble dye [15]. Oil red O positive staining was detected in the P. acnes-injected (Fig. 3G), but not the PBS-injected chamber (Fig. 3F). This shows that human sebocytes in a tissue chamber maintain their biological activity of lipid production after implantation into mice. The data suggest that implanted tissue chambers confer an immunological isolator, which allows human sebcoytes to survive in mice without rejection by the mouse immune system.
Figure 3.
Insertion of dermal trapped human sebocytes into a tissue chamber. DBCTS was used to create a supporting matrix to trap human SZ95 sebocyte cells as described in Section 2. A cell-depleted de-epidermized dead dermis were stained with H&E (A) and nucleus dye Hoechst 33258 (C) to ensure that all cells in dermis were completely depleted. Sebocytes were grown in the DBCTS by seeding cells (106 cells) on the surface of dead dermis (B, D, F, and G). H&E (B; arrows) and Hoechst 33258 (D; blue stains) indicated that cells grew on the surface (dash line) and distributed within the dermis 5 days after seeding. Dermis without seeding cells (A and C) served as a control. The sebocyte cell-trapped dermis was inserted into a tissue chamber (E). The tissue chamber bearing a sebocyte cell-trapped dermis (E, gray rectangle) was then implanted into an ICR mouse. Seven days after implantation, P. acnes (107 CFU/mL) or PBS was injected into tissue chamber. One day after injection with PBS (F) or P. acnes (G), sebocyte cell-trapped dermis was taken out of a chamber for oil red O staining. Oil red O positive stains (G, arrows) indicated that human sebocytes can survive and produce lipids within an implanted tissue chamber where sebocytes (E, green) interacted with P. acnes (E, black dots) and infiltrated host cells (E, blue). S: dermis surface. Bars (A and B): 0.5 µm (C and D): 20 µm; (F and G): 5 µm.
3.4 Quantitative proteomics analysis of tissue chamber fluids via ICPL labeling
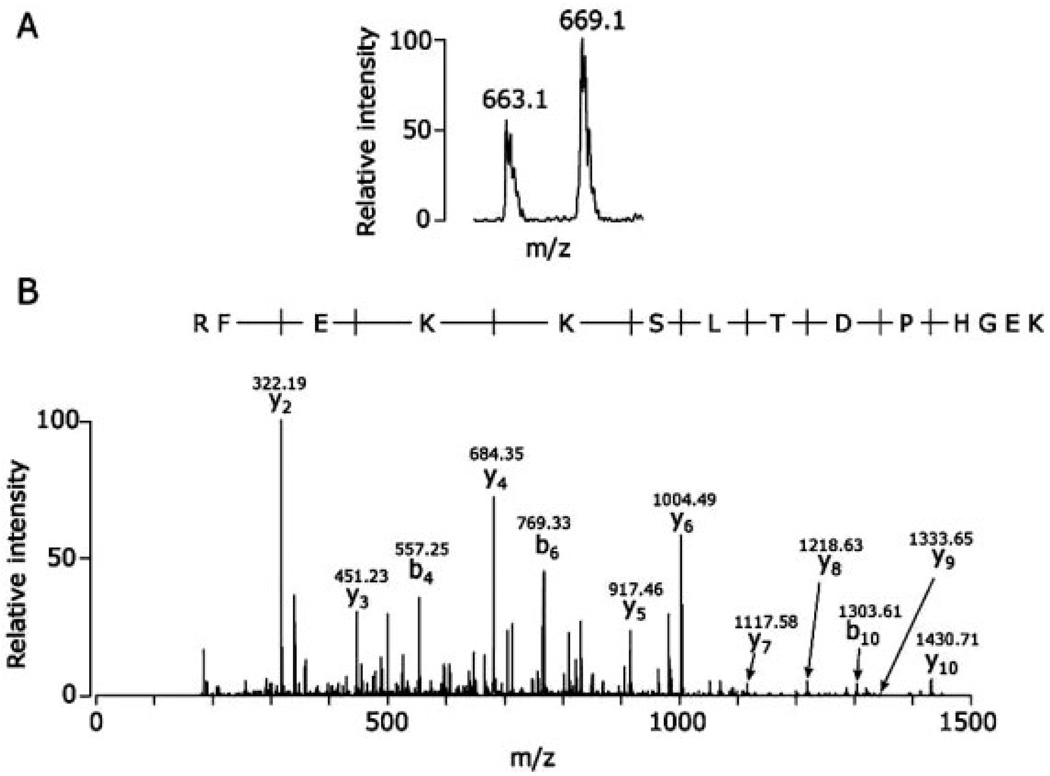
The human sebocytes encapsulated DBCTS created a humanized acne microenvironment in mice. Tissue chamber fluids containing proteins released from cells can be used to study the interactions among P. acnes, human sebocytes and mouse immune cells. Tissue chamber fluids obtained from P. acnes- and PBS-injected chambers were labeled with 13C-Nic-NHS (heavy) or 12C-Nic-NHS (light), respectively. Peptides corresponding to 13 proteins were thus sequenced and quantified. These proteins were derived from either mouse, human, or P. acnes (Table 1). Thirteen proteins are apolipoprotein A-I, complement C3, α polypeptide of fibrinogen, β chain of fibrinogen, α-2-macroglobulin, albumin, serine protease inhibitor A3K, serotransferrin, S100A9, kininogen-1, putative peroxiredoxin, proline iminopeptidase (PIPase), and ubiquitin. Proteins with ratios of heavy label-/light label- (H/L) greater than 1.5-fold or less than 0.6-fold were selected as a group of proteins that had different abundances after P. acnes injection (Table 1). Three of the proteins were upregulated (fibrinogen, α polypeptide; fibrinogen β chain; S100A9), and one was downregulated (serine protease inhibitor A3K). An internal peptide (MSMKIRPFFPQQ) of the fibrinogen β chain with a nonclassical tryptic cleavage could be derived from either ICR mice or inserted human sebocytes since the sequence of this peptide matched completely with fibrinogen beta chain in both mice and human. Two peptides derived from P. acnes were identified in tissue chamber fluids. A peptide (LQKFVAK) corresponding to a putative peroxiredoxin (AhpC/TSA family) was only labeled with 13C-Nic-NHS (heavy); however, a peptide (IIILDQR) assigned to PIPase was unlabeled with ICPL. Two internal peptides (LSKAGAEPAPER of kininogen-1 and LIFAGKQLEDGR of ubiquitin) were merely labeled with 13C-Nic-NHS (heavy) and 12C-Nic-NHS (light), respectively. A LC/MS spectrum of S100A9 indicated that two peaks at m/z values of 663.10 and 669.10 were derived from 12C-Nic-NHS ((light(L)- and 13C-Nic-NHS (heavy(H))-labeled RFEKKSLTDPHGEK (Fig. 4A), which was sequenced and illustrated in a CID spectrum (Fig. 4B). The ratio (H/L) of peak areas of the two-labeled peptides was approximately 1.5, suggestive of increased abundance of S100A9 after P. acnes stimulation. Although most of identified proteins in tissue chamber fluids were secreted proteins, cell matrix proteins, or plasma proteins, several intracellular proteins such as ubiquitin and P. acnes putative peroxiredoxin were also identified. The existence of intracellular proteins in tissue chamber fluids suggests that these proteins are discharged from dead cells or bacteria during infection of cells with P. acnes [24].
Table 1.
