Abstract
Mice with reduced expression of the NR1 subunit of the NMDA receptor (NR1 hypomorphic mice) display altered behavioral phenotypes that may relate to behavioral characteristics of schizophrenia. Altered phenotypes in the NR1 hypomorphs include marked deficits in species-typical behavioral interactions in tests of social aggression and social affiliation. To gain insight into neuroanatomical circuits disrupted by reduced NMDA receptor function, the present work compared regional brain activation in NR1 hypomorphic mice and their wild type controls after a resident-intruder test. Induction of Fos protein was used as an index of neuronal activation. Wild type mice exhibited robust induction of Fos in select brain regions, including specific nuclei of the hypothalamus and amygdala, lateral septum, and widespread regions of the cerebral cortex. Although the behavioral patterns were different for male and female mice, neuroanatomical patterns of Fos induction were remarkably similar for the two sexes. To determine socially specific components of Fos induction by the resident-intruder test, responses were compared for mice assessed in a test of general arousal and stress involving forced swim. Some common brain regions were activated by both tests but regionally specific differences were also found. The NR1 hypomorphic mice tested in the resident-intruder procedure displayed distinctly different behavioral interactions compared to the wild type mice and exhibited a significantly blunted Fos response in almost all brain regions. The mutant mice also exhibited reduced Fos in response to swim stress in specific brain regions. These data suggest that the NR1 hypomorphic mice have functional activation deficits in response to social challenge and swim stress.
Keywords: schizophrenia, animal model, NMDA, social interactions, cerebral cortex, amygdala
1. Introduction
NMDA antagonists produce a range of behavioral effects in humans that mimic positive, negative and cognitive symptoms of schizophrenia (Javitt and Zukin, 1991; Krystal et al., 1994; Lahti et al., 1995; Olney and Farber, 1995; Lahti et al., 2001). These findings suggest that endogenously reduced function of NMDA receptors could contribute to the pathophysiology of the illness. A mouse line that expresses low levels of the NR1 subunit of the NMDA receptor has been developed and provides a model of endogenous NMDA receptor hypofunction (Mohn et al., 1999). The mice are referred to as NR1 hypomorphic, since expression of the gene is reduced but not eliminated.
The NR1 hypomorphic mice (NR1neo/neo) exhibit a number of altered phenotypes that support their utility as a heuristic model for certain phenotypes of schizophrenia. Abnormal behaviors in The NR1neo/neo mice exhibit reduced locomotor habituation (Mohn et al., 1999), deficits in prepulse inhibition of acoustic startle (PPI) (Duncan et al., 2004; Fradley et al., 2005; Duncan et al., 2006), and increased sensitivity to amphetamine-induced disruption of PPI (Moy et al., 2006). The mutant mice have reduced physiological gating in a paired tone paradigm (Bickel et al., 2008) and impaired attentional modulation of auditory evoked potentials (Bickel et al., 2007). NR1neo/neo mice also show marked deficits in social behavior in tests of social affiliation and do not exhibit species-typical aggressive behavior in a resident-intruder paradigm (Mohn et al., 1999; Duncan et al., 2004).
The complete lack of normal social aggressive behavior of the NR1neo/neo mice in the resident intruder paradigm is in contrast to the hyper-aggressive responses of the mutants to human handling, hyperactivity in a novel environment, and normal performance and learning on a rotarod (Duncan et al., 2004). Thus, the behavioral responses of the NR1 mice in the resident-intruder test demonstrate fundamental deficits in a species-typical social behavior. The social dysfunction characteristic of schizophrenia can also be interpreted as severe deficits in species typical social behavior.
The functional circuitry of social-aggressive behavior has been investigated extensively by a variety of approaches (for reviews see (Nelson and Trainor, 2007); (Goodson, 2005). Immunocytochemical assessment of Fos has been especially valuable in this regard. Fos is induced in specific regions following the resident-intruder paradigm for rats (Veening et al., 2005; Veenema and Neumann, 2007), mice (Davis and Marler, 2004; Haller et al., 2006), and hamsters (Kollackwalker and Newman, 1995; Delville et al., 2000). Certain structures were shown to express Fos across these species in response to the resident-intruder tests, including the lateral septum, specific regions of the hypothalamus and amygdala, bed nucleus of the stria terminalis, and the medial preoptic area.
To provide insight into neuroanatomical circuits disrupted in the NR1neo/neo mice in responses to social-aggressive interactions, the present work investigated neuroanatomical activation patterns of Fos protein in the NR1+/+ and NR1neo/neo mice tested in the resident-intruder paradigm. To compare the neuroanatomical activation patterns in this test of social aggression with a non-social stress, Fos induction was also assessed in mice following a forced swim test.
