Abstract
The human adrenal reticularis produces the so-called adrenal androgens, dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEA-S). As opposed to the cortisol and aldosterone little is known regarding the mechanisms that regulate the production of the adrenal androgens. Several recent studies have shown that type II 3β-hydroxysteroid dehydrogenase (HSD3B2), cytochrome b5 (CYB5), and steroid sulfotransferase (SULT2A1) play an important role in the regulation of adrenal androgen production. Specifically, adrenal production of DHEA-S is correlated with reticularis expression of SULT2A1 and CYB5. In contrast, HSD3B2 has an inverse correlation with adrenal androgen production likely due to its unique ability to remove precursors from the pathway leading to DHEA. Therefore, its expression is limited to the adrenal glomerulosa/fasciculata but not in reticularis. The differential expression of these three proteins appears to be critical for reticularis function. In this review, we focus on studies that have begun to define the mechanisms regulating the transcription of these genes. Understanding the mechanisms controlling differential expression of these proteins should provide novel information about the human adrenal reticularis and its production of DHEA and DHEA-S.
Keywords: Adrenal, Androgen, Cytochrome b5, DHEA-sulfotransferase, 3β-hydroxysteroid dehydrogenase
II. Background & Significance
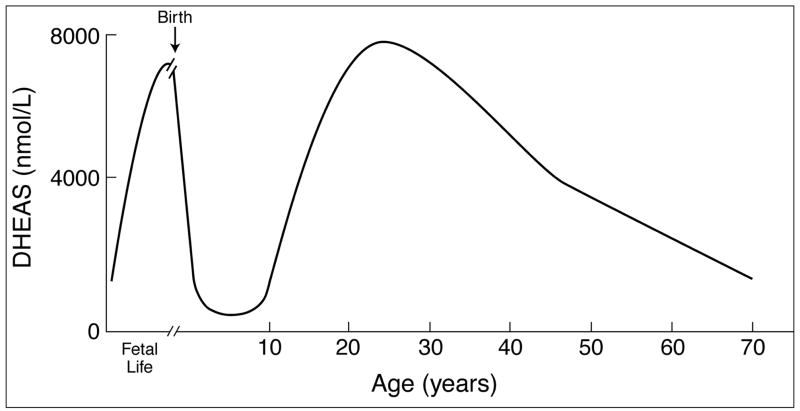
The fetal adrenal produces large amounts of dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS) during fetal development (Fig. 1). DHEA-S concentrations are also high in the newborn (Fig. 1) [1]. However, by age 1, the specialized fetal zone is lost and replaced by the definitive adrenal cortex, which initially synthesizes little DHEA-S (Fig. 1). Hence, DHEA-S production declines precipitously during the first months of life and remains low until adrenarche commences at about age 6–8 [2]. This rise in the circulating concentrations of DHEA and DHEA-S is the biochemical hallmark of adrenarche [3]. Importantly, the rise in DHEA-S occurs prior to the increase of either estrogens or androgens associated with puberty [4]. Circulating DHEA-S concentrations continue to rise and peak during the second decade of life, with levels being higher in males than in females (Fig. 1) [3, 5]. While circulating concentrations of DHEA and DHEA-S rise progressively in adrenarche, cortisol and ACTH concentrations do not change significantly in this period, indicating that adrenarche is not simply a global activation of the pituitary-adrenal axis.
Figure 1.
DHEA-S levels during human development, adrenarche and aging. This figure was modified from Rainey et al [43].