Identification of ICPL-labeled proteins in tissue chamber fluids
| Identified proteins | Accession number | Actual peptide mass (AMU) | Observed m/z | Charge state | Identified peptides and peptide ratios (H/L)a) |
|---|---|---|---|---|---|
| Peptides with both heavy and light labels | |||||
| Apolipoprotein A-I | Q00623 | 1204.64 | 602.83 | +2 | QKLQELQGR |
| Q00623 | 2562.36 | 854.79 | +3 | AKTHLKTLGEKARPALEDLR | |
| Complement C3 | P01027 | 1384.69 | 692.85 | +2 | TKKDTLPESR |
| P01027 | 1398.71 | 699.86 | +2 | KFISHIKCR | |
| Fibrinogen, α polypeptide | Q99K47 | 2319.27 | 773.76 | +3 | RKVIEKAQQIQALQSNVR 2.74 ± 0.11 |
| Fibrinogen β chain | Q8K0E8 P02675b) | 1614.79 | 807.80 | +2 | MSMKIRPFFPQQ 1.72 ± 0.23 |
| α-2-Macroglobulin | Q61838 | 1555.87 | 778.44 | +2 | QVVKTKVFQLR |
| Serum albumin | P07724 | 1124.60 | 562.80 | +2 | AFKAWAVAR |
| P02768b) | |||||
| P07724 | 1698.72 | 566.91 | +3 | MKCSSMQKFGER | |
| Serine protease inhibitor A3K* | P07759 | 2078.07 | 1039.54 | +2 | SVKVPMMKMKLLTTR 0.57 ± 0.10 |
| Serotransferrin | Q921|1 | 1360.65 | 680.83 | +2 | LLEACTFHKH |
| S100A9 | P31725 | 1986.95 | 662.99 | +3 | KEGHPDTLSKKEFR 1.53 ± 0.10 |
| Peptides with a heavy label only or no label | |||||
| Kininogen-1 | Q7M084 | 1336.70 | 668.85 | +2 | LSKAGAEPAPER |
| Putative peroxiredoxin (AhpC/TSA family protein) | Q6A772c) | 944.05 | 943.56 | +1 | LQKFVAK |
| PIPase | Q6A981c) | 870.21 | 869.53 | +1 | IIILDQR |
| Peptides with a light label only | |||||
| Ubiquitin [Rps27a] | P62991 P62988b) | 1451.76 | 726.39 | +2 | LIFAGKQLEDGR |
Indicated that proteins with higher abundance (greater than 1.5-folds) in fluids taken from a P. acnes-injected tissue chamber.
Indicated that proteins with lower abundance (less than 0.6-fold) in fluids taken from a P. acnes-injected tissue chamber.
Indicated that the ratios (mean ± SD) of peptides labeled with heavy (H; 13C6) and light (L; 12C6) forms of Nic-NHS. Only the values of H/L greater than 1.5- or less than 0.6-fold were displayed.
Indicated that mouse peptides with 100% identity with those in human.
Indicated that peptides derived from P. acnes.
Figure 4.
S100A9 is abundant in a P. acnes-injected tissue chamber. Fluids from PBS- and P. acnes- injected tissue chambers were collected as described in Section 2. After ICPL labeling and trypsin digestion, proteins in fluids were directly identified and sequenced by nano-LC MS. The light (L)-, and heavy (H)-labeled peptides (m/z values at 663.10 and 669.10) were derived from PBS-, and P. acnes- injected tissue chamber, respectively (A). Based on a MS/MS spectrum (B), the labeled peptide was assigned as an internal peptide (RFEKKSLTDPHGEK) of S100A9. The m/z value of each “y” and “b” ion in a MS/MS spectrum is indicated. The ratio (H/L) of the peak areas of two-labeled peptides is approximately 1.5, indicating that S100A9 is abundant in a P. acnes-injected tissue chamber.
4 Discussion
Subcutaneous implantation of a tissue chamber formatted as a perforated cylinder has been extensively utilized to investigate microbial infection [11, 13, 20]. The main advantage of implanted tissue chambers is the ability to locally analyze the host defense mechanisms. Data from our laboratory demonstrated that host phagocytes (Fig. 1), secretory factors including cytokines (Fig. 2) and extracellular fluids including interstitial fluids and plasma (Table 1) can influx into chambers. The implanted chamber surrounded by a thin layer of fibrous tissue prevented the leakage of injected bacteria. Thus, the implanted chamber created a tissue micro-environment where infiltrated host cells interact with pathogens. Elevation of MIP-2 cytokine in P. acnes-injected chamber was consistent with previous reports that recognition of P. acnes by Toll-like receptor 2 (TLR2) induces the production of proinflammatory cytokines [25].
