Abstract
Psoriasis is a chronic inflammatory disorder that is mediated by elements of the innate and adaptive immune systems. Its characteristic features in the skin consist of inflammatory changes in both dermis and epidermis, with abnormal keratinocyte differentiation and proliferation. Despite the elucidation of many aspects of psoriasis pathogenesis, some puzzling questions remain to be answered. A major question currently debated is if psoriasis is a primary abnormality of the epidermal keratinocyte or a reflection of dysregulated bone-marrow derived immunocytes. In this review we will focus on understanding the role of the innate immune system in psoriasis and how this provides a rational solution to address the origin of this multifactorial disease. Innate immunity is non-specific and genetically-based. It protects the body against the constant risk of pathogens through the use of rapidly mobilized defenses that are able to recognize and kill a wide variety of threats (bacteria, fungi, viruses, etc.). The key mechanisms of innate immune responses are the existence of receptors to recognize pathogens, and the production of factors that kill pathogens, such as antimicrobial peptides and proteins. Any combination of excessive sensitivity of the innate detection system, or dysregulation of the response system, can manifest both an epidermal phenotype and abnormal T-cell function. Thus, the multidimensional action of the innate immune system, its triggers, and its recently understood role in T-cell function, argue for an important role for innate mechanisms of recognition and response in the pathogenesis of psoriasis.
Introduction
Over 2–3% of the world-wide population suffers from psoriasis, a chronic inflammatory skin disease characterized by the formation of typical scaly plaques.1 Over the last decades, many researchers have tried to uncover the responsible elements of psoriasis pathogenesis. However, given difficulties in identifying a clear genetic locus associated with the disease, today psoriasis is considered a combination of genetic, immunologic and environmental factors. Psoriasis is recognized by a disturbed proliferation and differentiation of keratinocytes (hyperproliferation, orthokeratosis) accompanied by vascular alterations and epidermal infiltration of activated Th1 type lymphocytes and antigen-presenting cells together with a local Th1-type cytokine immune response.2,3 As a consequence of this diversity in etiology, the understanding of a multifactorial pathogenesis of psoriasis has evolved into a remarkable number of therapeutic concepts. These facts prompt further questions: Is the aberrant keratinocyte differentiation a primary defect or is it the consequence of an influx of pathogenic immunocytes?
Psoriatic plaques occur throughout the body and appear as scaly hypertrophic lesions of characteristic morphology. Because of the aberrant epidermal differentiation program in such lesions, psoriatic plaques lack a normal granular cell layer or intact stratum corneum.4 The cause of increased keratinocyte proliferation and disturbed cell maturation and thereby an impaired barrier defect), has been suggested as i) the consequence of high levels of cAMP, ii) a disturbed arachidonic acid metabolism, ii) high activity of proteases and decrease in anti-protease activity, iii) overexpression of growth factors iv) disturbed metabolism and efficiency of calcium and vitamin D.5–13 These theories may in part explain why glucocorticosteroids, vitamin D analogs, retinoids, and phototherapy are useful drugs in psoriasis treatment. However, many different types of skin barrier defects have been produced in transgenic mice but they do not develop a psoriatic plaque. For example in keratin-10 knockout mice hyperkeratosis is observed, IFN-β transgenic mice display profound barrier defects, but other typical signs of psoriasis, such as a loss of a granular layer or an angiogenic tissue reaction, are not present.14,15 On the other hand in double knockout mice for epidermal JunB/c-Jun kinase, showing a convincing psoriatic phenotype, S100A8 and S100A9 were both highly upregulated in pre-diseased skin.16 S100A8 and S100A9 are both antimicrobial proteins with chemotactic activity and a well known role in keratinocyte maturation and proliferation processes.17 These results were furthermore substantiated in vitro, showing that the S100A8 and S100A9 upregulation was increased by cre deletion of both Jun kinases in primary keratinocytes.16 Moreover, Zenz et al., excluded an exclusive role of T-cells in his mouse model, using also Rag2-deficient JunB/c-Jun double mutant mice that developed psoriasis-typical epidermal thickening, altered keratinocyte maturation, vascular dilatation and epidermal micro-abscesses in the absence of Band T-cells. Overall, these observations argue for the origin of psoriasis in the epidermis.
Much work also supports a role for the immunocyte, especially the T-cell, in the pathogenesis of psoriasis. The identification of T-cells in psoriatic lesions and the characterization of T-cell derived cytokines on inflammation and keratinocyte growth and differentiation became a new target of investigation and initiated a systematic search for therapies targeted to interfere with these activated T-cells, such as immunosuppressive drugs (phototherapy, cyclosporin, methotrexate). The psoriatic plaque is characterized by the presence of Th1 cytokines including TNF-β, IFN-β, IL-2.18 In addition to these T-cell derived cytokines, numerous antigen presenting cells (APCs) infiltrate psoriatic skin and produce inflammatory cytokines and chemokines. TNF-β has been identified as a promising target molecule and psoriasis patients treated with TNF-β inhibiting agents (Infliximab, Etarnercept) showed impressive decreases of their disease severity. The occurrence of rare but severe side effectsremains a problem for these treatment approaches. Many more soluble immune mediators including chemokines and their receptors have been recently identified in psoriatic plaques. CCL20, CCL22, CCL5 and CCL19 are only a few chemokines that deserve attention and may be new target molecules in psoriasis treatment.8,19
Reconciliation of the findings of the two approaches outlined above (the primary keratinocyte disorder and the immunocyte disorder) can be explained by the functions of the innate immune system.