III. Molecular Mechanism Regulating Adrenal Androgen Biosynthesis
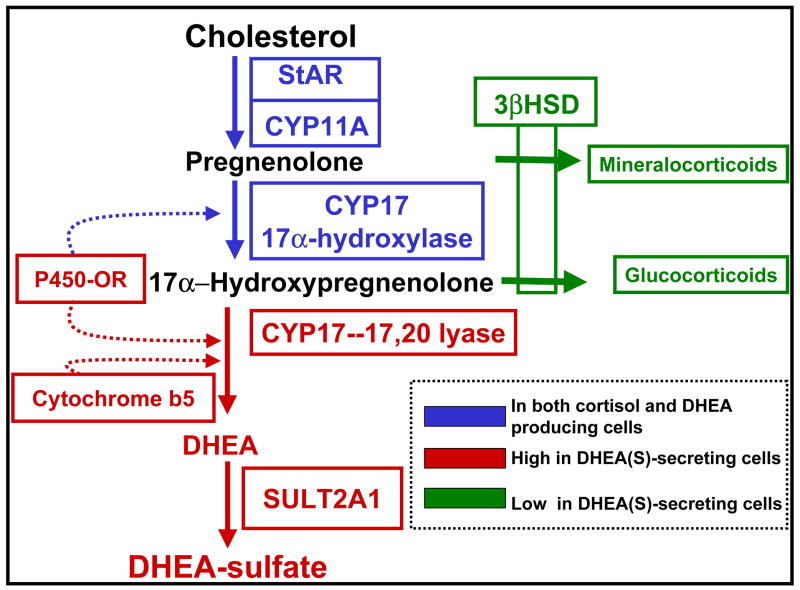
The synthesis of DHEA requires only two steroid-metabolizing enzymes, i.e. cholesterol side-chain cleavage enzyme (CYP11A) and 17α-hydroxylase/17,20 lyase (CYP17) plus the protein required for cholesterol transport into the mitochondria [steroidogenic acute regulatory protein (StAR)] (Fig. 2). However, the differential expression of StAR and these two enzymes within the adrenal does not appear to be a key factor in the regulation of adrenal androgen production. This is supported by the observation that StAR, CYP17 and CYP11A are present in the adrenal fasciculata and reticularis but only the reticularis produces significant amounts of DHEA [6]. In addition, StAR, CYP11A and CYP17 are present in infant and childhood adrenals that produce little DHEA/DHEA-S until adrenarche. If alterations in StAR, CYP11A and CYP17 are not the determining factors for DHEA/DHEA-S secretion, then what other enzymes are modified that would promote adrenal androgen production but not cortisol? Biochemical studies as well as immunohisotchemistry analysis of adrenals across the period of adrenarche support a role for three proteins in directing precursor steroids into DHEA/DHEA-S. Two are the steroidogenic enzymes 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2), and DHEA-sulfotransferase (SULT2A1), while the other is cytochrome b5 (CYB5). Our laboratory has focused on the mechanisms regulating the expression of each of these proteins with the goal of defining the molecular mechanisms that regulate human adrenal androgen production.
Figure 2.
Steroid pathway for DHEA-S production. Production relies on the coordinated expression of StAR, CYP11A, CYP17, and DHEA-sulfotransferase (SULT2A1). Also positively impacting DHEA-S biosynthesis is cytochrome b5 (CYB5), which can enhance the 17,20-lyase activity of CYP17. Negatively impacting the production of DHEA-S is 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2). This figure was modified from Rainey et al [43].