Implantation of a human islets-encapsulated permselective hollow fiber membrane into animals [26] and patients [27] has been applied for treatment of diabetes. Cells in encapsulated islets secreted insulin to normalize blood glucose levels in implanted receipts [27]. These results suggest that hollow fiber membranes served as immunoisolators that enhanced the survival of implanted human cells without rejection by receipt immune systems. When we inserted human SZ95 sebocytes into a tissue chamber, they survived and performed their biological activity of lipid production in the mice (Fig. 3) thus supporting the concept of an implanted chamber functioning as an immunoisolator. A de-epidermized dermis has been widely used as a scaffold to create a 3-D skin equivalent [28, 29]. We generated a humanized acne microenvironment by implanting a tissue chamber bearing a DBCTS into mice. Unlike other types of cell trappers such as PuraMatrix™ (chemical-based extracellular matrix) [30] and Gelfoam® (a gelatin derived from porcine skin) [31], DBCTS provided an nontoxic and nonimmunogenic cell scaffold because dead dermis used in DBCTS was prepared from the animal that was the same strain for implantation. Fluid was obtained one day after injection of P. acnes to a tissue chamber containing a sebocyte cell-trapped dermis (Fig. 3), while it was collected 3 days postbacterial injection into an empty chamber (Fig. 1). Exposure of P. acnes to sebocytes for one day instead of 3 days could decrease the cytotoxicity of bacterium to sebocytes. Furthermore, it has known that P. acnes is a slow-growing anaerobic bacterium [32]. We injected 20 µL of P. acnes or S. Staphylococcus epidermidis (ATCC 12228) (107 CFU/mL) into an empty chamber. Within the tissue chambers, P. acnes multiplied to 1.2 × 109 CFU/mL whereas S. epidermidis decreased to 2 × 106 CFU/mL 3 days after injection (data not shown). S. epidermidis (ATCC 12228) is an aerobic Gram-positive and nonbiofilm-forming bacterium [33]. Although the anaerobic condition within an implanted tissue chamber was undetermined, P. acnes, but S. epidermidis grew in chambers suggested that implanted tissue chambers offered favorable microenvironments for anaerobic bacteria. It has been known that P. acnes can form biofilms on various implanted biomaterials [34]. However, our data indicated P. acnes did not form biofilms on the surfaces of implanted and unimplanted tissue chambers (Fig. 1 of Supporting Information).
ICPL labeling technology was employed to quantify the differential proteomes of fluids drawn from PBS- and P. acnes-injected tissue chambers. Nicotinoylation created by ICPL labeling provided an active ester to interact with intact proteins prior to trypsin digestion, resulting in an increased accuracy in differential protein quantitation. Although ICPL labeled high abundant lysine residues, which normally enable to generate a higher coverage of the proteome with less side reactions, this labeling was restricted by several limitations. First, ICPL labeling may fail if N-terminus of the protein is blocked [35]. Second, the technology is only able to identify sites of PTM when it occurs in labeled peptides. Eleven mammalian proteins and two P. acnes proteins were identified and/or qualified via ICPL labeling incorporated with nano-LC-MS. Secreted proteins, cell matrix proteins, circulating plasma proteins as well as intracellular proteins were identified in tissue chamber fluids. This indicates that tissue chamber fluids contain secretomes as well as proteins derived from interstitial fluid and plasma [36, 37]. Intracellular proteins from host and P. acnes were also detectable in tissue chamber fluids, suggesting that dead cells and lysed P. acnes released intracellular proteins into chamber fluids. Although proteins secreted from human sebocytes and mouse cells are indistinguishable, sebocyte proteins may account for a small portion of identified proteins given that only 106 sebocytes were seeded onto the dermis. The establishment of comprehensive proteomes of various host cells, human sebocytes, and plasma in the future will help in distinguishing the cell sources of fluids collected by tissue chambers.