It was previously recognized that despite a compromised “structural shield” in psoriasis, the fully-developed psoriatic plaque provides a rather impressive chemical protective shield for the skin. This may be one explanation why psoriasis patients are rather resistant to invasion by infectious organisms.20,21 This observation led to the hypothesis that a local “chemical shield” provides psoriatic skin with resistance against microbial infections in contrast to patients with other inflammatory skin diseases, such as atopic dermatitis. Indeed, the compensatory response by immune cells together with the overexpression of innate immunity response genes in psoriasis are discussed as the molecular basis for this phenomenon of microbial resistance.22,23 Interestingly, many antimicrobial peptides and proteins (AMPs), endogenous natural antibiotics, were identified in psoriatic-scale extracts such as cathelicidin 24,25, S100-proteins 26, human beta-defensins (hBD) 22, RNase 7 26, and lysozyme 26 and many more (Table 1). In contrast, deficiency in expression of AMPs in skin lesions of patients with atopic dermatitis have been reported to account for the high occurrence of Gram-positive skin infections in those patients.25 Taking these observations into account, another question should be addressed when looking at psoriasis pathogenesis, namely whether the overexpression of AMPs is responsible for the psoriasis phenotype. Focussing on the broad range properties of many cutaneous antimicrobial peptides and proteins, such as proteinase inhibitors, chemokines, and neuropeptides, one could assume that these AMPs may contribute to psoriasis pathogenesis by recruiting immune cells to the epidermis, mediating a proinflammatory immune response and initiating proliferation of blood vessels and keratinocytes.27,28
Table 1.
Summary of antimicrobial peptides and proteins that are overexpressed in psoriatic skin, their inducers, and their expression patterns.
| AMPs overexpressed in psoriasis | Inducers of AMP expression | Expression in cells, tissues, skin diseases | Reference |
|---|---|---|---|
| hBD-2 | TNF-α, IFN-γ, IL-1, Fusobacterium nucleatum, Pseudomonas aeruginosa, calcium, vitamin D | Epidermal keratinocytes, psoriasis | 21; 22; 59; 61; 68; 69 |
| hBD-3 | INF-α, IFN-γ | epidermal keratinocytes | |
|
| |||
| Cathelicidin/LL-37 | Vitamin D | epidermal keratinocytes, T-cells, monocytes, neutrophils, psoriasis, wound fluids, LE, contact dermatitis | 59; 60; 70; 71; 72; 73 |
|
| |||
| S100A7 (psoriasin) | Calcium, vitamin D, retinoid acid, Escherichia coli, Staphylococus aureus TNF-α, IFN-γ, IL-1β, | epidermal keratinocytes, breast cancer, oral sqamous cell carcinoma | 26; 62; 63; 79; 80; 82; 83; 84; 85; 86; 87; 88, 89, 90; 91 |
| S100A8 (calgranulin A) | UVA | Epidermal keratinocytes, wound healing, neutrophils, monocytes, sqamous cell carcinoma | |
| S100A9 (calgranulin B) | Epidermal keratinocytes, wound healing, neutrophils, monocytes, sqamous cell carcinoma | ||
| S100A12 (calgranulin C) | IL-6 | Epidermal keratinocytes, neutrophils, lymphocytes, monocytes | |
| S100A15 | Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, TNF-α IFN-γ, IL-1β, calcium, TPA | Epidermal keratinocytes | |
|
| |||
| RNase 7 | Pseudomonas aeruginisa, Staph aureus, E. coli, Streptococcus pyogenes, TNF-α, IFN-γ, IL-β | Epidermal keratinocytes, respiratory tract, genitourinary tract, gut | 26; 92 |
|
| |||
| Lysozyme | Leukocytes, monocytes, macrophages, epidermal keratinocytes | 92; 93 | |
Abbreviations used: IFN (interferon), TNF (tumor necrosis factor), IL (interleukin), UVA (ultraviolett A), LE (lupus erythematodes), TPA (12-0-tetradecanoylphorbol-13-acetat)
These observations challenge to review the role innate immunity and the role of antimicrobial peptides and proteins in psoriasis.
General principle of innate host defense
The immune system is designed to protect the host from infection and other insults. There are two major approaches that the immune system uses to protect the host: the innate and the adaptive system, which both contribute to the pathophysiology of psoriasis. In this review, the innate immune response system of psoriasis will be highlighted.
Innate immunity is non-specific and genetically-based. It protects the body against the constant risk of pathogens, through the use of rapidly mobilized defenses that are able to recognize and kill a wide variety of threats (bacteria, fungi, viruses, etc.). The key mechanisms of innate immune responses are 1) the existence of receptors to recognize pathogens 29,30 and 2) to employ mechanisms that kill pathogens, such as antimicrobial peptides and proteins. The major cellular elements of innate immunity in the skin are granulocytes, dendritic cells (DCs), macrophages and keratinocytes. The innate immune system triggers a sequence of factors that results in the production of cytokines, chemokines, endogenous antimicrobial substances, the activation of immune cells and transcription factors, and in the end initiates the killing of the pathogenic microbe and activation of adaptive immunity.
The innate immune system senses invading microbes through their pathogen associated molecular patterns (PAMPs) via pathogen recognition receptors (PRRs), such as Toll like receptors (TLRs). TLRs are structurally related to the Drosophila Toll receptor and are expressed in human keratinocytes.31,32 As effectors of innate immunity, antimicrobial peptides (AMPs) play a predominant role providing the first line of defense in the skin against invading microbes.33 The production of antimicrobial peptides are the phylogenetically oldest innate immune responses.29,34 AMPs directly kill a broad spectrum of microbes including Gram-positive and Gram-negative bacteria, fungi and certain viruses. They have been identified in resident cells, such as keratinocytes, as well as in infiltrating cells and, are more than simple antibiotics. Their additional function as proteinase inhibitors, chemokines, neuropeptides and much more might be overlooked even more important to skin biology.