2. Results
2.1.1 Social behavior in the resident-intruder test
In confirmation of previous work (Duncan et al. 2004), the NR1 hypomorphic mice had deficient aggressive or dominant responses, but retained investigatory sniffing directed toward the partner mouse (Table 1). Aggressive behavior, characterized by overt attacks with biting on the back or neck, was only observed in the male wild type mice. Two of the male NR1+/+ pairs had high levels of aggressive behavior (with an average of 11 attack bouts across a 10-min test), while the other two pairs in this group had no attack responses. Instead, the low-aggression pairs had 1–2 bouts of dominant mounting. All five of the female NR1+/+ pairs showed from 4–7 bouts of dominant mounting during the resident-intruder test. Overall, these results indicate that the male and female wild type groups had different behavioral phenotypes in response to a social challenge, and that both male and female NR1neo/neo mice failed to show species-typical aggressive or dominant responses.
Table 1.
Social behavior phenotype during the resident-intruder test. Data shown are mean number of bouts ± SEM for pairs of NR1+/+ or NR1neo/neo mice during a 15-min observation period.
| Malesa | Femalesb | |||
|---|---|---|---|---|
| NR1+/+ | NR1neo/neo | NR1+/+ | NR1neo/neo | |
| Aggressive responses | 5.5±3.2 | 0±0 | 0±0 | 0±0 |
| Dominant mounting | 0.8±0.5 | 0±0 | 5.4±0.6 | 0±0 |
| Investigatory sniffing | 15.8±1.3 | 10.8±0.9 | 14.0±2.9 | 17.5±1.8 |
Data taken from four pairs of each genotype.
Data taken from five pairs of NR1+/+ and four pairs of NR1neo/neo.
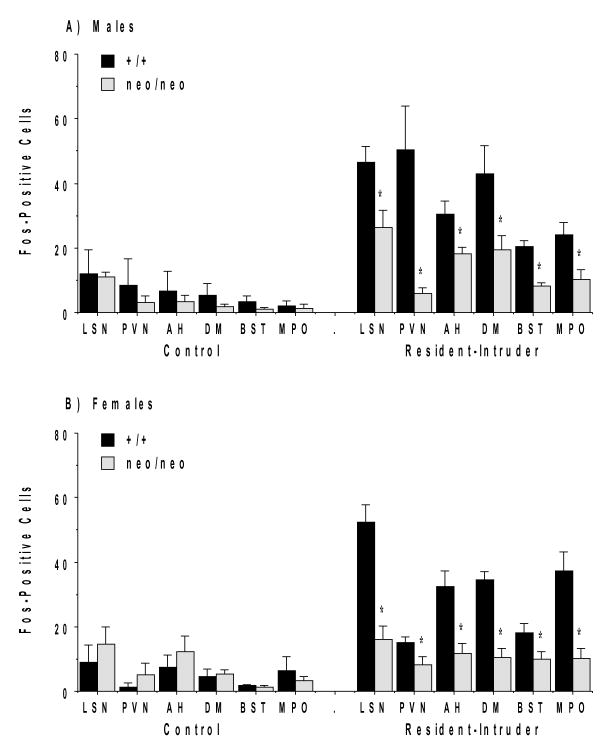
2.2 Reduced Fos induction in NR1neo/neo mice following the resident-intruder test
The exposure to an unfamiliar mouse in the resident-intruder test led to robust induction of Fos protein in select brain regions of the wild type mice. Significant deficits in Fos expression were observed in the NR1 hypomorphic mice for most of these regions after the social challenge. Surprisingly, given the difference in behavioral phenotype in males and females, the pattern of Fos induction was similar across the two sexes. The overall 3-way ANOVAs did not indicate significant effects of sex in any of the regions examined. The main effect of test condition (either control or resident-intruder) in the 3-way ANOVAs was significant for region of interest examined, except the jaw region of the somatosensory cortex.