It has been shown that P. acnes induces an elevation of proinflammatory cytokines in acne lesions [1, 25]. Although we found that MIP-2 in tissue chamber fluid was measurable by ELISA assay (Fig. 2), we did not detect any cytokines by nano-LC-MS (Table 1). The cytokines are typically present at relatively low concentrations in the Pg/mL range. Nano-LC-MS may not be sensitive enough to detect cytokines. Thus, using immunodetection assays such as ELISA or cytokines antibody arrays [38] may be the best way to obtain cytokine profiles in tissue chamber fluid. The abundance of S100A9 significantly increased after P. acnes injection. S100A9, also known as myeloid-related protein (MRP14), is a member of the S100 family of low molecular weight (9–13 kDa) proteins that are characterized by the presence of two calcium-binding motifs of helix-loop-helix conformation [39]. S100A9 is known to exert a variety of roles by forming a heterodimer with S100A8 (MRP8). The S100A8/A9 heterodimer (also called calprotectin) was originally identified as an antimicrobial protein in neutrophils [40]. Both S100A8 and S100A9 contain histidine-based zinc-binding sequences (His-X-X-X-His motif), which are involved in the antimicrobial activity of S100A8/A9 [41]. In addition to the antimicrobial activity, this heterodimer protein has an imperative role in recruitment of leukocytes to the site of inflammation [42, 43]. Thus, the increase of S100A9 in a P. acnes-injected chamber may be a host antimicrobial response and/or an induction of attracting other immune cells collaboratively against P. acnes infection. Unlike with S100A9, the level of the secreted serine protease inhibitor A3K was significantly decreased after P. acnes injection. Despite the fact that members of serine protease inhibitor family can be induced in macrophages during bacterial infection [44, 45], it has been reported that bacteria are capable of subverting the function of proteases, activators or inhibitors for their own benefits [46]. Thus, the decrease of serine protease inhibitor A3K in tissue chamber fluids may be a consequence of cells infected with bacteria.
The fact that only two P. acnes proteins, putative peroxiredoxin (AhpC/TSA family) and PIPase (EC 3.4.11.5) were identified in the tissue chamber fluids may be due to few proteins being released from P. acnes 1 day postinjection. The insufficient amount of proteins in chamber fluids also may contribute to the failure in labeling with either light (12C6) or heavy (13C6) forms of Nic-NHS for protein quantitation. PIPase is a peptidase catalyzing the removal of N-terminal proline from low- and high-molecular weight peptides [47]. Microbial PIPases can digest the proteins or peptides of host tissue into amino acids and then be used as nutrients [47]. PIPase is especially important for the ability of some pathogenic bacteria to degrade collagen, a major component of the extracellular matrix that supports most tissues. Thus, PIPase of P. acnes may play a critical role in the breakdown of host tissues for bacterial invasion.
5 Concluding remarks
In summary, the utilization of DBCTS as a scaffold in combination with a tissue chamber allows the harboring of the host cells, P. acnes and human sebocytes in vivo and therefore the mimicry of a microenvironment of acne lesions. In response to P. acnes infection, host cells and human sebocytes can release various soluble factors including secreted proteins (e.g., pro-inflammatory cytokines) and proteins discharged from lyzed cells. The mimicked acne microenvironment effectively entraps these soluble factors which thus become detectable before they are diluted into circulating systems. It has been reported that protein abundances could be different for proteins that are present in the tissue microenvironment versus in the circulating system [48]. Identification of proteins in the acne microenvironment provided us with a glimpse of local interactions of bacteria with the host biological environment. Proteomics identified proteins including various host proteins (fibrinogen, S100A9, serine protease inhibitor A3K) and P. acnes proteins (putative peroxiredoxin and PIPase), and provide new targets for treatment of acne vulgaris. Most importantly, the humanized acne microenvironment may confer a valuable animal model for screening novel anti-acne drugs and vaccines.
Acknowledgments
T. Nakatsuji and Y Shi have contributed equally to this project, and both should be considered as first authors. This work was supported by National Institutes of Health Grants (R01-AI067395-01, R21-R022754-01, R21-I58002-01 and 1R41AR056169-01) and a Center for Proteolytic Pathway Grant (RR020843). We thank C. Niemeyer for critical reading of the manuscript.