Toll-like receptors (TLRs)
The innate immune response is the initial nonspecific response to a microbial attack. As one member of pattern recognition receptors, Toll-like-receptors (TLRs) are used by cells of the innate immune system to recognize molecular structures, so called pathogen associated molecular patterns (PAMPs). PAMPs are conserved by microbes and are essential for the survival or pathogenecity of microorganisms.29 Typical examples of PAMPs include lipopolysaccharide (LPS) from Gram-negative bacteria, lipoteichoic acids and peptidoglycan of Gram-positive bacteria and mannans of yeasts/fungi.
TLRs are the mammalian homologues of the Drosophila Toll family that control the dorso-ventral patterning in the developing embryo and the antimicrobial response in the adult fly.35 Structurally, TLR family members are characterized by the presence of a leucine-rich extracellular domain and a highly conserved intracellular Toll/Interleukin (IL)-1 receptor (TIR) domain.36 While the extracellular part is responsible for specific ligand-recognition, the intracellular part mediates the signal transduction.
TLR2 is a heterodimer that associates with TLR1 or TLR6 and CD14 to recognize lipopeptides from bacteria, peptidoglycan (PGN) and lipoteicheic acid (LTA), TLR3 recognizes double-stranded RNA (dsRNA) produced during virus replication, while TLR4/CD14 is activated by lipopolysaccharide (LPS) derived from Gram-negative bacteria. Flagellin is recognized by TLR5, viral single stranded RNA (ssRNA) by TLR 7 and TLR8. TLR9 recognizes unmethylated CpG DNA primarily found in bacteria. TLR11 recognizes uropathogenic E. coli.37,38 Moreover, further ligands for many TLRs have been found, including endogenous proteins like heat-shock-proteins, and small fragments of hyaluronan.39,40 The common downstream signalling pathway of activated TLRs leads to activation of nuclear factor (NF)-βB via myeloid differentiation protein (MyD88)- IRAK-TRAF6- signal cascade ultimately triggering the transcription of chemokines, proinflammatory cytokines, antimicrobial substances, and upregulation of cell surface molecules involved in the initiation of adaptive immune responses to pathogens and antimicrobial peptides and proteins.29 TLR3 in contrast activates NF-βB without the MyD88 pathway and TLR4 is only partially dependent on MyD88 for signalling.
Few authors have conceived that TLRs may be important in psoriasis pathogenesis. Earlier, an association between psoriasis and streptococcal infections was discovered.41,42 not aware of the presence of TLRs as potential receptors for PAMPs. Later, it was proposed that microorganisms on the skin are directly implicated in psoriasis pathogenesis and aggravation 43–45 and it was suggested that certain microorganisms induce and/or exacerbate disease process through the activation of TLRs in the skin.46 It was found that TLR1, TLR2, and TLR5 are constitutively expressed in healthy epidermal keratinoyctes, while TLR3 and TLR4 are almost absent. TLR1 and TLR2 are expressed throughout the epidermis with higher expression in the basal keratinocytes and TLR5 is exclusively expressed in the basal cell layer. In contrast, in lesional psoriatic skin strong TLR1 staining is observed in keratinocytes of the upper epidermis. TLR2 is highly expressed on keratinoyctes of the upper epidermis, but not in the basal layer, while TLR5 is downregulated in basal keratinocytes of psoriatic patients. TLR3 and TLR4 are weakly expressed in healthy and psoriatic skin. Other authors however, reported that TLRs are not differently expressed in psoriatic compared to atopic skin.47
The role of TLRs in the pathogenesis of psoriasis is not fully understood. Too many questions remain to be clarified concerning TLR induced immune response. Until today, many studies have been performed on TLR expression, some with conflicting results.48,49 In conclusion, detection of TLR gene expression is not consistent with its activation and its biological function. For example, IL-8 but not TLR2, is transcriptionally upregulated upon stimulation with Candida albicans. However, pre-treatment of NHEK with TLR2 and TLR4 antibodies abrogated Candida albicans killing activity of NHEK.50
Topical application of Imiquimod, a TLR7 agonist, triggered aggravation of a psoriatic plaque.51 Its antiviral and antitumoral effects is considered the consequence of type 1 IFN-β/β induction.52 This observation offers new sights into the pathophysiology of psoriasis, since a defined TLR activation has not been observed as a trigger for psoriasis.
Antimicrobial peptides and proteins in the skin
AMPs are mostly small cationic molecules binding to and interacting with the negatively charged membranes of microbes and are thereby able to kill. The exact mechanisms by which antimicrobial peptides kill microbes are still not fully understood. It is proposed that AMPs may form pores in the membrane of microbes which leads to lysis of the pathogenic threat.53 AMPs that are made in the skin provide a soluble barrier that forms an impediment to infection. In case of infection, inflammation or injury, many antimicrobial peptides in the skin are upregulated due to increased synthesis by keratinocytes and deposition from degranulation of recruited neutrophils. Interestingly, AMPs are abundantly expressed in psoriatic skin 22,25,54,55, while they are present in only very low concentrations in skin from atopic dermatitis patients.21,25 Clinically, expression levels of these natural antibiotics correlate with the susceptibility to skin infections, because atopic dermatitis patients suffer from frequent occurring skin infections, while psoriatic patients do not. These observations indicate that 1) AMP expression may depend on cytokine profiles present in the micromilieu of inflamed skin, 2) that AMP dysregulation may cause disease. Psoriasis and atopic dermatitis (AD) are the two most common chronic inflammatory skin diseases found in Caucasian population. However, their mechanisms for skin inflammation are quite different. While psoriasis is known to be associated with a Th1 type cytokine pattern and involves predominantly T-cells and neutrophils, AD is believed to exhibit a Th2-type directed cytokine pattern contributing to the high IgE levels and eosinophilia characteristics in this condition. On the other hand, recent studies hint that AMPs function also as chemokines, proteinase inhibitors, or neuropeptides rather than simple antibiotics.56 AMPs are involved in wounding, wound healing and vascularization 57,58, can induce a proinflammatory cytokine response 27 and are involved in skin differentiation processes, since antimicrobial peptides and proteins expression in epidermal keratinocytes are inducible by high calcium concentrations, retinoid acid, and 1,25(OH)-vitamin D3.59–62 Thereby it is possible that an imbalance or dysregulation of AMPs itself will lead to the formation of psoriatic lesions by recruiting inflammatory cells to certain areas of the skin, accumulate a proinflammatory cytokine response and trigger angiogenesis and keratinocyte proliferation. In line with this hypothesis is the fact that AMPs show a certain distribution on human healthy skin, that reflects the predicted areas of psoriasis development.63 Moreover, the frequent discussed Koebner phenomenen (formation of a psoriatic plaque after trauma of unaffected skin) could be explained by the presence of AMPs in human skin: injury, the trigger for the Koebner phenomenen, is known to induce upregulation of AMPs in the skin and could thereby trigger the formation of a psoriatic plaques in a genetic predisposed individual. Many different AMPs have been described so far, of which some will be presented in the following.