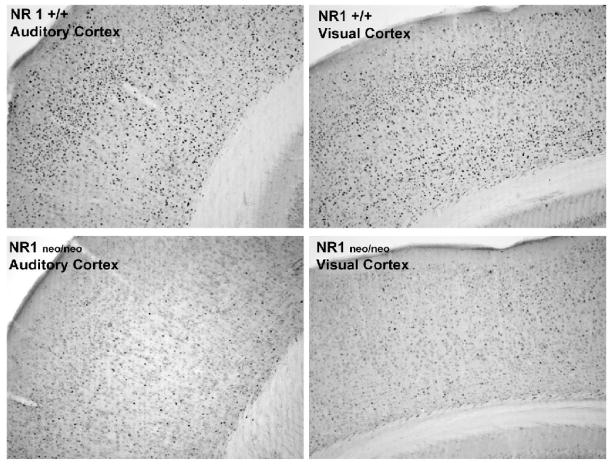
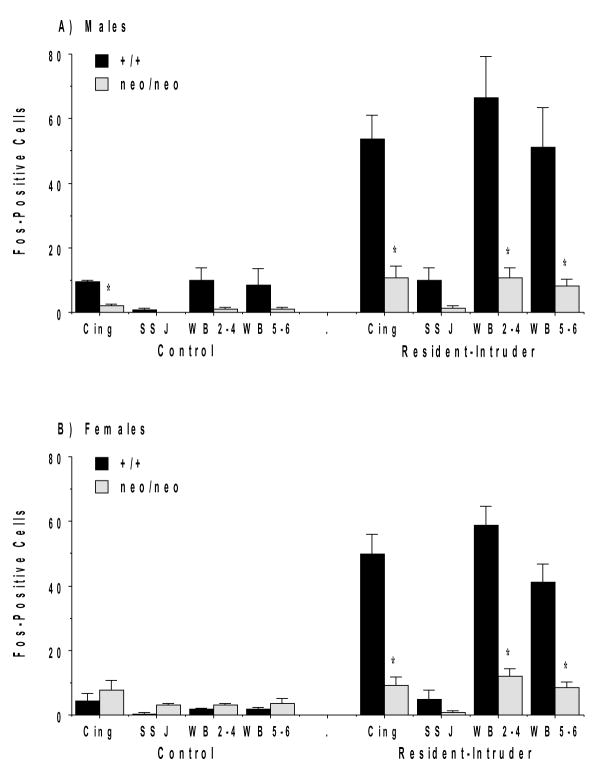
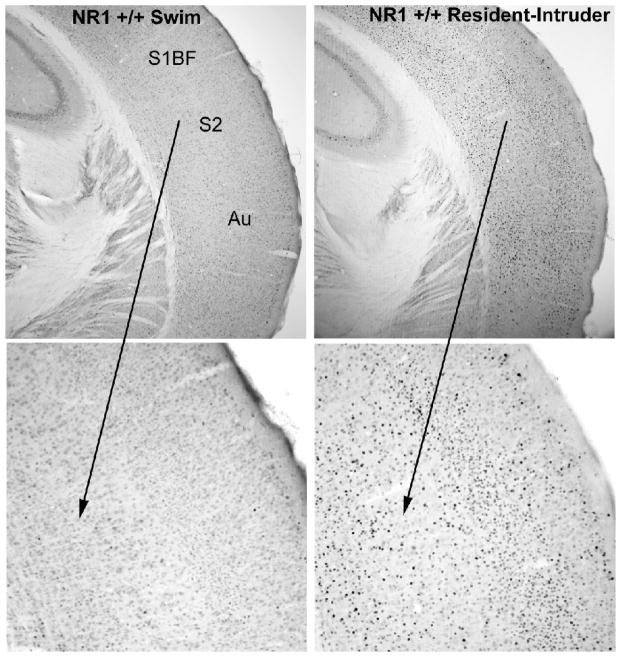
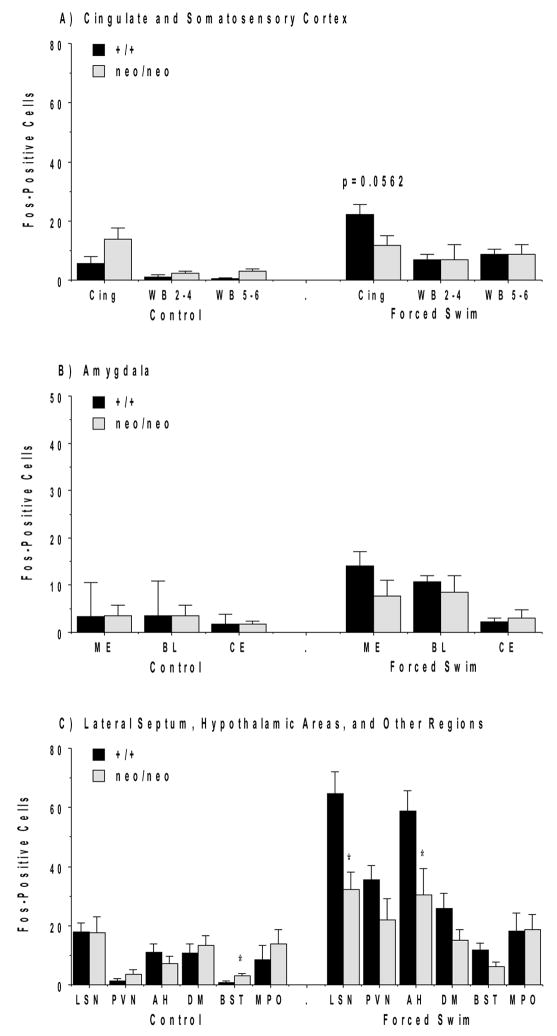
The social challenge led to intensely stained cells in widespread sensory cortical regions, with clear reductions in the mutant mice (Figure 1). Quantitative analysis of Fos-positive cells demonstrated that the NR1neo/neo mice had significantly decreased Fos responses in limbic (cingulate) and sensory (whisker barrel) cortical regions (Figure 2). Significant effects were not observed in the jaw region of the somatosensory cortex (SS J in Figure 2).
