Abstract
Context
There now exist multiple reports of a constellation of language, personality, and social-behavioral features present among relatives that mirror the symptom domains of autism, but much milder in expression. Studies of this ‘broad autism phenotype’ (BAP) may provide a potentially important, complementary approach for detecting the genes causing autism and defining associated neural circuitry, by identifying more refined phenotypes which can be measured quantitatively in both affected and unaffected individuals, and which are tied to functioning in particular regions of the brain.
Objective
To gain insights into neuropsychological features that index genetic liability to autism.
Design
Case-control.
Setting
General community.
Participants
Thirty-eight high-functioning individuals with autism and parents of autistic individuals, both with and without the BAP (N=83), as well as control groups.
Main Outcome Measures
A comprehensive battery of neuropsychological tasks tapping social cognition, executive function, and global/local processing strategies (central coherence).
Results
Both individuals with autism and parents with the BAP differed from controls on measures of social cognition, with performance in the other two domains more similar to controls.
Conclusions
Data suggest that the social cognitive domain may be an important target for linking phenotype to cognitive process to brain structure in autism, and may ultimately provide insights into genes involved in autism.
Autism is a severe, life-long developmental disorder that compromises functioning across multiple domains, including social behavior, language, sensory function, and ritualistic/repetitive behaviors and interests. While the etiology of autism is complex and not fully understood, strong evidence from twin and family studies points toward a large genetic contribution, with heritability estimates as high as 90% (1, 2). Twin and family studies have also shown that genetic liability appears to be expressed among unaffected relatives of people with autism through features that are milder, but qualitatively similar, to the defining characteristics of autism.
The first observation of such subclinical traits can be credited to child psychiatrist Leo Kanner, whose original narrative descriptions of autism also noted among relatives a strong preoccupation with “abstractions of a scientific, literary, or artistic nature, and limited in genuine interest in people.” (1943, p. 250). A decade later, Leon Eisenberg further described relatives, and fathers in particular, as “perfectionistic to an extreme … pre-occupied with detailed minutiae to the exclusion of concern for over-all meanings” (1957, p. 721). These early observations were unfortunately misinterpreted as evidence faulting parents’ behaviors in the etiology of autism, and it would be another 20 years before this myth would be dispelled by the landmark twin study of Folstein and Rutter (3).
This study not only detected markedly higher concordance rates of autism among monozygotic twins than dizygotic, but also found even higher MZ concordance for a more broadly defined phenotype including subclinical language and cognitive features. Family studies following up on these striking findings have repeatedly confirmed the presence of such subclinical phenotypes among relatives, now referred to as constituting a ‘broad autism phenotype’ or ‘BAP’. The features of the BAP closely parallel the core symptom domains in autism (i.e., impaired social functioning, language and communication deficits, and restricted/repetitive interests, respectively), yet are subtle in expression and not usually associated with any functional impairment (4). Importantly, whereas by definition autism involves impairment across all three symptom domains, evidence suggests that these features may decouple and segregate independently in unaffected (with autism) relatives (5–7), consistent with the observation that they appear to be uncorrelated in neurotypical populations (8). Studies of relatives may therefore help to disentangle the complex autistic phenotype to identify component traits more amenable to neurocognitive and genetic dissection than the full clinical syndrome.
The present study is an attempt to inform the neuropsychological basis of autism and the BAP via detailed neuropsychological assessment of high-functioning individuals with autism and parents of autistic individuals (both with and without the BAP). We investigate performance within the three principal neuropsychological domains that have each been proposed as key cognitive abilities in which impairments may explain the autistic phenotype -- social cognition, central coherence and executive function. Autistic individuals’ impairments in each of these domains have been reported [for reviews see (8, 9)], and an emerging literature has begun to document parallel performance patterns among unaffected relatives (10–14).
We assessed performance using a battery of tasks selected to assess processing comprehensively within a given domain, and chose our battery with an eye towards links to studies of subjects with circumscribed neurological lesions that could shed light on specific neural structures that are involved in autism, and potentially the BAP. For instance, several of our social cognition tasks had been shown to tap amygdala function, a structure that has also been hypothesized to play a role in autism (15–17). We focused on high-functioning adults with autism such that an identical battery of tasks might be administered to both the parent and autistic groups, thus affording direct comparisons relative to respective control groups.
To summarize, the goals of this study were 1) to provide an in-depth characterization of the neuropsychological profile of autism and the BAP; 2) to identify patterns of performance within and across neuropsychological domains which were common to both autistic individuals and parents; and 3) to identify cosegregation between clinical phenotype and neuropsychological functioning that might serve to carve out specific phenotypic subtypes and that could form the basis for stronger genotype-phenotype associations.
Methods
Participants
Participants included 36 high-functioning individuals with autism, 41 autism controls (neurotypical individuals with no family history of autism), 83 autism parents, and 32 parent controls (i.e., neurotypical parents with no family history of autism or developmental delays). Intact nuclear families were targeted to afford analysis of parent-child correlations. Twenty-two intact families were successfully recruited (i.e., 44 parents and their high-functioning autistic child). The remaining families included either a single parent and their autistic child (n=15), or only parents (n=24) who participated without their child. Control groups (both parent and autism controls) were recruited through local advertisement, and were screened to be similar in demographic characteristics to the autism and autism-parent groups (see Table 1).
Table 1.