Abbreviations
- CFU
colony forming unit
- DBCTS
dermis-based cell-trapped system
- H&E
hematoxylin and eosin
- ICPL
isotope-coded protein label
- MIP-2
macrophage-inflammatory protein-2
- Nic-NHS
N-nicotinoyloxy-succinimid
- PIPase
proline iminopeptidase
Footnotes
The authors have declared no conflict of interest.
References
- 1.Zouboulis CC. Acne and sebaceous gland function. Clin. Dermatol. 2004;22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ochsendorf F. Systemic antibiotic therapy of acne vulgaris. J. Dtsch. Dermatol. Ges. 2006;4:828–841. doi: 10.1111/j.1610-0387.2006.06053.x. [DOI] [PubMed] [Google Scholar]
- 3.Gloor M, Wasik B, Becker A, Hoffler U. Inhibition of lipase activity in antibiotic-resistant Propionibacterium acnes strains. Dermatology. 2002;205:260–264. doi: 10.1159/000065856. [DOI] [PubMed] [Google Scholar]
- 4.Layton AM, Dreno B, Gollnick HP, Zouboulis CC. A review of the European Directive for prescribing systemic isotretinoin for acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2006;20:773–776. doi: 10.1111/j.1468-3083.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 5.Webster GF, Ruggieri MR, McGinley KJ. Correlation of Propionibacterium acnes populations with the presence of triglycerides on nonhuman skin. Appl. Environ. Microbiol. 1981;41:1269–1270. doi: 10.1128/aem.41.5.1269-1270.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano K, Kiyokane K, Benvenuto-Andrade C, Gonzalez S. Real-timereflectance confocal microscopy, a noninvasive tool for in vivo quantitative evaluation of comedolysis in the rhino mouse model. Skin Pharmacol. Physiol. 2007;20:29–36. doi: 10.1159/000096169. [DOI] [PubMed] [Google Scholar]
- 7.Mirshahpanah P, Maibach HI. Models in acnegenesis. Cutan. Ocul. Toxicol. 2007;26:195–202. doi: 10.1080/15569520701502815. [DOI] [PubMed] [Google Scholar]
- 8.Takaoki M, Kawaji H. Impaired antibody response against T-dependent antigens in rhino mice. Immunology. 1980;40:27–32. [PMC free article] [PubMed] [Google Scholar]
- 9.Toho M, Uchida Y, Miyamoto I, Ogawa T. Immunohistochemical studies of the acne-like inflammatory model. Jikken Dobutsu. 1990;39:531–537. doi: 10.1538/expanim1978.39.4_531. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. Pathogenesis of foreign body infection: Description and characteristics of an animal model. J. Infect. Dis. 1982;146:487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- 11.Kristian SA, Lauth X, Nizet V, Goetz F, et al. Alanylation of teichoic acids protects Staphylococcus aureus against Toll-like receptor 2-dependent host defense in a mouse tissue cage infection model. J. Infect. Dis. 2003;188:414–423. doi: 10.1086/376533. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Elmets CA, Smith JW, Liu YT, et al. Quantitative proteomes and in vivo secretomes of progressive and regressive UV-induced fibrosarcoma tumor cells: Mimicking tumor microenvironment using a dermis-based cell-trapped system linked to tissue chamber. Proteomics. 2007;7:4589–4600. doi: 10.1002/pmic.200700425. [DOI] [PubMed] [Google Scholar]
- 13.Kristian SA, Golda T, Ferracin F, Cramton SE, et al. The ability of biofilm formation does not influence virulence of Staphylococcus aureus and host response in a mouse tissue cage infection model. Microb. Pathog. 2004;36:237–245. doi: 10.1016/j.micpath.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Huang CM, Wang CC, Barnes S, Elmets CA. In vivo detection of secreted proteins from wounded skin using capillary ultrafiltration probes and mass spectrometric proteomics. Proteomics. 2006;6:5805–5814. doi: 10.1002/pmic.200600163. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Kang Q, Wang DM. Constitutive coactivator of peroxisome proliferator-activated receptor (PPARgamma), a novel coactivator of PPARgamma that promotes adipogenesis. Mol. Endocrinol. 2007;21:2320–2333. doi: 10.1210/me.2006-0520. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt A, Kellermann J, Lottspeich F. A novel strategy for quantitative proteomics using isotope-coded protein labels. Proteomics. 2005;5:4–15. doi: 10.1002/pmic.200400873. [DOI] [PubMed] [Google Scholar]