Figure 1.
Photomicrographs of Fos induction in the auditory (left panels) and visual cortex (right panels) in NR1+/+ and NR1neo/neo mice following the resident-intruder test.
Figure 2.
Counts of Fos-positive cells in male (A) and female (B) NR1+/+ and NR1neo/neo mice following the resident-intruder test in cerebral cortical regions. For this figure and other figures showing for Fos cell counts, data are means + SEM, with N = 3 mice per control group, and 8–10 mice per social challenge group. Data for some brain regions were not available for one male NR1+/+ mouse in the resident-intruder test group, due to experimenter error. Abbreviations: Cing, cingulate cortex; SS J, jaw region of the somatosensory cortex layers 5 and 6; WB 2–4, whisker barrel region of somatosensory cortex layers 2–4; WB 5–6, whisker barrel region of somatosensory cortex layers 5 and 6. *p<0.05.
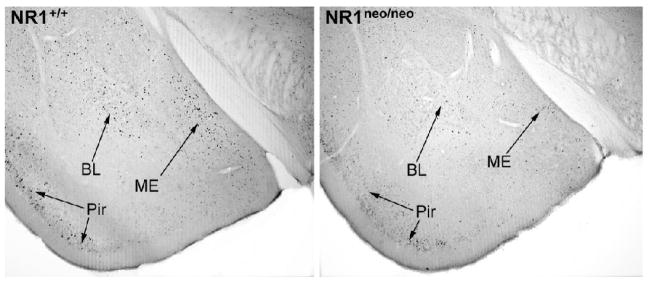
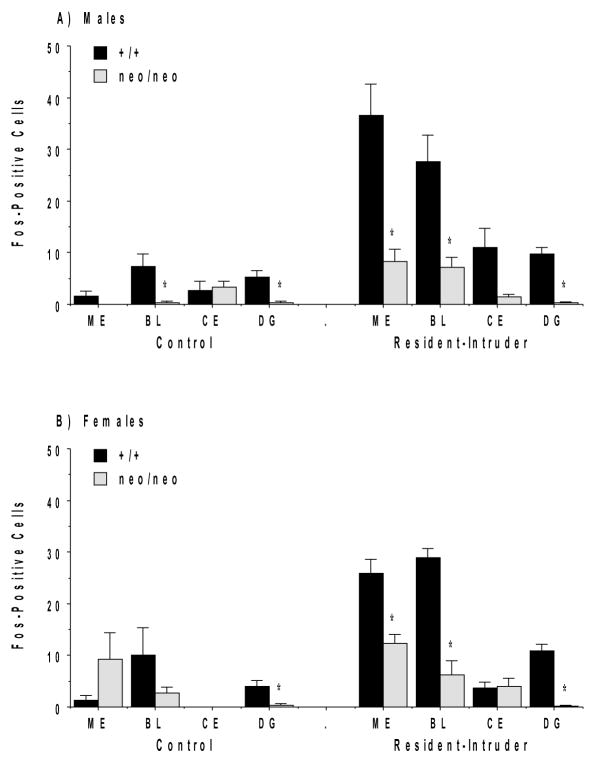
Reduced Fos induction in the NR1 mice compared to wild type mice was also observed in specific nuclei of the amygdala (Figure 3). Quantification of Fos-positive neurons demonstrated that the NR1 mutant mice had significantly less Fos induction in the medial and basolateral nuclei of the amygdala and in the dentate gyrus.
Figure 3.