Demographic Characteristic
| Autism/Control Groups | Parent Groups | |||
|---|---|---|---|---|
| Autism | Controls | Autism Parents | Controls | |
| N | 36 | 41 | 83 | 32 |
| Sex | ||||
| (M:F) | 29:7 | 34:7 | 37:44 | 13:19 |
| Age | ||||
| ¯x (sd) | 21.5 (5.5) | 23.4 (5.6) | 46.6 (6.7) | 46.7 (7.5) |
| Full Scale IQ | ||||
| ¯x (sd) | 101.2 (18.1) | 108.3 (15.0) | 117.5 (11.2) | 121.2 (10.8) |
| Mean education | ||||
| level (in grades) | 11.2 | 11.7 | 13.8 | 13.4 |
| Race | ||||
| White | 86% | 86% | 85% | 76% |
| Other | 3% | 14% | 2% | 3% |
| Not reported | 11% | -- | 13% | 21% |
Note: Race was self-reported and collected for purposes of reporting sample characteristics to NIH.
To ensure that all participants were capable of completing the same battery of tasks, only autistic individuals >16 years of age, with performance IQ ≥ 80 on the Wechsler Abbreviated Scale of Intelligence (18) were included. The Autism Diagnostic Interview, Revised (19) and the Autism Diagnostic Observational Schedule, Revised (20), were administered to all participants. Clinical diagnoses were assigned according to current DSM-IV criteria (21). All participants gave informed consent to participate in accordance with a protocol approved by the Institutional Review Board of the University of North Carolina.
Clinical Assessment of the Broad Autism Phenotype
Characteristics of the BAP were assessed through clinically based interviews using the Modified Personality Assessment Schedule-Revised, or MPAS-R. This instrument has been used in a number of studies to define key features of the BAP (5, 6, 22). Interviewers guide participants through a number of questions to probe personality characteristics and dispositions relevant to autism and the BAP, namely, rigid or perfectionistic personality, and socially aloof or untactful personality. These traits are thought to parallel the ritualistic/repetitive and social symptom domains of autism, respectively. Such features have been shown to reliably distinguish autism relatives from controls (5).
Ratings were assigned by two independent raters, by combining information from the subject and informant (typically a spouse) interviews according to specified rules. Ratings are based on behavioral examples given by the subject and/or informants in response to a number of probes. Characteristics are rated either as present (2), absent (0) or unknown (1). BAP (+) status was assigned for ratings of ‘present’ (2), and BAP (−) status assigned for scores of 0 or 1 (absent, unknown). Readers are referred elsewhere (5, 6) for additional details on rating procedures. Inter-rater agreement exceeded 85% for all BAP personality traits. Disagreements were resolved by a third independent rater. Control parents were included as a population control for contrasts with parents of autistic individuals and were not administered the MPAS.
Neuropsychological Measures
Measures of social cognition, executive function, and central coherence were selected based on 1) their theoretical and empirical ties to the neuropsychological domains of interest; 2) psychometric properties, including reliability and indication that each was suitable for individuals with autism, parents, and controls without danger of floor or ceiling effects; and 3) evidence from fMRI and/or lesion studies suggesting associations with particular structures or regions of the brain thought to be important for the three domains and putatively implicated also in autism (23, 24). Tasks are described below.
Social cognition
These tasks have been described in detail elsewhere (citations provided below) and so are reviewed briefly below.
The Reading the Mind in the Eyes Task (10), involves inferring psychological states from viewing slides depicting only the eye region of faces. Participants choose one of four responses provided and the proportion of correct answers serves as the index of performance. Impaired performance on this task has been reported in individuals with autism and their relatives (10, 11, 25, 26) and has been tied to the amygdala, medial prefrontal cortex, and superior temporal gyrus (27–30).
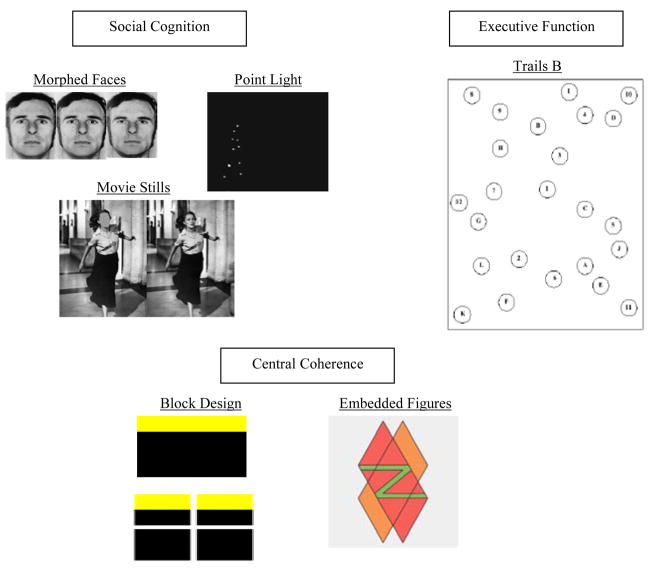
Morphed Faces Task assesses the ability to identify emotions from faint facial expressions generated by ‘morphing’, or linearly modifying, a neutral facial expression into progressively prototypical expressions of happiness, sadness, and fear. Thus, low-morphed stimuli are those closest to ‘neutral’, and therefore displaying the most subtle degree of emotional expression (see Figure 1). Scoring is based on performance relative to norms (32). Original stimuli are from the Ekman faces (31). Lesion studies implicate amygdala function in performance on the morphed faces task (32).
Trustworthiness of Faces (33, 34) involves assessing the trustworthiness of faces differing in emotional expression, gender, gaze direction, etc. Scoring was based on normative data (see 44). Lesion and imaging studies indicate that this task taps amygdala function (35).
The Movie Stills Task (36) measures one’s reliance on facial information to discern the emotional content of complex scenes. The accuracy of subjects’ emotional judgments (based on norms) is compared when still movie scenes are presented with faces digitally erased vs. when faces are intact. The basic emotions of fear, anger, and sadness are assessed. Adolphs & Tranel (36) demonstrated that individuals with bilateral amygdala damage fail to benefit from the presentation of facial information in this task, showing similar accuracy across the masked and unmasked faces trials.