1) CYB5 and the 17,20-lyase activity of CYP17
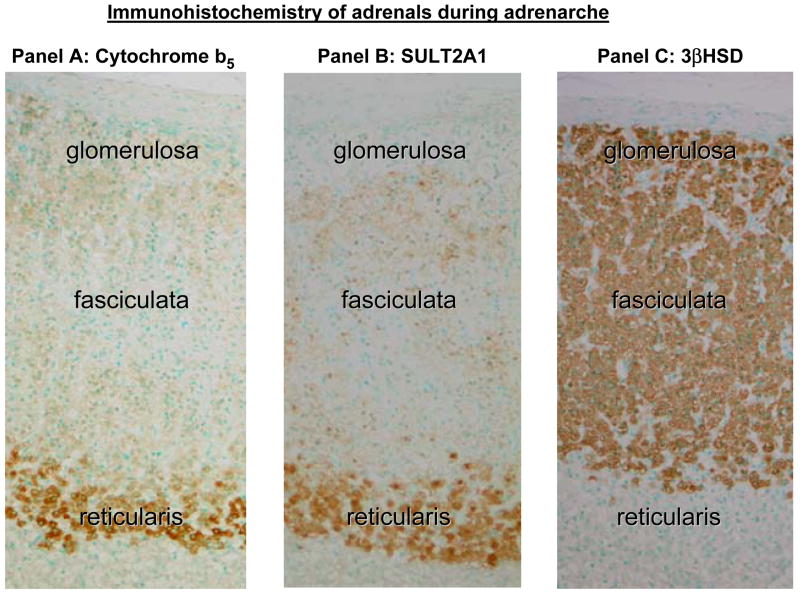
In human adrenal glands, the CYP17 enzyme is found in both the fasciculata and reticularis zones of the cortex, consistent with its role in both cortisol and DHEA synthesis (Fig. 2). However, the CYP17 enzyme has the ability to catalyze both 17α-hydroxylase and 17,20-lyase reactions [7–9]. In the adrenal fasciculata, CYP17 accomplishes the 17α-hydroxylase reaction with diminished 17,20-lyase activity in, while it promotes both 17α-hydroxylase and 17,20-lyase activities in the adrenal reticularis. On this mechanism, CYB5 is regarded as an important regulator of CYP17 function. The CYB5 is expressed in two different isoforms generated by alternative splicing from the same mRNA. One isoform is cytoplasmic and is found primarily in reticulocytes, while the larger isozyme localizes to the endoplasmic reticulum. It is known that localization of cytochrome b5 protein was most remarkable in the zona reticularis in the human adrenal gland (Fig. 3) [10]. It is suggested that human CYB5 acts principally as an allosteric effector that interacts primarily with the CYP17/oxidoreductase complex to stimulate 17,20-lyase activity [11]. The reticularis-specific expression of CYB5 appears to be a key factor in promoting adrenal androgen production and recent studies have started to better define the factors regulating this gene’s transcription.
Figure 3.
Immunohistochemistry for cytochrome b5 (CYB5), DHEA-sulfotransferase (SULT2A1) and 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) in human adrenal cortex. Both SULT2A1 and CYB5 immunoreactivities are strongly detected in the cytoplasm of adrenocortical cells in the reticularis. Immunoreactivity of SULT2A1 is weak in the fasciculata and not detected in the glomerulosa. CYB5 was similarly week in the fasciculata and glomerulosa. On the other hand, reactivity for HSD3B2 was marked in the glomerulosa and fasciculata, but negative in the reticularis. This figure was modified from Rainey et al [43].
The study of the CYB5 promoter in HepG2 and K562 cells by Li and his coworkers and recent work done by Miller et al. have defined a number of transcription factors that regulate CYB5 gene transcription [12, 13]. According to the Miller study, CYB5 mRNA arises from multiple transcriptional start sites 83 to 122 base pairs upstream from the ATG translational start codon [13]. To better define the potential factors that would lead to adrenal reticularis specific expression, this group identified the roles of several factors, including Sp3, GATA6, SF-1 in the regulation of CYB5 transcription [13]. Two of these factors, GATA6 and SF-1, are also associated with the regulation of another reticularis enzyme, SULT2A1. As these factors are not preferentially expressed in the adrenal reticularis additional experiments are needed to determine if there are reticularis specific regulatory mechanisms for these transcription factors or if other yet to be defined zonal factors determine CYB5 reticularis expression..
2) SULT2A1
Humans are presently known to express at least five cytosolic sulfotransferase (ST) enzymes, of which only two can use steroids as substrates. Steroid sulfaction can occur through the activity of SULT2A1, commonly known as steroid sulfotransferase or cholesterol sulfotransferase SULT2B1. SULT2A1 is predominantly expressed in the cytoplasm of adrenocortical cells in the reticularis and its substrates include pregnenolone, 17α hydroxypregnenolone, and DHEA (Fig. 2, 3). During fetal development each of these steroids are used as substrates resulting in high circulating levels of the corresponding sulfated products; however, in adults the predominant substrate is DHEA resulting in DHEA-S. The production of sulfonated DHEA is so high during certain periods of our lifespan that DHEA-S is quantitatively the most abundant hormone secreted by the human adrenal. While the enzymatic activity of SULT2A1 has been studied in some detail, only recently have studies focused on the regulation of human SULT2A1 expression. Herein, we discuss the role of three transcription factors, steroidogenic factor 1 (SF1 or NR5A1), GATA-6, and estrogen-related receptor α (ERRα) in the regulation of SULT2A1 transcription.