- 17.Sarioglu H, Brandner S, Jacobsen C, Meindl T, et al. Quantitative analysis of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced proteome alterations in 5L rat hepatoma cells using isotope-coded protein labels. Proteomics. 2006;6:2407–2421. doi: 10.1002/pmic.200500680. [DOI] [PubMed] [Google Scholar]
- 18.Kamphorst JJ, van der Heijden R, DeGroot J, Lafeber FP, et al. Profiling of endogenous peptides in human synovial fluid by NanoLC-MS: Method validation and peptide identification. J. Proteome Res. 2007;6:4388–4396. doi: 10.1021/pr0704534. [DOI] [PubMed] [Google Scholar]
- 19.Elias JE, Haas W, Faherty BK, Gygi SP. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat. Methods. 2005;2:667–675. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
- 20.Voermans M, van Soest JM, van Duijkeren E, Ensink JM. Clinical efficacy of intravenous administration of marbofloxacin in a Staphylococcus aureus infection in tissue cages in ponies. J. Vet. Pharmacol. Ther. 2006;29:555–560. doi: 10.1111/j.1365-2885.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 21.Leyden JJ, Del Rosso JQ, Webster GF. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: Focus on antibiotic resistance. Cutis. 2007;79:9–25. [PubMed] [Google Scholar]
- 22.Thiboutot D, Jabara S, McAllister JM, Sivarajah A, et al. Human skin is a steroidogenic tissue: Steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1) J. Invest. Dermatol. 2003;120:905–914. doi: 10.1046/j.1523-1747.2003.12244.x. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer H, Schalla W, Hagele W, Stuttgen G. P. acnes and the chemistry of sebum. Acta. Derm. Venereol. Suppl. (Stockh) 1980;89:23–26. [PubMed] [Google Scholar]
- 24.Chen YL, Yu C, Lei HY. Propionibacterium acnes induces acute TNFalpha-mediated apoptosis of hepatocytes followed by inflammatory T-cell-mediated granulomatous hepatitis in mice. J. Biomed. Sci. 1999;6:349–356. doi: 10.1007/BF02253524. [DOI] [PubMed] [Google Scholar]
- 25.Jugeau S, Tenaud I, Knol AC, Jarrousse V, et al. Induction of toll-like receptors by Propionibacterium acnes. Br. J. Dermatol. 2005;153:1105–1113. doi: 10.1111/j.1365-2133.2005.06933.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Iwata H, Shimizu H, Takagi T, et al. Comparative studies of in vitro and in vivo function of three different shaped bioartificial pancreases made of agarose hydrogel. Biomaterials. 1994;15:113–120. doi: 10.1016/0142-9612(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 27.Scharp DW, Swanson CJ, Olack BJ, Latta PP, et al. Protection of encapsulated human islets implanted without immunosuppression in patients with type I or type II diabetes and in nondiabetic control subjects. Diabetes. 1994;43:1167–1170. doi: 10.2337/diab.43.9.1167. [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Shi Y, Chen Y, Zhao H, et al. In vitro and in vivo biological performance of collagen-chitosan/silicone membrane bilayer dermal equivalent. J. Mater. Sci. Mater. Med. 2007;18:2185–2191. doi: 10.1007/s10856-007-3088-4. [DOI] [PubMed] [Google Scholar]
- 29.Hata K. Current issues regarding skin substitutes using living cells as industrial materials. J. Artif. Organs. 2007;10:129–132. doi: 10.1007/s10047-006-0371-y. [DOI] [PubMed] [Google Scholar]