The Point Light Task (37) assesses the ability to make emotional attributions from biological motion stimuli. Participants are shown visual displays of locomotion from the movements of light-emitting diodes attached to the main joints of moving people, and asked to judge the emotion best conveyed by each display. Both basic emotions and complex judgments of trustworthiness are assessed. Accuracy was evaluated based on normative data (42). Lesion and fMRI studies indicate that processing of basic emotions from biological motion may tap the right somatosensory cortex and superior temporal sulcus (38), whereas judging complex emotions or personality traits such as trustworthiness appears to rely on the medial prefrontal cortex (37, 39).1
Figure 1.
Examples of neurocognitive tasks.
Executive Function
We assessed the executive skills of planning, set shifting and cognitive control through two well established tasks: the Tower of Hanoi and the Trailmaking Task. These tasks are believed to tap prefrontal cortical regions (40, 41).
The Tower of Hanoi involves planning a sequence of moves that transfer an initial configuration of rings onto a particular peg, abiding by certain rules. Impairments on this task have been observed in autism and among relatives of autistic individuals; although very high-functioning individuals have also been reported to perform exceptionally well (42).
The Trailmaking Task, is a measure of set shifting and cognitive control of interference in which sequences of numbers and letters must be alternately used to trace to an end point as quickly as possible. This measure is widely employed in brain injury/lesion studies (e.g., see metanalytic review by Morgan et al. (43)). As in typical neuropsychological evaluations, we also included a more simplistic version (Trails A) which does not involve set shifting, but instead only tracing of letters in a sequence without alternation involved. Analyses examine the difference between the set shifting (Trails B) and the basic (Trails A) tasks.
Weak Central Coherence, is a theory concerning the detail-focused cognitive style prevalent among individuals with autism, assessed through tasks which index the salience of parts over wholes, and relative insignificance of overall gestalt. Global, integrative processing skills are predominantly mediated by the right hemispheric areas (44–46), whereas local featural processing is associated with left hemisphere functioning (46).
The Embedded Figures Test (47) involves identifying simple figures embedded within a complex design. Performance is assessed for accuracy and time. Individuals with autism and their relatives have been shown to complete this task in shorter time than controls, which is believed to index their local processing bias (12).
The Sentence Completion Task (14), pits demands for global sentence meaning against the tendency to give locally cued associative responses by requiring participants to complete a number of sentence stems (e.g., “The sea tastes of salt and ____”), and examining responses for local (e.g., “pepper”) quality, which are globally meaningless in the whole sentence context, or global, semantically meaningful sentence completions (e.g., “water”). Responses are scored as either global, local, or other non-global errors (e.g., semantically inappropriate responses were sometimes given by participants). Prior work has documented a tendency for individuals with autism to more often respond with local sentence completions (14).
The Block Design Task (14, 48) assesses reliance on the relative salience of parts over wholes, and relative insignificance of a design’s overall gestalt. Participants’ task is to construct a series of designs using blocks based on a model that is either presented complete or presegmented, with the latter condition breaking the gestalt and rendering processing of figure-parts easier. Unlike controls, individuals with autism tend to perform comparably across the two conditions (48) and similar response styles have been documented among fathers of autistic individuals (14).
Analysis plan
For each domain we examined differences between individuals with autism vs. autism controls, and differences between autism parents and parent controls. Within the autism parent group, specific comparisons were conducted to investigate differences between individuals with the BAP and those without. We hypothesized that specific BAP traits would co-segregate with performance in conceptually similar neuropsychological domains (e.g., parents displaying social BAP features would demonstrate differences on social cognitive tasks, whereas those who exhibited the rigid/perfectionistic personality traits were hypothesized to show differences on tasks of executive skills, which involve cognitive flexibility). Within the autistic group, where both rigid/ritualistic behavior and social impairment are defining features, we expected that performance would be impaired across all domains: social cognition, executive function, and central coherence.
Analyses were performed using SAS 9.1. Descriptive statistics were performed to assess raw data distributions for normality. General linear models were used to examine group differences for each outcome2. All possible interactions between groups and the within subject variables were included in the models, as were interactions with age, sex, and IQ. To help control type I error rates, interactions were not interpreted unless significant at p <.05. Where interactions were not significant, only main effects are reported. In cases where interactions with sex were detected, separate estimates and tests of group differences for males and females were calculated using post-estimation procedures. To ensure specificity of findings related to BAP status, all BAP features (social and rigid/perfectionistic) were included in the models.
Results
Frequency of BAP traits among autism parents
Twenty-two autism parents were identified as positive for social BAP features,34 rated positive for the rigid/pefectionistic BAP traits, and 40 showed neither characteristic, and are referred to as BAP (−). There were 5 mothers and 8 fathers who displayed both rigid and social BAP traits. Whereas fathers more often showed features of the social BAP (41% of Fathers vs. only 16% of Mothers; χ2 = 6.75, p = .009), rigid/perfectionistic BAP traits were observed in roughly equal numbers of fathers and mothers(43% of fathers vs. 41% of mothers; χ2 = .14, p = .70). As noted in Analyses, above, findings related to BAP status reported below control for the co-occurrence of the BAP features.
Performance across Neuropsychological Domains
Table 2 summarizes findings across domains and specific patterns are described below by domain. For each task, results are first presented for autism vs. control comparisons, followed by parent group comparisons (social or rigid/perfectionistic BAP (+) vs. BAP (−) vs. controls) . As noted previously, parent comparisons examined the specific hypothesis that performance would vary by BAP status. Thus, findings are reported for BAP (+) vs. BAP (−) vs. controls. Adjusted mean differences, standard errors, and p values are presented in Tables 3 (social cognition) and 4 (executive functioning and central coherence). For measures of social cognition, all tasks except the Eyes Test were analyzed as z-scored differences from norms, resulting in mean differences in standard deviations (i.e., a mean difference of ‘1.0’ reflects a full standard deviation difference between groups).