The well-known orphan nuclear receptor SF1 is a critical factor in the regulation of transcription of the genes encoding the adrenal cytochrome P450 enzymes [14, 15]. We demonstrated that specific SF1 binding cis-regulatory elements are necessary for trans-activation of SULT2A1 promoter [16]. However, two observations oppose the argument that SF1 is the key factor causing zonal expression of SULT2A1 in the adrenal reticularis. First, SF1 does not exhibit elevated expression in the zona reticularis compared to the other adrenal zones. Secondly, SF1-regulated genes, such as CYP17, are expressed in both the fasciculata and reticularis. While SULT2A1 joins the large number of steroid-metabolizing genes that can be regulated by SF1, it would appear that other factors contribute to the elevated levels seen in the zona reticularis.
The GATA family of transcription factors has been demonstrated to be robust regulators of transcription in a wide variety of tissues including the heart and gonads [17–21]. Recent studies also indicate that GATA-6 is highly expressed in the adult and fetal adrenal cortex [22]. We previously demonstrated that GATA-6 works in synergy with SF1 to maximally increase expression of SULT2A1 [23]. However, GATA-6 expression is not limited to the adrenal reticularis suggesting that it is also not the key to understanding zonal expression of SULT2A1.
ERRα is a member of a subfamily of orphan nuclear receptors that are structurally and functionally related to estrogen receptors. We have demonstrated that expression of ERRα in the adult adrenal and SULT2A1 promoter activity is more responsive to ERRα via three functional regulatory cis-elements sharing sequence similarity to binding sites of SF1 [24]. Its expression is elevated in the inner zones of the adrenal cortex suggesting that it may play a role in reticularis expression of SULT2A1. However, the mechanisms regulating this apparent orphan receptor is still poorly defined and further research is needed to determine if its expression correlates with increases in DHEA-S biosynthesis as seen in the fetal adrenal or at the time of adrenarche.
3) 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2)
The enzyme 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) is essential for the adrenal biosynthesis of mineralocorticoids (aldosterone) and glucocorticoids (cortisol) but its expression is inversely associated with adrenal androgens (Fig. 2). There are two isoforms of HSD3B but it is only the type II form (HSD3B2) that is expressed in the lomerulosa and fasciculate of adrenal glands (Fig. 3). In the adrenal gland, HSD3B2 catalyzes the oxidation and isomerization of 3β-hydroxy-5-ene (Δ5) steroids into 3-keto-4-ene (Δ4) steroids leading directly to the production of progesterone and 17α-hydroxyprogesterone from pregnenolone and 17α-hydroxypregnenolone precursors, respectively [25].
HSD3B2 influences the production of aldosterone, cortisol, and DHEA by competing with CYP17 for the metabolism of pregnenolone and 17α-hydroxypregnenolone. [26]. High HSD3B2 expression combined with low CYP17 activity will favor aldosterone synthesis and oppose cortisol and adrenal androgen synthesis. Conversely, low HSD3B2 expression coupled with high CYP17 activity will favor adrenal androgen production. The human adrenal produces DHEA at high levels within the fetal adrenal and in the zona reticularis of the adult adrenal. Both of these tissues express low levels of HSD3B2 mRNA and protein [6, 27–32]. Thus, a detailed understanding of the mechanisms that regulate HSD3B2 expression would help in efforts to understand adrenal physiology.