Human β-defensins
Defensins contain six cysteine residues that form characteristic disulfide bridges. Disulfide bridge alignment and molecular structure separate this major antimicrobial peptide family into β-, β-, and β-defensins. β-Defensins are expressed by human neutrophils, which are also referred to as human neutrophil peptides 1 through 4. Human defensins (hD) 5 and 6 are abundantly expressed in Paneth cells of small intestinal crypts and in cells of the female urogenital tract.64 Human β-defensins contain three disulfide bridges. The four best known human β-defensins (hBD), hBD-1 to -4 have been identified in various cell types including epithelia and peripheral blood cells.65,66 HBD-1 is constitutively expressed in epithelia, whereas hBD-2 is highly upregulated in inflamed skin including psoriasis. HBD-3, which like HBD-2 was purified from psoriatic skin, is inducible in a variety of tissues.66 β-Defensins have a broad-spectrum antimicrobial activity and additional immune-related functions.
HBD-1 and hBD-2 have antimicrobial activity directed against Gram-negative bacteria but are less effective against Gram-positive bacteria, hBD-3 is active against preferentially Gram-positive bacteria. Only hBD-2 and hBD-3 are both strongly expressed in psoriatic skin.21,22 Several studies on the regulation of expression of β-defensins have been published. HBD-2 and hBD-3 expression have been reported to be inducible by TNF-β and IFN-β and can be inhibited by Th2 cytokines, such as IL-4 and IL-13 in normal human epidermal keratinocytes.21,22 Other authors describe that IL-1 was the strongest inducer of hBD-2, while IFN-β does not induce hBD-2 but is the strongest inducer of hBD-3.67 However, the typical Th1 cytokine pattern of psoriasis and low levels of Th2 cytokines may account for the high expression of hBD in psoriatic skin and low expression levels in AD.
Previous studies showed that hBD-2 was also inducible by bacteria, such as Fusobacterium nucleatum 68 and Pseudomonas aeruginosa.69 Hence, the mechanisms of hBD-2 induction by bacteria are not clear. Most likely, it is mediated via PAMPs which are known activators of eligible PRRs.
Furthermore hBD-2 expression is induced by high calcium concentrations and 1,25(OH)-vitamin D3. 59,61
Human Cathelicidin
Cathelicidins form a distinct class of proteins present in the innate immunity of mammals. Similar to defensins, they act as precursor molecules that can release an antimicrobial peptide after cleveage. Their precursors contain a highly conserved N-terminal “cathelin” domain flanked by a signal peptide domain on its N-terminus and by an antimicrobial peptide region on its C-terminus. In man, one cathelicidin gene, named CAMP has been identified as a coding region for the 18kD pre-pro-protein hCAP18, which includes the C-terminal human antimicrobial peptide LL-37. Cathelicidin LL-37 is expressed by various types of cells, tissues and body fluids such as epidermal keratinocytes and intestinal cells 60, T cells and monocytes 70, wound fluids 71, bronchoalveolar lavage fluids 72, and vernix caseaosa of newborns.73 Furthermore LL-37 expression is increased in psoriasis 74 and other inflammatory skin disorders such as lupus erythematodes and contact dermatitis 24, but is downregulated in atopic dermatitis.74 Vitamin D has been recently found a potent inducer of LL-37 in cultured human keratinocytes, but not in other human epidermal cells such as colonocytes.59,60 Stimulation of human myeloid cells with vitamin D also upregulated LL-37.75 Regulation of human LL-37 occurs via a consensus vitamin D responsive element in the LL-37 promoter. Interestingly, induction of cathelicidin is absent in murine cells probably due to the absence of a vitamin D responsive element in the murine cathelicidin promoter.75 This observation may be important for the role of LL-37 in psoriasis since vitamin D has many biological effects, including calcium regulation, stimulation of cellular differentiation, inhibition of proliferation, antioxidative, antitumorigenic, and immune modulatory functions.75,76 Interestingly, other mediators of epidermal keratinocyte differentiation, such as high calcium and retinoid acid are not able to upregulate LL-37 in NHEK, in contrast to hBD-2.60 Vitamin D metabolism in psoriatic patients has been a matter of dispute, because one group of investigators observed no difference in levels of circulating 1,25(OH)2D3 between psoriatic and normal patients, whereas another group found reduced concentrations in psoriatic patients associated with higher disease severity.13 These findings suggest the existence of an inverse relationship between severity of psoriasis and serum vitamin D. However, the importance of vitamin D in the pathogenesis of psoriasis needs to be further elucidated.