Table 2.
Summary of Findings
| Autism Parents | ||
|---|---|---|
| Social Cognition | ||
| Eyes Task | ** | |
| Morphed Faces | ** | |
| Trustworthiness of Faces | ** | |
| Movie Stills | ** | |
| Point Light Basic Emotions | *- | |
| Point Light Trustworthiness | ** | |
| Executive Function | ||
| Tower of Hanoi | - | - |
| Trailmaking Test | - | - |
| Central Coherence | ||
| Embedded Figures | - | - |
| Sentence Completion | * | - |
| Block Design | - | - |
Notes:
All parent findings for measures of social cognition were detected in the social BAP (+) group only (i.e., no differences between BAP (−) and control groups were detected).
No associations with BAP status were detected for any measures of executive function or central coherence, nor was performance related to parent status in general (i.e., autism vs. control).
denotes predicted difference detected at p < .05
Table 3.
Mean differences on measures of social cognition
| Autism/Control Groups |
Parent Groups1 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AU vs Control | BAP (+) vs BAP (−) | BAP (+) vs Controls | BAP (−) vs Controls | |||||||||
| Mean Difference |
SE | p | Mean Difference |
SE | p | Mean Difference |
SE | p | Mean Difference |
SE | p | |
| Eyes Task | −6.71 | 2.71 | 0.016 | −8.99 | 2.29 | 0.000 | −8.82 | 2.43 | 0.001 | 0.17 | 1.84 | 0.925 |
| Morphed Faces | ||||||||||||
| Happy | 0.37 | 0.26 | 0.158 | |||||||||
| Low morphedness | −0.18 | 0.29 | 0.532 | −0.31 | 0.31 | 0.308 | −0.13 | 0.24 | 0.584 | |||
| High morphedness | 0.08 | 0.41 | 0.840 | 0.09 | 0.43 | 0.826 | 0.01 | 0.34 | 0.971 | |||
| Sad | −0.22 | 0.25 | 0.380 | |||||||||
| Low morphedness | −0.62 | 0.36 | 0.085 | −0.71 | 0.38 | 0.065 | −0.09 | 0.30 | 0.773 | |||
| High morphedness | 0.23 | 0.43 | 0.594 | 0.17 | 0.45 | 0.706 | −0.06 | 0.36 | 0.873 | |||
| Afraid | −0.79 | 0.30 | 0.008 | |||||||||
| Low morphedness | −0.77 | 0.36 | 0.034 | −0.74 | 0.37 | 0.050 | 0.02 | 0.29 | 0.939 | |||
| High morphedness | 0.34 | 0.45 | 0.451 | −0.22 | 0.47 | 0.636 | −0.56 | 0.37 | 0.128 | |||
| Trustworthiness of Faces | ||||||||||||
| Positive faces | 0.56 | 0.16 | <.001 | −0.19 | 0.14 | 0.190 | −0.30 | 0.15 | 0.045 | −0.12 | 0.11 | 0.296 |
| Negative faces | −0.19 | 0.16 | 0.240 | −0.65 | 0.14 | <.0001 | −0.67 | 0.15 | <.0001 | −0.02 | 0.11 | 0.824 |
| Movie Stills | ||||||||||||
| Without faces | −0.04 | 0.03 | 0.206 | 0.01 | 0.03 | 0.788 | 0.02 | 0.03 | 0.482 | 0.01 | 0.02 | 0.568 |
| Sad | −0.15 | 0.06 | 0.014 | |||||||||
| Angry | −0.11 | 0.05 | 0.047 | |||||||||
| Afraid | 0.13 | 0.04 | 0.001 | |||||||||
| With faces | −0.06 | 0.03 | 0.052 | −0.08 | 0.03 | 0.013 | −0.07 | 0.03 | 0.032 | 0.01 | 0.02 | 0.774 |
| Sad | −0.09 | 0.06 | 0.127 | |||||||||
| Angry | −0.13 | 0.05 | 0.010 | |||||||||
| Afraid | 0.03 | 0.04 | 0.395 | |||||||||
| Point Light Basic Emotions | −0.03 | 0.02 | 0.106 | −0.01 | 0.02 | 0.631 | 0.02 | 0.02 | 0.169 | |||
| Positive emotions | −0.10 | 0.03 | 0.003 | |||||||||
| Negative emotions | −0.04 | 0.03 | 0.168 | |||||||||
| Point Light Trustworthiness | ||||||||||||
| Positive stimuli | −0.43 | 0.14 | 0.004 | −0.37 | 0.14 | 0.008 | −0.28 | 0.14 | 0.053 | 0.09 | 0.11 | 0.438 |
| Negative stimuli | 0.12 | 0.16 | 0.475 | 0.35 | 0.15 | 0.022 | 0.26 | 0.16 | 0.100 | −0.09 | 0.12 | 0.485 |
Notes: Scores for the Eyes Test represent difference in % correct. Scores for all other measures represent z-scored differences from norms. Mean difference scores are adjusted for covariates of age, sex, and IQ. Means are presented for primary components of each task unless significant interactions were detected. Significant differences appear in blue.
BAP (+) defined by social traits measured on the MPAS-R
Though not described below in the interest of space, we note here that parent-child correlations were examined but did not reveal significant associations across domains or specific tasks.
Social Cognition
The Reading the Mind in the Eyes Test
Autism
The autistic group performed significantly less accurately than controls (see Table 3).
Parents
Significant differences were also detected among parents, but these differences were only present among those parents with the social BAP (hereafter referred to as BAP (+)). The BAP (+) group was less accurate than controls and BAP (−) parents. There were no significant differences between controls and BAP (−) parents or parents showing the rigid feature of the BAP. A significant effect of sex was detected among parents, revealing that differences were more profound among BAP (+) mothers than fathers.