Recent studies suggest that HSD3B2 transcription is regulated in part by the nuclear receptor hormone family NR4A. The NGFIB family, which includes NGFIB (Nur77 or NR4A1), NURR1 (Nur-related factor 1 or NR4A2), and NOR1 (neuron-derived orphan receptor 1 or NR4A3) [33, 34], appears to play an important role in the coordinated regulation of the hypothalamic/pituitary/adrenal axis [35–39]. We previously demonstrated the role of the NGFIB family of orphan nuclear receptors in the regulation of HSD3B2 transcription via the consensus NGFIB-binding response element (NBRE) located at −131/-Adrenal Androgen Biosynthesis 124 [39]. Moreover, it is confirmed that within adult and fetal adrenal gland NGFIB expression paralleled expression of HSD3B2 [39]. On the other hand, the expression of NGFIB was inversely correlated with the ability of the tissues to produce DHEA [39]. This inverse correlation between adrenal androgen production and the expression of NGFIB and HSD3B2 appears to be a unifying link for the production of DHEA by the fetal adrenal and adult adrenal reticularis as postulated by Goto et al. [40].
Fos and Jun are members of the AP-1 transcription factors. The Jun family members modify transcription by binding specific response elements as dimers or heterodimers with Fos [41]. Jun and Fos can also form dimers with other transcription factors such as members of the ATF/CREB, C/EBP, and Maf families of proteins [41]. The proximal region of the HSD3B2 promoter contains two consensus AP-1 cis-elements, which are capable of binding fos/jun [41]. It is demonstrated that fos/jun has only a small effect on reporter activity of HSD3B2; however, the fos-jun heterodimer strongly acts in synergy with the orphan receptor SF-1 to stimulate reporter activity [41]. We find much higher levels of fos and jun expression in cortisol producing tissue compared to DHEA producing tissue (unpublished observation). These data show a correlation between HSD3B2 and fos/jun that needs further study.
Martin et al. reported that GATA-4 and GATA-6 physically interact with the nuclear receptors, steroidogenic factor 1, and liver receptor homolog 1 to synergistically activate HSD3B2 [42]. In their study, it was reported that the human HSD3B2 promoter, which contains four consensus GATA elements, constitutes an important target for GATA factors. These factors are sufficient to activate transcription from the −1073 bp HSD3B2 promoter fragment and blockade endogenous GATA expression and activity in adrenal steroidogenic cells [42]. In addition, it is shown that the proximal GATA element located at −196 bp is sufficient to confer GATA responsiveness of the HSD3B2 promoter and is required for full HSD3B2 promoter activity in adrenal steroidogenic cells [42]. However GATA6, the major GATA family member found in the post-natal adrenal does not show a zonal distribution that would correlate with HSD3B3 expression. Thus while GATA6 appears to be a potent activator of HSD3B2 transcription its role in zonal expression is unlikely.
IV. Summary & Future Direction
We have made progress in defining the differences in adrenocortical cortisol and DHEA producing cells. Of note are the critical changes in the expression of steroid-metabolizing enzymes involved in both cortisol and DHEA-S biosynthesis. We propose that three key proteins, namely cytochrome b5 (CYB5), DHEA-sulfotransferase (SULT2A1), and 3β-hydroxysteroid dehydrogenase (HSD3B2) develop a clear adrenal zone-specific pattern of expression that lead to a transition from cortisol producing cell to DHEA-S producing cell. The molecular mechanisms regulating differential expression of these genes should provide important clues to the physiologic regulators of DHEA-S biosynthesis. Recent studies indicate clear differences in the mechanisms that regulate transcription of HSD3B2 verses CYB5 and SULT2A1. These studies support a role for transcription factors NGFI-B, GATA-6, and ERRα in the differential expression of these key genes. Based on these studies it appears that the fasciculate and reticularis zones preferentially control the expression of specific steroid metabolizing enzymes through their transcription.