Cathelicidin LL-37 mounts antimicrobial activity against a wide range of microbes (Group A streptococci, Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecium, Escherichia coli, different types of Salmonella, and Candida albicans) and in higher dosage antimicrobial activity was observed also against sphirochaete.77 The antimicrobial activitiy of cathelicidin in vivo has been proven in a mouse model. Mice deficient in the cathelicidin protein CRAMP, the murine equivalent to human LL-37, were more susceptible to severe infection caused by group A Streptococcus than their wildtype littermates.78
S100 proteins
S100 proteins comprise a family of low molecular weight (9–13 kDa) proteins that are characterized by the presence of calcium-binding EF-hands. Fourteen out of 21 S100 genes are located within the epidermal differentiation complex on chromosome 1.q21 and 13 S100 proteins are expressed in normal and/or diseased epidermis. Certain S100 proteins are overexpressed in skin cancer, metastasis, psoriasis, arthritis, wound healing, inflammation and cellular stress.79,80
For S100A7, S100A8 (calgranulin A), S100A9 (calgranulin B), and S100A12 a role in innate immunity has been demonstrated so far.63,81,82 For S100A15, which is highly homologous to S100A7 (93% identity), a role in innate immunity is proposed as well.83 Interestingly, all of the recent mentioned S100 proteins are highly overexpressed in psoriatic skin 26,84–87 or are elevated in serum from psoriatic patients.17
S100A7 mounts antimicrobial activity directed preferentially against Escherichia coli.63 S100A8 is regulated by LPS derived from Gram negative bacteria and interleukin (IL)-1β. Calprotectin consisting of a heterodimer of 2 individual peptide chains -migration inhibitory factor-related protein (MRP)-8 (S100A8, calgranulin A) and MRP-14 (S100A9, calgranulin B)- has been proposed to play a role in antifungal innate host defense of epithelia.82,88,89 Increased calprotectin expression is also observed in herpes simplex virus-and Epstein-Barr virus-infected oral keratinocytes 88 and antimicrobial activity of calprotection was found against Borrelia burgdorferi.90 S100A12, also called human calgranulin C, has been implicated as an important component of the host responses that limits the parasite burden during filarial nematode infections.81 Furthermore, in keratinocytes S100A7 62 as well as S100A15 have been shown to be strongly upregulated upon stimulation with high calcium and proinflammatory cytokines.91 Beside of their antimicrobial properties many S100 proteins are involved in the regulation of epidermal maturation and are highly upregulated in psoriatic skin.17,91
Conclusion
Skin innate immune defense is greatly enhanced by an antimicrobial peptide barrier that is activated when physical barriers fail to prevent a microbial attack. Under physiological conditions, low levels of antimicrobial peptides are present in the skin. In certain disease states, such as psoriasis, AMP levels are increased. AMPs which are expressed by resident keratinocytes and immunocytes, may link the two main hypotheses about psoriasis pathogenesis as either a primary keratinocyte disorder or an immunocyte-mediated chronic skin inflammatory disorder. AMPs are more than simple antibiotics, they can trigger chemotaxis, angiogenesis, and keratinocyte proliferation, which are all important features in psoriasis pathogenesis.
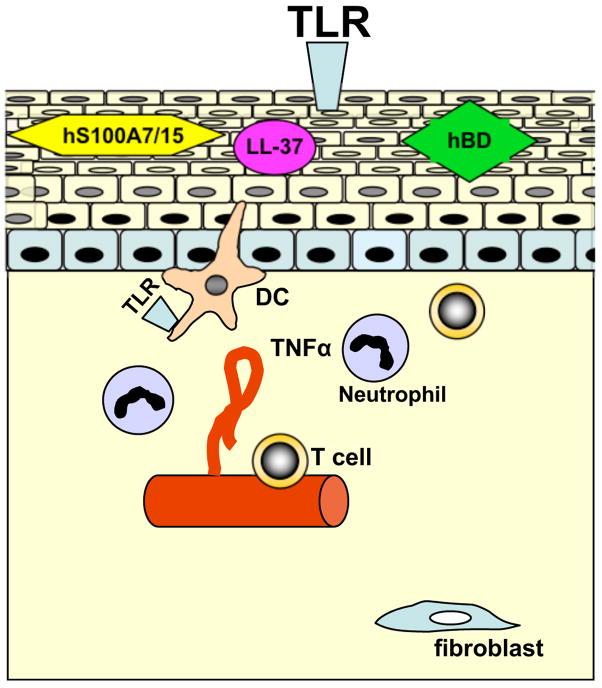
Figure 1.
Schematic summary of important innate immunity events in the pathophysiology of psoriasis. The fully developed psoriatic plaque comprises structural and molecular changes in the dermis and epidermis, that results in resistance against infections, apoptosis, and differentiation. Either triggered by an exogenous event disrupting barrier function (with or without bacterial infection) or by endogenous-derived infitration of activated immunocytes, the formation of a psoriatic lesion occurs. Toll-like receptors (TLR) may recognize pathogens and thereby orchestrate a vitious circle of innate immune response genes, including antimicrobial peptides and proteins (AMPs). Such AMPs including cathelicidin (LL-37) human beta defensins (hBD), human S100A7 (psoriasin) and human S100A15 (hS100A7/15) have not only antimicrobial activity, but also act as chemokines and can alter adaptive immune cell function, including thus of dendritic cells (DC) and T-cells. Thereby, AMPs that are expressed by resident keratinocytes as well as by immunocytes, may link the two main hypotheses of psoriasis pathogenesis discussed as a primary keratinocyte disorder or as an immunocyte-derived chronic inflammation disorder.