Morphed Faces
Autism
The autistic group was less accurate at identifying fearful expressions across all variations of expression intensity, both high and low morphedness (i.e., faces closer to prototypical facial expression and therefore presumably easier to decipher, as well as those closest to neutral/faintly expressed). No differences were observed for happy or sad faces.
Parents
Analysis revealed significantly lower accuracy identifying fearful faces among the social BAP (+) group, but only when expressions were most faintly expressed. That is, at low levels of morphedness parents in the social BAP (+) group were significantly less accurate in identifying fearful expressions than the other parent groups (controls, BAP (−), rigid BAP). As observed in the autistic group, no significant differences were observed for happy or sad stimuli.
Trustworthiness of faces
Autism
The autistic group differed from controls in judging the trustworthiness of faces. Individuals with autism differed from norms significantly more than controls only on the negatively valenced slides (i.e., on ‘unfriendly’ stimuli). Relative to controls, individuals with autism significantly over-rated the trustworthiness of negatively valenced faces (i.e., they rated ‘unfriendly’ faces as overly friendly).
Parents
The social BAP (+) group differed from norms significantly more than controls and BAP (−) parents on the positively valenced stimuli, where they rated ‘friendly’ faces as significantly less trustworthy than the other parent groups. In other words, relative to the other groups’ ratings, they rated these faces as somewhat threatening. No significant differences were detected between the other parent groups.
Movie stills
Autism
The autistic group differed significantly from controls across all emotions, though patterns differed for each condition, faces omitted vs. faces present. When stimuli were shown without faces, the autistic group differed from controls on all emotions – they were less accurate identifying sadness and anger, and better than controls at identifying fearful scenes. When faces were present, individuals with autism were less accurate identifying anger, and no longer showed any advantage identifying fear, suggesting they benefited less from viewing facial information than did controls.
Parents
Findings among parents were consistent with this pattern – across all emotions, BAP (+) parents were significantly less accurate than controls and BAP (−) groups only when faces were present. Thus, whereas controls and parents without the social BAP became more accurate in judging emotions when facial expressions were revealed, parents with the social BAP did not show this increase in accuracy, suggesting they were less apt to take advantage of facial information when judging the emotional content of complex scenes. BAP (−) and control groups performed similarly to each other.
Point light displays
Autism
In the basic emotion condition, individuals with autism were significantly less accurate at identifying positive emotions from point light displays. Differences were also observed in the more complex emotion condition involving judgments of trustworthiness. The autistic group showed less sensitivity to positively valenced stimuli, failing to modulate their ratings as stimuli became increasingly positive.
Parents
Parent groups showed no differences in their ratings of the basic emotions, but the social BAP (+) group exhibited differences in judgments of trustworthiness for both positively and negatively valenced slides. Whereas controls and parents without the social BAP judged positively valenced slides as trustworthyand negative displays less so, the social BAP (+) group appeared relatively insensitive to variations in positive and negative valence, rating these stimuli as ‘neutral’.
Executive Function
Tower of Hanoi
Autism
There were no significant differences in time or number of moves required to complete the correct tower configuration.
Parents
No significant differences were observed among parent groups. Parents with the rigid/perfectionistic traits of the BAP parents performed similarly to BAP (−) and control parents. Parents who displayed the social BAP also performed comparably to BAP (−) and control groups.
Trailmaking
Autism
There was no significant difference between the autistic and control groups in time to complete the trailmaking task.
Parents
Parents of autistic individuals performed comparably to controls, and there were no significant differences associated with BAP status (rigid/perfectionistic or social BAP).
Central Coherence
Embedded Figures
Autism
There were no significant differences between autistic and control groups on either the mean time to completion or the likelihood of arriving at a correct answer.
Parents
The autism parent group performed comparably to controls on time and accuracy, and there were no associations with any BAP features.
Sentence Completion Task
Autism
Significant differences were detected in the frequency of errors and time to completion – the autistic group produced more non-global responses (i.e., local and other non-global completions) and took longer to respond than controls.
Parents
Parent group comparisons revealed no differences in response type, but autism parents (both rigid/perfectionistic BAP (+) and BAP (−)) responded significantly faster than controls.
Block Design
Autism
The autistic group took significantly longer than controls to complete designs when blocks were segmented. No significant difference was observed when blocks were presented as a whole.
Parents
There were no significant differences between parent groups, and no associations with any BAP features.
Discussion
Summary of findings
Results suggest that measures of social cognition most robustly differentiate performance of individuals with autism and parents with the social BAP from controls and BAP (−) parents. As illustrated in Table 2, on five of the six social cognitive measures differences were observed in both individuals with autism and parents with the social BAP, and the autistic group demonstrated differences from controls on all six tasks. By contrast, measures of executive function and central coherence did not differentiate groups as clearly. These findings both validate the concept of the social BAP and highlight the potential utility of neuropsychological measures of social cognition in studies of the brain and gene basis of autism. Importantly, parents with the social BAP are not impaired clinically, yet show neurocognitive patterns similar to those observed in autism.
Our data suggest that specific social cognitive profiles shared by individuals with autism and a subgroup of parents (i.e., those with the social BAP) may be wide ranging, involving a number of different social cognitive skills -- making complex social judgments of trustworthiness from facial information (trustworthiness of faces), interpreting the emotional content of complex scenes with and without affective facial information (movie stills), inferring emotions from very subtle variations in facial expression (morphed faces), and interpreting complex emotional content from biological motion (point light).
Importantly, patterns of performance were in some instances qualitatively different in the autistic and parent groups. In the trustworthiness of faces task, for instance, individuals with autism judged negative faces as significantly ‘more positive’ than controls, whereas BAP (+) parents judged positively valenced faces as ‘more negative’ than controls and BAP (−) parents. Such a divergence in performance demonstrates how neurocognitive characteristics may manifest variably in autism and the BAP. Below we discuss further the implications of these findings for studies of the brain and gene basis of autism, and conclude with limitations and questions for future studies.