Acknowledgments
This work was supported by awards from the National Institutes of Health (DK043140 and DK068850 to WER).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yuen BH, Mincey EK. Human chorionic gonadotropin, prolactin, estriol, and dehydroepiandrosterone sulfate concentrations in cord blood of premature and term newborn infants: relationship to the sex of the neonate. Am J Obstet Gynecol. 1987;156(2):396–400. doi: 10.1016/0002-9378(87)90291-2. [DOI] [PubMed] [Google Scholar]
- 2.DePeretti E, Forest MG. Unconjugated dehydroepiandrosterone plasma levels in normal subjects from birth to adolescence in humans: the use of a sensitive radioimmunoassay. J Clin Endocrinol Metab. 1976;43(5):982–991. doi: 10.1210/jcem-43-5-982. [DOI] [PubMed] [Google Scholar]
- 3.Smith MR, Rudd BT, Shirley A, Rayner PH, Williams JW, Duignan NM, Bertrand PV. A radioimmunoassay for the estimation of serum dehydroepiandrosterone sulphate in normal and pathological sera. Clin Chim Acta. 1975;65(1):5–13. doi: 10.1016/0009-8981(75)90328-9. [DOI] [PubMed] [Google Scholar]
- 4.Ducharme JR, Forest MG, DePeretti E, Sempe M, Collu R, Bertrand J. Plasma adrenal and gonadal sex steroids in human pubertal development. J Clin Endocrinol Metab. 1976;42(3):468–476. doi: 10.1210/jcem-42-3-468. [DOI] [PubMed] [Google Scholar]
- 5.Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53(6):739–747. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- 7.Bradshaw KD, Waterman MR, Couch RT, Simpson ER, Zuber MX. Characterization of complementary deoxyribonucleic acid for human adrenocortical 17 alpha-hydroxylase: a probe for analysis of 17 alpha- hydroxylase deficiency. Mol Endocrinol. 1987;1(5):348–354. doi: 10.1210/mend-1-5-348. [DOI] [PubMed] [Google Scholar]
- 8.Kagimoto M, Winter JS, Kagimoto K, Simpson ER, Waterman MR. Structural characterization of normal and mutant human steroid 17 alpha- hydroxylase genes: molecular basis of one example of combined 17 alpha- hydroxylase/17,20 lyase deficiency. Mol Endocrinol. 1998;2(6):564–570. doi: 10.1210/mend-2-6-564. [DOI] [PubMed] [Google Scholar]
- 9.Swart P, Estabrook RW, Mason JI, Waterman MR. Catalytic activity of human and bovine adrenal cytochromes P-450 17 alpha, lyase expressed in Cos 1 cells. Biochem Soc Trans. 1989;17(6):1025–1026. doi: 10.1042/bst0171025. [DOI] [PubMed] [Google Scholar]
- 10.Yanase T, Sasano H, Yubisui T, Sakai Y, Takayanagi R, Nawata H. Immunohistochemical study of cytochrome b5 in human adrenal gland and in adrenocortical adenomas from patients with Cushing’s syndrome. Endocr J. 1998;45(1):89–95. doi: 10.1507/endocrj.45.89. [DOI] [PubMed] [Google Scholar]
- 11.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273(6):3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 12.Li XR, Giordano SJ, Yoo M, Steggles AW. The isolation and characterization of the human cytochrome b5 gene. Biochem Biophys Res Commun. 1995;209(3):894–900. doi: 10.1006/bbrc.1995.1582. [DOI] [PubMed] [Google Scholar]
- 13.Huang N, Dardis A, Miller WL. Regulation of cytochrome b5 gene transcription by Sp3, GATA-6, and steroidogenic factor 1 in human adrenal NCI-H295A cells. Mol Endocrinol. 2005;19(8):2020–2034. doi: 10.1210/me.2004-0411. [DOI] [PubMed] [Google Scholar]
- 14.Penhoat A, Sanchez P, Jaillard C, Langlois D, Begeot M, Saez JM. Human proopiomelanocortin-(79–96), a proposed cortical androgen-stimulating hormone, does not affect steroidogenesis in cultured human adult adrenal cells. J Clin Endocrinol Metab. 1991;72(1):23–26. doi: 10.1210/jcem-72-1-23. [DOI] [PubMed] [Google Scholar]