Footnotes
Conflict of interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113:1664–75. doi: 10.1172/JCI22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKay IA, Leigh IM. Altered keratinocyte growth and differentiation in psoriasis. Clin Dermatol. 1995;13:105–14. doi: 10.1016/0738-081x(95)93817-8. [DOI] [PubMed] [Google Scholar]
- 3.Wrone-Smith T, Mitra RS, Thompson CB, Jasty R, Castle VP, Nickoloff BJ. Keratinocytes derived from psoriatic plaques are resistant to apoptosis compared with normal skin. Am J Pathol. 1997;151:1321–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Ghadially R, Reed JT, Elias PM. Stratum corneum structure and function correlates with phenotype in psoriasis. J Invest Dermatol. 1996;107:558–64. doi: 10.1111/1523-1747.ep12582813. [DOI] [PubMed] [Google Scholar]
- 5.Blessing M, Schirmacher P, Kaiser S. Overexpression of bone morphogenetic protein-6 (BMP-6) in the epidermis of transgenic mice: inhibition or stimulation of proliferation depending on the pattern of transgene expression and formation of psoriatic lesions. J Cell Biol. 1996;135:227–39. doi: 10.1083/jcb.135.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikai K. Psoriasis and the arachidonic acid cascade. J Dermatol Sci. 1999;21:135–46. doi: 10.1016/s0923-1811(99)00042-0. [DOI] [PubMed] [Google Scholar]
- 7.Karvonen SL, Korkiamaki T, Yla-Outinen H, Nissinen M, Teerikangas H, Pummi K, et al. Psoriasis and altered calcium metabolism: downregulated capacitative calcium influx and defective calcium-mediated cell signaling in cultured psoriatic keratinocytes. J Invest Dermatol. 2000;114:693–700. doi: 10.1046/j.1523-1747.2000.00926.x. [DOI] [PubMed] [Google Scholar]
- 8.Krueger JG. The immunologic basis for the treatment of psoriasis with new biologic agents. J Am Acad Dermatol. 2002;46:1–23. doi: 10.1067/mjd.2002.120568. quiz 23–6. [DOI] [PubMed] [Google Scholar]
- 9.Kuijpers AL, Zeeuwen PL, de Jongh GJ, van de Kerkhof PC, Alkemade HA, Schalkwijk J. Skin-derived antileukoproteinase (SKALP) is decreased in pustular forms of psoriasis. A clue to the pathogenesis of pustule formation? Arch Dermatol Res. 1996;288:641–7. doi: 10.1007/BF02505272. [DOI] [PubMed] [Google Scholar]
- 10.Magert HJ, Drogemuller K, Raghunath M. Serine proteinase inhibitors in the skin: role in homeostasis and disease. Curr Protein Pept Sci. 2005;6:241–54. doi: 10.2174/1389203054065374. [DOI] [PubMed] [Google Scholar]
- 11.Moussali H, Bylaite M, Welss T, Abts HF, Ruzicka T, Walz M. Expression of hurpin, a serine proteinase inhibitor, in normal and pathological skin: overexpression and redistribution in psoriasis and cutaneous carcinomas. Exp Dermatol. 2005;14:420–8. doi: 10.1111/j.0906-6705.2005.00300.x. [DOI] [PubMed] [Google Scholar]
- 12.Nanney LB, Stoscheck CM, Magid M, King LE., Jr Altered [125I]epidermal growth factor binding and receptor distribution in psoriasis. J Invest Dermatol. 1986;86:260–5. doi: 10.1111/1523-1747.ep12285389. [DOI] [PubMed] [Google Scholar]
- 13.Staberg B, Oxholm A, Klemp P, Christiansen C. Abnormal vitamin D metabolism in patients with psoriasis. Acta Derm Venereol. 1987;67:65–8. [PubMed] [Google Scholar]
- 14.Carroll JM, Crompton T, Seery JP, Watt FM. Transgenic mice expressing IFN-gamma in the epidermis have eczema, hair hypopigmentation, and hair loss. J Invest Dermatol. 1997;108:412–22. doi: 10.1111/1523-1747.ep12289702. [DOI] [PubMed] [Google Scholar]
- 15.Porter RM, Leitgeb S, Melton DW, Swensson O, Eady RA, Magin TM. Gene targeting at the mouse cytokeratin 10 locus: severe skin fragility and changes of cytokeratin expression in the epidermis. J Cell Biol. 1996;132:925–36. doi: 10.1083/jcb.132.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–75. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 17.Benoit S, Toksoy A, Ahlmann M, Schmidt M, Sunderkötter C, Foell D, et al. Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br J Dermatol. 2006;155:62–6. doi: 10.1111/j.1365-2133.2006.07198.x. [DOI] [PubMed] [Google Scholar]
- 18.Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752–9. doi: 10.1046/j.1523-1747.1999.00749.x. [DOI] [PubMed] [Google Scholar]
- 19.Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621–32. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 20.Christophers E, Henseler T. Contrasting disease patterns in psoriasis and atopic dermatitis. Arch Dermatol Res. 1987;279 (Suppl):S48–51. doi: 10.1007/BF00585919. [DOI] [PubMed] [Google Scholar]
- 21.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–9. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 22.Harder J, Bartels J, Christophers E, Schröder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 23.Nickoloff BJ. Skin innate immune system in psoriasis: friend or foe? J Clin Invest. 1999;104:1161–4. doi: 10.1172/JCI8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 25.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 26.Harder J, Schröder JM. Psoriatic scales: a promising source for the isolation of human skin-derived antimicrobial proteins. J Leukoc Biol. 2005;77:476–86. doi: 10.1189/jlb.0704409. [DOI] [PubMed] [Google Scholar]
- 27.Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, et al. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005;174:4271–8. doi: 10.4049/jimmunol.174.7.4271. [DOI] [PubMed] [Google Scholar]
- 28.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 30.Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest. 2001;107:13–9. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kollisch G, Kalali BN, Voelcker V, Wallich R, Behrendt H, Ring J, et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–41. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mempel M, Voelcker V, Kollisch G, Plank C, Rad R, Gerhard M, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J Invest Dermatol. 2003;121:1389–96. doi: 10.1111/j.1523-1747.2003.12630.x. [DOI] [PubMed] [Google Scholar]