Implications for understanding the brain and gene basis of autism and the BAP
As with other studies of the BAP, these results may help to narrow the empirical target from an otherwise highly complex and heterogeneous phenotype (i.e., a diagnosis of autism), to more specific component features that are likely to be more amenable to genetic and neurobiological investigation. And, because our measures constitute quantitative indices of neuropsychological functioning in both affected and unaffected individuals, studies implementing these measures may profit from larger sample sizes and resulting increases in power.
As these tasks derived from lesion and fMRI studies, and have therefore been linked with specific brain regions, we may also speculate on the neuroanatomical structures that could underlie observed patterns of performance, and which may be mediated by autism susceptibility genes. Lesion and imaging studies strongly implicate amygdala function in performance on each of the social cognitive tasks with which autistic individuals and parents with the BAP demonstrated differences (27, 28, 32, 34, 36). And, while a number of neural mechanisms have been implicated in the pathophysiology of autism (for review, see (50)) the amygdala has figured prominently in hypotheses concerning the basis of the social impairments of the disorder (e.g, (15, 17). Our findings support this link, and further suggest that the amygdala may play a role in the subtle social-behavioral manifestation of genetic liability to autism, among unaffected relatives. Results also support involvement of the medial prefrontal cortex, (linked to performance on the ‘eyes’ and ‘pointlight’ tasks (37, 39)) and the superior temporal gyrus, which has also been linked to performance on the eyes test (27–30).
Questions and limitations
It will be important for future work to replicate these findings with larger samples. Indeed, the possibility of false positives cannot be ruled out; however, we note that all differences on social cognitive measures were consistent with hypotheses. Nonetheless, studies with larger samples will be important for clarifying some intriguing and unexpected results, such as the stronger differences detected among BAP (+) mothers vs. controls than fathers on the Eyes Test. It will also be important to investigate the specificity of findings to autism, as there is suggestion of phenotypic overlap between autism and other neurogenetic disorders (e.g., schizophrenia)(51). Such work should inform both genetic and neurobiological studies of these disorders.
The lack of group differences within the domains of executive function and central coherence remains puzzling, particularly given the large body of research demonstrating executive control deficits and a local processing bias/limited drive for central coherence associated with autism (for reviews see (52, 53). However, some other studies have also failed to detect differences in thesedomains (54, 55), raising the possibility that effects within these domains are more subtle and/or heterogeneous. Also, the net cast by our task battery was not as broad as some other studies’ and this may have hindered our ability to capture group differences.
A related issue concerns the neurocognitive correlates of the rigid/perfectionistic features of the BAP. It is possible that this feature may not constitute as valid a construct as the social BAP, or alternatively, may relate to additional cognitive mechanisms not considered here (e.g., sensorimotor processing, which has been associated with repetitive behaviors in autism (56)).
A final question is why we did not detect parent-child associations in social cognition or the other domains. This could be due to insufficient variation within the autistic group, where most individuals were unequivocally impaired on social cognition measures. And while we targeted intact families, we were only able to recruit 22 such families, raising the possibility that we lacked power to detect such associations. Future studies may therefore benefit by including more heterogeneous autistic groups, and larger samples of intact families.
In sum, our findings raise some important questions for future studies, and at the same time add to current understanding of the neuropsychological basis of autism and the BAP. Results support further study of the component features of the BAP and the neuropsychological characteristics that underlie the components of this construct, which may add important new information about the psychological, neural, and genetic mechanisms underlying autism. From this study, social cognition has emerged as a promising candidate for future studies incorporating the BAP approach and more direct measures of neurocognitive functions, such as structural and functional imaging.
Table 4.
Mean differences for executive function and central coherence tasks
| Autism/Control Groups |
Parent Groups1 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Au vs Control | BAP (+) vs BAP (−) | BAP (+) vs Controls | BAP (−) vs Controls | |||||||||
| Mean Difference |
SE | p | Mean Difference |
SE | p | Mean Difference |
SE | p | Mean difference |
SE | p | |
| EXECUTIVE FUNCTION | ||||||||||||
| Tower of Hanoi | ||||||||||||
| Number of moves | 4.21 | 3.56 | 0.242 | −0.55 | 2.18 | 0.803 | 1.98 | 2.37 | 0.406 | 2.53 | 1.81 | 0.166 |
| Time to complete | 27.76 | 15.95 | 0.087 | 2.31 | 17.12 | 0.893 | 18.95 | 18.36 | 0.305 | 16.64 | 13.95 | 0.237 |
| Trailmaking Task | ||||||||||||
| Time to complete | 2.10 | 4.50 | 0.642 | 3.21 | 3.87 | 0.408 | −1.63 | 4.14 | 0.695 | −4.84 | 3.06 | 0.116 |
| CENTRAL COHERENCE | ||||||||||||
| Embedded Figures | ||||||||||||
| Accuracy (# correct) | 0.12 | 0.22 | 0.597 | −0.11 | 0.25 | 0.665 | −0.04 | 0.25 | 0.877 | 0.07 | 0.18 | 0.705 |
| Time | −0.19 | 1.45 | 0.897 | −1.08 | 1.90 | 0.571 | 1.29 | 1.84 | 0.484 | 2.37 | 1.44 | 0.102 |
| Sentenced Completion2 | ||||||||||||
| #Non-global responses | 1.30 | 0.43 | 0.003 | 0.32 | 0.40 | 0.420 | 0.33 | 0.42 | 0.438 | 0.00 | 0.33 | 0.991 |
| Response time | 1.87 | 0.92 | 0.045 | −0.35 | 0.39 | 0.363 | −1.45 | 0.56 | 0.011 | −1.10 | 0.53 | 0.039 |
| Block Design | ||||||||||||
| Whole -Time | 2.04 | 3.14 | 0.594 | 2.79 | 4.06 | 0.494 | 1.78 | 4.36 | 0.684 | −1.01 | 2.75 | 0.715 |
| Segmented - Time | 2.79 | 1.13 | 0.045 | −0.26 | 0.89 | 0.769 | −0.12 | 0.97 | 0.903 | 0.14 | 0.70 | 0.839 |
Notes: Mean difference scores adjusted for covariates of age, sex, and IQ. Significant p values appear in blue.