- 30.Kim MS, Yeon JH, Park JK. A microfluidic platform for 3-dimensional cell culture and cell-based assays. Biomed. Microdevices. 2007;9:25–34. doi: 10.1007/s10544-006-9016-4. [DOI] [PubMed] [Google Scholar]
- 31.Hong L, Peptan I, Clark P, Mao JJ. Ex vivo adipose tissue engineering by human marrow stromal cell seeded gelatin sponge. Ann. Biomed. Eng. 2005;33:511–517. doi: 10.1007/s10439-005-2510-7. [DOI] [PubMed] [Google Scholar]
- 32.Störmer M, Kleesiek K, Dreier J. Propionibacterium acnes lacks the capability to proliferate in platelet concentrates. Vox Sang. 2008;94:193–201. doi: 10.1111/j.1423-0410.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang YQ, Ren SX, Li HL, Wang YX, et al. Genome-based analysis of virulence genes in a nonbiofilm-forming Staphylococcus epidermidis strain (ATCC 12228) Mol. Microbiol. 2003;49:1577–1593. doi: 10.1046/j.1365-2958.2003.03671.x. [DOI] [PubMed] [Google Scholar]
- 34.Bayston R, Ashraf W, Barker-Davies R, Tucker E, et al. Biofilm formation by Propionibacterium acnes on biomaterials in vitro and in vivo: Impact on diagnosis and treatment. J. Biomed. Mater. Res. A. 2007;81:705–709. doi: 10.1002/jbm.a.31145. [DOI] [PubMed] [Google Scholar]
- 35.Asara JM, Zhang X, Zheng B, Christofk HH, et al. InGel Stable-Isotope Labeling (ISIL): A strategy for mass spectrometry-based relative quantification. J. Proteome. Res. 2006;5:155–163. doi: 10.1021/pr050334t. [DOI] [PubMed] [Google Scholar]
- 36.Anderson NL, Anderson NG. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Liu AY, Loriaux P, Wollscheid B, et al. Mass spectrometric detection of tissue proteins in plasma. Mol. Cell. Proteomics. 2007;6:64–71. doi: 10.1074/mcp.M600160-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Huang RP. An array of possibilities in cancer research using cytokine antibody arrays. Expert Rev. Proteomics. 2007;4:299–308. doi: 10.1586/14789450.4.2.299. [DOI] [PubMed] [Google Scholar]
- 39.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 2006;396:201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, et al. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–765. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 41.Loomans HJ, Hahn BL, Li QQ, Phadnis SH, Sohnle PG. Histidine-based zinc-binding sequences and the antimicrobial activity of calprotectin. J. Infect. Dis. 1998;177:812–814. doi: 10.1086/517816. [DOI] [PubMed] [Google Scholar]
- 42.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: A novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 43.Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc. Res. Tech. 2003;60:569–580. doi: 10.1002/jemt.10299. [DOI] [PubMed] [Google Scholar]
- 44.Hill RE, Hastie ND. Accelerated evolution in the reactive centre regions of serine protease inhibitors. Nature. 1987;326:96–99. doi: 10.1038/326096a0. [DOI] [PubMed] [Google Scholar]
- 45.Hamerman JA, Hayashi F, Schroeder LA, Gygi SP, et al. Serpin 2a is induced in activated macrophages and conjugates to a ubiquitin homolog. J. Immunol. 2002;168:2415–2423. doi: 10.4049/jimmunol.168.5.2415. [DOI] [PubMed] [Google Scholar]
- 46.Bergmann S, Hammerschmidt S. Fibrinolysis and host response in bacterial infections. Thromb. Haemost. 2007;98:512–520. [PubMed] [Google Scholar]
- 47.Walter R, Simmons WH, Yoshimoto T. Proline specific endo- and exopeptidases. Mol. Cell. Biochem. 1980;30:111–127. doi: 10.1007/BF00227927. [DOI] [PubMed] [Google Scholar]
- 48.Celis JE, Gromov P, Cabezon T, Moreira JM, et al. Proteomic characterization of the interstitial fluid perfusing the breast tumor microenvironment: A novel resource for biomarker and therapeutic target discovery. Mol. Cell. Proteomics. 2004;3:327–344. doi: 10.1074/mcp.M400009-MCP200. [DOI] [PubMed] [Google Scholar]