- 33.Gallo RL, Nizet V. Endogenous production of antimicrobial peptides in innate immunity and human disease. Curr Allergy Asthma Rep. 2003;3:402–9. doi: 10.1007/s11882-003-0074-x. [DOI] [PubMed] [Google Scholar]
- 34.Hancock RE, Scott MG. The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A. 2000;97:8856–61. doi: 10.1073/pnas.97.16.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson KV, Nüsslein-Volhard C. Information for the dorsal--ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature. 1984;311:223–7. doi: 10.1038/311223a0. [DOI] [PubMed] [Google Scholar]
- 36.Gay NJ, Keith FJ. Drosophila Toll and IL-1 receptor. Nature. 1991;351:355–6. doi: 10.1038/351355b0. [DOI] [PubMed] [Google Scholar]
- 37.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–63. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 38.Lauw FN, Caffrey DR, Golenbock DT. Of mice and man: TLR11 (finally) finds profilin. Trends Immunol. 2005;26:509–11. doi: 10.1016/j.it.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–84. doi: 10.1074/jbc.M310859200. [DOI] [PubMed] [Google Scholar]
- 41.Cohen Tervaert WC, Esseveld H. The occurrence of beta-hemolytic streptococci in the throat of patients with psoriasis vulgaris; observations on their significance in the pathogenesis of this disease. Ned Tijdschr Geneeskd. 1969;113:2215–8. [PubMed] [Google Scholar]
- 42.Whyte HJ, Baughman RD. Acute Guttate Psoriasis and Streptococcal Infection. Arch Dermatol. 1964;89:350–6. doi: 10.1001/archderm.1964.01590270036008. [DOI] [PubMed] [Google Scholar]
- 43.Kanda N, Tani K, Enomoto U, Nakai K, Watanabe S. The skin fungus-induced Th1- and Th2-related cytokine, chemokine and prostaglandin E2 production in peripheral blood mononuclear cells from patients with atopic dermatitis and psoriasis vulgaris. Clin Exp Allergy. 2002;32:1243–50. doi: 10.1046/j.1365-2745.2002.01459.x. [DOI] [PubMed] [Google Scholar]
- 44.Leung DY, Hauk P, Strickland I, Travers JB, Norris DA. The role of superantigens in human diseases: therapeutic implications for the treatment of skin diseases. Br J Dermatol. 1998;139 (Suppl 53):17–29. doi: 10.1046/j.1365-2133.1998.1390s3017.x. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt A. Malassezia furfur: a fungus belonging to the physiological skin flora and its relevance in skin disorders. Cutis. 1997;59:21–4. [PubMed] [Google Scholar]
- 46.Baker BS, Ovigne JM, Powles AV, Corcoran S, Fry L. Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br J Dermatol. 2003;148:670–9. doi: 10.1046/j.1365-2133.2003.05287.x. [DOI] [PubMed] [Google Scholar]
- 47.Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DY. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol. 2003;112:1195–202. doi: 10.1016/j.jaci.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 48.Song PI, Park YM, Abraham T, Harten B, Zivony A, Neparidze N, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. J Invest Dermatol. 2002;119:424–32. doi: 10.1046/j.1523-1747.2002.01847.x. [DOI] [PubMed] [Google Scholar]
- 49.Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J Dermatol Sci. 2002;30:185–94. doi: 10.1016/s0923-1811(02)00105-6. [DOI] [PubMed] [Google Scholar]
- 50.Pivarcsi A, Bodai L, Rethi B, Kenderessy-Szabo A, Koreck A, Szell M, et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15:721–30. doi: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- 51.Gilliet M, Conrad C, Geiges M, Cozzio A, Thurlimann W, Burg G, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490–5. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- 52.Reiter MJ, Testerman TL, Miller RL, Weeks CE, Tomai MA. Cytokine induction in mice by the immunomodulator imiquimod. J Leukoc Biol. 1994;55:234–40. doi: 10.1002/jlb.55.2.234. [DOI] [PubMed] [Google Scholar]
- 53.Oren A, Taylor JM. The subcellular localization of defensins and myeloperoxidase in human neutrophils: immunocytochemical evidence for azurophil granule heterogeneity. J Lab Clin Med. 1995;125:340–7. [PubMed] [Google Scholar]
- 54.Fellermann K, Stange EF. Defensins -- innate immunity at the epithelial frontier. Eur J Gastroenterol Hepatol. 2001;13:771–6. doi: 10.1097/00042737-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Ong PY, Leung DY. Atopic dermatitis. Clin Allergy Immunol. 2002;16:355–79. [PubMed] [Google Scholar]
- 56.Yang D, Chertov O, Oppenheim JJ. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J Leukoc Biol. 2001;69:691–7. [PubMed] [Google Scholar]
- 57.Barak O, Treat JR, James WD. Antimicrobial peptides: effectors of innate immunity in the skin. Adv Dermatol. 2005;21:357–74. doi: 10.1016/j.yadr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 58.Shaykhiev R, Beisswenger C, Kandler K, Senske J, Puchner A, Damm T, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L842–8. doi: 10.1152/ajplung.00286.2004. [DOI] [PubMed] [Google Scholar]
- 59.Wang T-T, Nestel F, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 60.Schauber J, Dorschner R, Yamasaki K, Brouha B, Gallo R. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pernet I, Reymermier C, Guezennec A, Branka JE, Guesnet J, Perrier E, et al. Calcium triggers beta-defensin (hBD-2 and hBD-3) and chemokine macrophage inflammatory protein-3 alpha (MIP-3alpha/CCL20) expression in monolayers of activated human keratinocytes. Exp Dermatol. 2003;12:755–60. doi: 10.1111/j.0906-6705.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 62.Martinsson H, Yhr M, Enerback C. Expression patterns of S100A7 (psoriasin) and S100A9 (calgranulin-B) in keratinocyte differentiation. Exp Dermatol. 2005;14:161–8. doi: 10.1111/j.0906-6705.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 63.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schröder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J, et al. Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol. 2002;3:583–90. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 65.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–22. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 66.Harder J, Bartels J, Christophers E, Schröder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 67.Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schröder JM. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004;123:522–9. doi: 10.1111/j.0022-202X.2004.23234.x. [DOI] [PubMed] [Google Scholar]