BAP (+) defined as rigid/perfectionistic as measured on the MPAS-R
Sentence completion coefficients are from a logistic regression model
Acknowledgments
This project was funded by STAART Center Grant #1 U54 MH66418 to JP. ML acknowledges support from Autism Speaks and KL2RR025746 from the National Center for Research Resources.
We gratefully acknowledge the contributions of the families and individuals who participated in this research. We are also grateful to Dr. Francesca Happé for her guidance in use of measures of central coherence, and to Morgan Parlier, M.S.W., for her considerable efforts overseeing and participating in data collection and coding for this project, as well as the members of the UNC Clinical Core for their work assisting in recruitment and testing. This project was funded by STAART Center Grant #1 U54 MH66418 to JP. ML acknowledges support from Autism Speaks and KL2RR025746 from the National Center for Research Resources. ML had full access to all of the data in this study and takes responsibility for its integrity and the accuracy of the data analysis.
Footnotes
These stimuli were kindly provided by Dr. A. Heberlein, Harvard University
Most of the tasks are complexly designed with repeated within-subject measures that are defined by the emotion (happy, sad, angry, scared), intensity of emotion (neutral – extreme), and/or degree of difficulty (as in the morphed faces, high vs. low morphedness). To account for such complexities, a repeated measures mixed model was fit for each outcome. Three alternative within-person covariance structures were considered in guiding model selection: unstructured, compound symmetric heterogeneous, and compound. Bayesian information criterion (BIC) scores were used to evaluate these alternative covariance structures and guided selection of the most appropriate model
References
- 1.Freitag CM. The genetics of autistic disorders and its clinical relevance: a review of the literature. Mol Psychiatry. 2007;12(1):2–22. doi: 10.1038/sj.mp.4001896. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AR, State MW. Recent advances in the genetics of autism. Biol Psychiatry. 2007;61(4):429–37. doi: 10.1016/j.biopsych.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18(4):297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 4.Piven J. In: The Genetics of Personality: The Example of the Broad Autism Phenotype, in Molecular Genetics and the Human Personality. Belmaker BJERP, editor. Washington DC: American Psychiatric Publishing, Inc; 2002. pp. 43–62. [Google Scholar]
- 5.Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across multiple- and single-incidence families of autistic individuals. American Journal of Human Genetics: Neuropsychiatric Genetics. 2007 doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piven J, Palmer P, Landa R, Santangelo S, Jacobi D, Childress D. Personality and language characteristics in parents from multiple-incidence autism families. Am J Med Genet. 1997;74:398–411. [PubMed] [Google Scholar]
- 7.Pickles A, Starr E, Kazak S, Bolton P, Papanikolaou K, Bailey A, Goodman R, Rutter M. Variable expression of the autism broader phenotype: Findings from extended pedigrees. Journal of Child Psychology and Psychiatry. 2000;41:491–502. [PubMed] [Google Scholar]
- 8.Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9(10):1218–20. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- 9.Sigman M, Spence SJ, Wang AT. Autism from developmental and neuropsychological perspectives. Annu Rev Clin Psychol. 2006;2:327–55. doi: 10.1146/annurev.clinpsy.2.022305.095210. [DOI] [PubMed] [Google Scholar]
- 10.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The Reading the Mind in the Eyes Test Revised Version: A Study with Normal Adults, and Adults with Asperger Syndrome or High-functioning Autism. J Child Psychol Psychiat. 2001;42:241–251. [PubMed] [Google Scholar]
- 11.Losh M, Piven J. Social-Cognition and the Broad Autism Phenotype: Identifying Genetically Meaningful Phenotypes. Journal of Child Psychology and Psychiatry. 2007 doi: 10.1111/j.1469-7610.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- 12.Bolte S, Poustka F. The broader cognitive phenotype of autism in parents: how specific is the tendency for local processing and executive dysfunction? J Child Psychol Psychiatry. 2006;47(6):639–45. doi: 10.1111/j.1469-7610.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 13.Baron-Cohen S, Hammer J. Parents of children with Asperger syndrome: what is the cognitive phenotype? The Journal of Cognitive Neuroscience. 1997;9:548–554. doi: 10.1162/jocn.1997.9.4.548. [DOI] [PubMed] [Google Scholar]
- 14.Happe F, Briskman J, Frith U. Exploring the cognitive phenotype of autism: weak central coherence in parents and siblings of children with autism: I. Experimental Tests. Journal of Child Psychology and Psychiatry. 2001;42:299–307. [PubMed] [Google Scholar]
- 15.Baron-Cohen S, Ring H, Bullmore ET, Wheelwright S, Ashwin C, Williams SCR. The amygdala theory of autism. Neuroscience and Biobehavioral Reviews. 2000;24:355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 16.Howard M, Cowell PE, Boucher J, Broks P, Mayes A, Farrant A, Roberts N. Convergent neuroanatomical and behavioral evidence of an amygdala hypothesis of autism. NeuroReport. 2000;11:2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- 17.Loveland KA, Bachevalier J, Pearson DA, Lane DM. Fronto-limbic functioning in children and adolescents with and without autism. Neuropsychologia. 2008;46(1):49–62. doi: 10.1016/j.neuropsychologia.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) 1999. [Google Scholar]
- 19.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview--Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental Syndromes. Journal of Autism and Developmental Syndromes. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 20.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- 21.APA. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington DC: 1994. [Google Scholar]