- 15.Weber A, Clark AJ, Perry LA, Honour JW, Savage MO. Diminished adrenal androgen secretion in familial glucocorticoid deficiency implicates a significant role for ACTH in the induction of adrenarche. Clin Endocrinol (Oxf) 1997;46(4):431–437. doi: 10.1046/j.1365-2265.1997.1580969.x. [DOI] [PubMed] [Google Scholar]
- 16.Saner KJ, Suzuki T, Sasano H, Pizzey J, Ho C, Strauss JF, 3rd, Carr BR, Rainey WE. Steroid sulfotransferase 2A1 gene transcription is regulated by steroidogenic factor 1 and GATA-6 in the human adrenal. Mol Endocrinol. 2005;19(1):184–197. doi: 10.1210/me.2003-0332. [DOI] [PubMed] [Google Scholar]
- 17.Molkentin JD. The zinc finger-containing transcription factors GATA-4, -5, and -6. Ubiquitously expressed regulators of tissue-specific gene expression. J Biol Chem. 2000;275(50):38949–38952. doi: 10.1074/jbc.R000029200. [DOI] [PubMed] [Google Scholar]
- 18.Charron F, Paradis P, Bronchain O, Nemer G, Nemer M. Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression. Mol Cell Biol. 1999;19(6):4355–4365. doi: 10.1128/mcb.19.6.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Sedgwick T, Shi YB, Evans T. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol. 1998;18(5):2901–2911. doi: 10.1128/mcb.18.5.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heikinheimo M, Ermolaeva M, Bielinska M, Rahman NA, Narita N, Huhtaniemi IT, Tapanainen JS, Wilson DB. Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology. 1997;138(8):3505–3514. doi: 10.1210/endo.138.8.5350. [DOI] [PubMed] [Google Scholar]
- 21.Ketola I, Pentikainen V, Vaskivuo T, Ilvesmaki V, Herva R, Dunkel L, Tapanainen JS, Toppari J, Heikinheimo M. Expression of transcription factor GATA-4 during human testicular development and disease. J Clin Endocrinol Metab. 2000;85(10):3925–3931. doi: 10.1210/jcem.85.10.6900. [DOI] [PubMed] [Google Scholar]
- 22.Kiiveri S, Liu J, Westerholm-Ormio M, Narita N, Wilson DB, Voutilainen R, Heikinheimo M. Differential expression of GATA-4 and GATA-6 in fetal and adult mouse and human adrenal tissue. Endocrinology. 2002;143(8):3136–3143. doi: 10.1210/endo.143.8.8939. [DOI] [PubMed] [Google Scholar]
- 23.Jimenez P, Saner K, Mayhew B, Rainey WE. GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology. 2003;144(10):4285–4268. doi: 10.1210/en.2003-0472. [DOI] [PubMed] [Google Scholar]
- 24.Seely J, Amigh KS, Suzuki T, Mayhew B, Sasano H, Giguere V, Laganiere J, Carr BR, Rainey WE. Transcriptional regulation of dehydroepiandrosterone sulfotransferase (SULT2A1) by estrogen-related receptor alpha. Endocrinology. 2005;146(8):3605–3613. doi: 10.1210/en.2004-1619. [DOI] [PubMed] [Google Scholar]
- 25.Simard J, de Launoit Y, Labrie F. Characterization of the structure-activity relationships of rat types I and II 3 beta-hydroxysteroid dehydrogenase/delta 5 -delta 4 isomerase by site-directed mutagenesis and expression in HeLa cells. J Biol Chem. 1991;266(23):14842–14845. [PubMed] [Google Scholar]
- 26.Conley AJ, Bird IM. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56(4):789–799. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Yang L, Suwa T, Casson PR, Hornsby PJ. Differentially expressed genes in zona reticularis cells of the human adrenal cortex. Mol Cell Endocrinol. 2001;173(1–2):127–134. doi: 10.1016/s0303-7207(00)00404-4. [DOI] [PubMed] [Google Scholar]
- 28.Rainey WE, Carr BR, Wang ZN, Parker CR., Jr Gene profiling of human fetal and adult adrenals. J Endocrinol. 2001;171(2):209–215. doi: 10.1677/joe.0.1710209. [DOI] [PubMed] [Google Scholar]