- 68.Chung WO, Dale BA. Innate immune response of oral and foreskin keratinocytes: utilization of different signaling pathways by various bacterial species. Infect Immun. 2004;72:352–8. doi: 10.1128/IAI.72.1.352-358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, et al. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002;118:275–81. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 70.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–93. [PubMed] [Google Scholar]
- 71.Frohm M, Gunne H, Bergman AC, Agerberth B, Bergman T, Boman A, et al. Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem. 1996;237:86–92. doi: 10.1111/j.1432-1033.1996.0086n.x. [DOI] [PubMed] [Google Scholar]
- 72.Agerberth B, Grunewald J, Castanos-Velez E, Olsson B, Jornvall H, Wigzell H, et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med. 1999;160:283–90. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
- 73.Marchini G, Lindow S, Brismar H, Stabi B, Berggren V, Ulfgren AK, et al. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol. 2002;147:1127–34. doi: 10.1046/j.1365-2133.2002.05014.x. [DOI] [PubMed] [Google Scholar]
- 74.Ong PY, Hamid QA, Travers JB, Strickland I, Al Kerithy M, Boguniewicz M, et al. Decreased IL-15 may contribute to elevated IgE and acute inflammation in atopic dermatitis. J Immunol. 2002;168:505–10. doi: 10.4049/jimmunol.168.1.505. [DOI] [PubMed] [Google Scholar]
- 75.Gombart AF, Luong QT, Koeffler HP. Vitamin D compounds: activity against microbes and cancer. Anticancer Res. 2006;26:2531–42. [PubMed] [Google Scholar]
- 76.Bikle DD. Vitamin D and skin cancer. J Nutr. 2004;134:3472S–3478S. doi: 10.1093/jn/134.12.3472S. [DOI] [PubMed] [Google Scholar]
- 77.Dürr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–25. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 78.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 79.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–68. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 80.Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. J Invest Dermatol. 2004;123:23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]
- 81.Gottsch JD, Eisinger SW, Liu SH, Scott AL. Calgranulin C has filariacidal and filariastatic activity. Infect Immun. 1999;67:6631–6. doi: 10.1128/iai.67.12.6631-6636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sohnle PG, Hunter MJ, Hahn B, Chazin WJ. Zinc-reversible antimicrobial activity of recombinant calprotectin (migration inhibitory factor-related proteins 8 and 14) J Infect Dis. 2000;182:1272–5. doi: 10.1086/315810. [DOI] [PubMed] [Google Scholar]
- 83.Büchau AS, Kukova G, Lewerenz V, Wolf R, Ruzicka T, Walz M. Human S100A15 is regulated by calcium, cytokines, and bacterial compounds. J Invest Dermatol. 2006;126(supplement 4):69. [Google Scholar]
- 84.Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem. 2003;51:675–85. doi: 10.1177/002215540305100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mirmohammadsadegh A, Tschakarjan E, Ljoljic A, Bohner K, Michel G, Ruzicka T, et al. Calgranulin C is overexpressed in lesional psoriasis. J Invest Dermatol. 2000;114:1207–8. doi: 10.1046/j.1523-1747.2000.00005-2.x. [DOI] [PubMed] [Google Scholar]
- 86.Semprini S, Capon F, Tacconelli A, Giardina E, Orecchia A, Mingarelli R, et al. Evidence for differential S100 gene over-expression in psoriatic patients from genetically heterogeneous pedigrees. Hum Genet. 2002;111:310–3. doi: 10.1007/s00439-002-0812-5. [DOI] [PubMed] [Google Scholar]
- 87.Wolf R, Mirmohammadsadegh A, Walz M, Lysa B, Tartler U, Remus R, et al. Molecular cloning and characterization of alternatively spliced mRNA isoforms from psoriatic skin encoding a novel member of the S100 family. Faseb J. 2003;17:1969–71. doi: 10.1096/fj.03-0148fje. [DOI] [PubMed] [Google Scholar]
- 88.Eversole LR, Miyasaki KT, Christensen RE. Keratinocyte expression of calprotectin in oral inflammatory mucosal diseases. J Oral Pathol Med. 1993;22:303–7. doi: 10.1111/j.1600-0714.1993.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 89.Steinbakk M, Naess-Andresen CF, Lingaas E, Dale I, Brandtzaeg P, Fagerhol MK. Antimicrobial actions of calcium binding leucocyte L1 protein, calprotectin. Lancet. 1990;336:763–5. doi: 10.1016/0140-6736(90)93237-j. [DOI] [PubMed] [Google Scholar]
- 90.Lusitani D, Malawista SE, Montgomery RR. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect Immun. 2003;71:4711–6. doi: 10.1128/IAI.71.8.4711-4716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolf R, Voscopoulos CJ, FitzGerald PC, Goldsmith P, Cataisson C, Gunsior M, et al. The mouse S100A15 ortholog parallels genomic organization, structure, gene expression, and protein-processing pattern of the human S100A7/A15 subfamily during epidermal maturation. J Invest Dermatol. 2006;126:1600–8. doi: 10.1038/sj.jid.5700210. [DOI] [PubMed] [Google Scholar]
- 92.Harder J, Schröder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;227:46779–84. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- 93.Chen VL, france DS, Martinelli GP. De novo synthesis of lysozyme by human epidermal cells. J Invest Dermatol. 1986;87:585–7. doi: 10.1111/1523-1747.ep12455834. [DOI] [PubMed] [Google Scholar]