- 22.Murphy M, Bolton P, Pickles A, Fombonne E, Piven J, Rutter M. Personality Traits of the Relatives of Autistic Probands. Psychological Medicine. 2000;30:1411–1424. doi: 10.1017/s0033291799002949. [DOI] [PubMed] [Google Scholar]
- 23.Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10(4):259–71. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- 24.Valdizan JR. [Cognitive functions and neuronal networks in the social brain] Rev Neurol. 2008;46 (Suppl 1):S65–8. [PubMed] [Google Scholar]
- 25.Baron-Cohen S, Wheelwright S, Jolliffe T. Is there are a ‘language of the eyes’? Evidence from normal adults and adults with autism or Asperger Syndrome. Visual Cognition. 1997;4:311–332. [Google Scholar]
- 26.Dorris L, Espie CA, Knott F, Salt J. Mind-reading difficulties in the siblings of people with Asperger’s syndrome: evidence for a genetic influence in the abnormal development of a specific cognitive domain. Journal of Child Psychology and Psychiatry. 2004;45:412–418. doi: 10.1111/j.1469-7610.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 27.Adolphs R, Baron-Cohen S, Tranel D. Impaired Recognition of social emotions following amygdala damage. Journal of Cognitive Neuroscience. 2002;14:1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 28.Baron-Cohen S, Ring H, Wheelwright S, Bullmore E, Brammer M, Simmons A, Williams S. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 29.Happe F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, Dolan R, Frackowiak R, Frith C. Theory of mind in the brain: evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- 30.Stone V, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10:640–656. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- 31.Ekman P, Friesen W. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists; 1976. [Google Scholar]
- 32.Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. J Cogn Neurosci. 2004;16(3):453–62. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- 33.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 34.Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. Journal of Cognitive Neuroscience. 2001;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- 35.Adolphs R. Neural Mechanisms for Recognition of Emotion. Current Opinion in Neurobiology. 2002 [Google Scholar]
- 36.Adolphs R, Tranel D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia. 2003;41(10):1281–9. doi: 10.1016/s0028-3932(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 37.Heberlein AS, Adolphs R, Tranel D, Damasio H. Cortical regions for judgments of emotions and personality traits from point-light walkers. J Cogn Neurosci. 2004;16(7):1143–58. doi: 10.1162/0898929041920423. [DOI] [PubMed] [Google Scholar]
- 38.Pelphrey KA, Morris JP. Brain Mechanisms for Interpreting the Actions of Others From Biological-Motion Cues. Curr Dir Psychol Sci. 2006;15(3):136–140. doi: 10.1111/j.0963-7214.2006.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heberlein AS, Saxe RR. Dissociation between emotion and personality judgments: convergent evidence from functional neuroimaging. Neuroimage. 2005;28(4):770–7. doi: 10.1016/j.neuroimage.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 40.Duncan J. Disorganization of behavior after frontal lobe damage. Cognitive Neuropsychology. 1986;3:271–290. [Google Scholar]
- 41.Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- 42.Baron-cohen S, Wheelwright S, Stone V, Rutherford M. A mathematician, a physicist, an da computer scientist with Asperger Syndrome: performance on folk psychology and folk physics test. Neurocase. 1999;5:475–483. [Google Scholar]
- 43.Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clin Psychol Rev. 2000;20(1):113–36. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- 44.Hellige JB. Hemispheric asymmetry for visual information processing. Acta Neurobiol Exp (Wars) 1996;56(1):485–97. doi: 10.55782/ane-1996-1151. [DOI] [PubMed] [Google Scholar]
- 45.Proverbio AM, Minniti A, Zani A. Electrophysiological evidence of a perceptual precedence of global vs. local visual information. Brain Res Cogn Brain Res. 1998;6(4):321–34. doi: 10.1016/s0926-6410(97)00039-6. [DOI] [PubMed] [Google Scholar]
- 46.Weissman DH, Woldorff MG. Hemispheric asymmetries for different components of global/local attention occur in distinct temporo-parietal loci. Cereb Cortex. 2005;15(6):870–6. doi: 10.1093/cercor/bhh187. [DOI] [PubMed] [Google Scholar]
- 47.Witkin H, Oltman P, Raskin E, Karp S. A Manual for the Embedded Figure Test. Consulting Psychologists Press. California: 2001. [Google Scholar]
- 48.Shah A, Frith U. An islet of ability in autistic children: a research note. J Child Psychol Psychiatry. 1983;24(4):613–20. doi: 10.1111/j.1469-7610.1983.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 49.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. 1996. [Google Scholar]
- 50.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64(7):945–50. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sasson N, Tsuchiya N, Hurley R, Couture SM, Penn DL, Adolphs R, Piven J. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia. 2007;45(11):2580–8. doi: 10.1016/j.neuropsychologia.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Happe F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- 53.Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8(1):26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Kaland N, Smith L, Mortensen EK. Brief Report: cognitive flexibility and focused attention in children and adolescents with Asperger syndrome of High-functioning Autism as measured on the computerized version of the Wisconsin Card Sorting Test. Journal Of Autism and Developmental Disorders. 2007 doi: 10.1007/s10803-007-0474-1. [DOI] [PubMed] [Google Scholar]
- 55.Scheeren AM, Stauder JE. Broader autism phenotype in parents of autistic children: reality or myth? J Autism Dev Disord. 2008;38(2):276–87. doi: 10.1007/s10803-007-0389-x. [DOI] [PubMed] [Google Scholar]
- 56.Perry W, Minassian A, Lopez B, Maron L, Lincoln A. Sensorimotor gating deficits in adults with autism. Biol Psychiatry. 2007;61(4):482–6. doi: 10.1016/j.biopsych.2005.09.025. [DOI] [PubMed] [Google Scholar]