- 29.Doody KM, Carr BR, Rainey WE, Byrd W, Murry BA, Strickler RC, Thomas JL, Mason JI. 3 beta-hydroxysteroid dehydrogenase/isomerase in the fetal zone and neocortex of the human fetal adrenal gland. Endocrinology. 1990;126(5):2487–2492. doi: 10.1210/endo-126-5-2487. [DOI] [PubMed] [Google Scholar]
- 30.Parker CR, Jr, Faye-Petersen O, Stankovic AK, Mason JI, Grizzle WE. Immunohistochemical evaluation of the cellular localization and ontogeny of 3 beta-hydroxysteroid dehydrogenase/delta 5–4 isomerase in the human fetal adrenal gland. Endocr Res. 1995;21(1–2):69–80. doi: 10.3109/07435809509030422. [DOI] [PubMed] [Google Scholar]
- 31.Narasaka T, Suzuki T, Moriya T, Sasano H. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Mol Cell Endocrinol. 2001;174(1–2):111–120. doi: 10.1016/s0303-7207(00)00445-7. [DOI] [PubMed] [Google Scholar]
- 32.Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1996;81(10):3558–3565. doi: 10.1210/jcem.81.10.8855801. [DOI] [PubMed] [Google Scholar]
- 33.Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97(2):161–163. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 34.Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20(5):689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- 35.Murphy EP, Conneely OM. Neuroendocrine regulation of the hypothalamic pituitary adrenal axis by the nurr1/nur77 subfamily of nuclear receptors. Mol Endocrinol. 1997;11(1):39–47. doi: 10.1210/mend.11.1.9874. [DOI] [PubMed] [Google Scholar]
- 36.Wilson TE, Mouw AR, Weaver CA, Milbrandt J, Parker KL. The orphan nuclear receptor NGFI-B regulates expression of the gene encoding steroid 21–hydroxylase. Mol Cell Biol. 1993;13(2):861–868. doi: 10.1128/mcb.13.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang SF, Chung BC. Difference in transcriptional activity of two homologous CYP21A genes. Mol Endocrinol. 1995;9(10):1330–1336. doi: 10.1210/mend.9.10.8544841. [DOI] [PubMed] [Google Scholar]
- 38.Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. 2004;18(2):279–290. doi: 10.1210/me.2003-0005. [DOI] [PubMed] [Google Scholar]
- 39.Bassett MH, Suzuki T, Sasano H, De Vries CJ, Jimenez PT, Carr BR, Rainey WE. The orphan nuclear receptor NGFIB regulates transcription of 3beta-hydroxysteroid dehydrogenase. implications for the control of adrenal functional zonation. J Biol Chem. 2004;279(36):37622–37630. doi: 10.1074/jbc.M405431200. [DOI] [PubMed] [Google Scholar]
- 40.Goto M, Piper HK, Marcos J, Wood PJ, Wright S, Postle AD, Cameron IT, Mason JI, Wilson DI, Hanley NA. In humans, early cortisol biosynthesis provides a mechanism to safeguard female sexual development. J Clin Invest. 2006;116(4):953–960. doi: 10.1172/JCI25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leers-Sucheta S, Morohashi K, Mason JI, Melner MH. Synergistic activation of the human type II 3β-hydroxysteroid dehydrogenase/delta 5 - delta 4 isomerase promoter by the transcription factor steroidogenic factor-1/adrenal 4-binding protein and phorbol ester. J Biol Chem. 1997;272(12):7960–7967. doi: 10.1074/jbc.272.12.7960. [DOI] [PubMed] [Google Scholar]
- 42.Martin LJ, Taniguchi H, Robert NM, Simard J, Tremblay JJ, Viger RS. GATA factors and the nuclear receptors, steroidogenic factor 1/liver receptor homolog 1, are key mutual partners in the regulation of the human 3beta-hydroxysteroid dehydrogenase type 2 promoter. Mol Endocrinol. 2005;19(9):2358–2370. doi: 10.1210/me.2004-0257. [DOI] [PubMed] [Google Scholar]
- 43.Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13(6):234–239. doi: 10.1016/s1043-2760(02)00609-4. [DOI] [PubMed] [Google Scholar]