Photomicrographs of Fos induction in the amygdala in NR1+/+ and NR1neo/neo mice following the resident-intruder paradigm. BL, basolateral nucleus; ME, medial nucleus; Pir, piriform cortex.
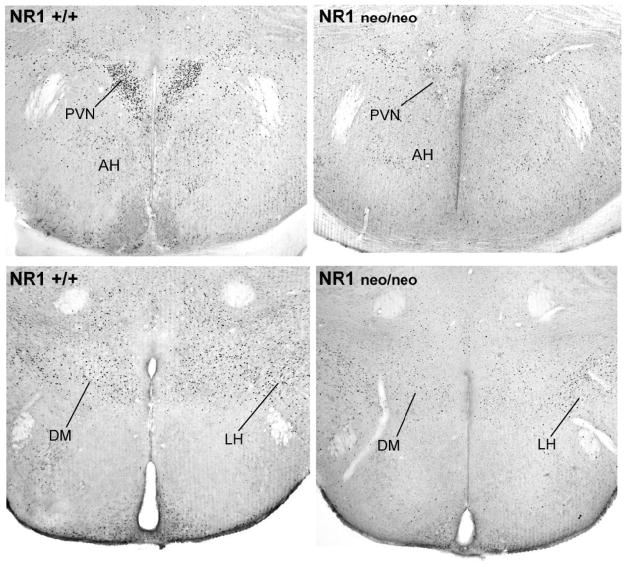
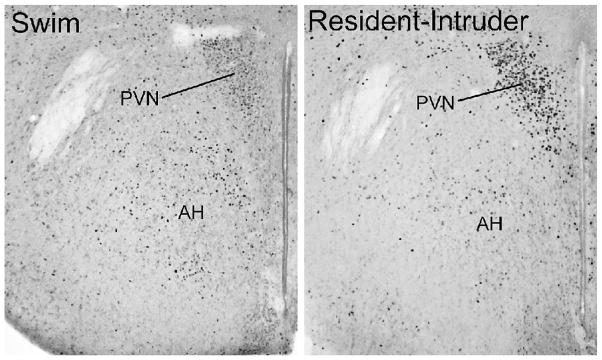
Other regions where the social challenge induced robust induction of Fos in NR1+/+ mice included specific hypothalamic regions and the lateral septum. Exposure to the social stimulus led to robust Fos expression in the magnocellular paraventricular (PVN) and dorsomedial nuclei of the hypothalamus (Figure 5). Quantification of the Fos-positive cells showed that the mutant group had consistently lower induction of Fos in all areas of the hypothalamus, medial preoptic area, and lateral septal nucleus (LSN), in both males and females (Figure 6A and 6B). 3-way ANOVAs indicated significant main effects of genotype, as well as interactions between genotype and test condition (control or resident-intruder), for each of the regions (Table 2).
Figure 5.
Photomicrographs of Fos induction in the hypothalamus in NR1+/+ and NR1neo/neo mice following the resident-intruder test. Two different levels of the hypothalamus (approximately 0.9 mm and 1.5 mm caudal to bregma) are shown respectively in the top and bottom panels. PVN, magnocellular paraventricular nucleus of the hypothalamus; AH, anterior hypothalamic area; DM, dorsomedial nucleus; LH, lateral hypothalamic area.
Figure 6.
Fos induction in the lateral septum, nuclei of the hypothalamus, and other regions in NR1+/+ and NR1neo/neo mice following the resident-intruder test. Abbreviations: LSN, lateral septal nucleus; PVN, magnocellular paraventricular nucleus of the hypothalamus; AH, anterior hypothalamic area; DM, dorsomedial nucleus; BST, bed nucleus of the stria terminalis; MPO, medial preoptic area.
Table 2.
F values for the statistical analysis of genotype main effects and genotype × test condition interaction effects on Fos expression in different regions of brain.
| Regiona | df | Genotype | Genotype × Test Conditionb |
|---|---|---|---|
| __ | |||
| Cing | 1,38 | 21.67, p<0.0001 | 17.9, p=0.0001 |
| WB 2–4 | 1,37 | 22.17, p<0.0001 | 16.42, p=0.0003 |
| WB 5–6 | 1,37 | 13.22, p=0.0008 | 9.96, p=0.0032 |
| ME | 1,37 | 8.83, p=0.0052 | 16.25, p=0.0003 |
| BL | 1,37 | 29.45, p<0.0001 | 7.39, p=0.0099 |
| DG | 1,37 | 76.02, p<0.0001 | 12.18, p=0.0013 |
| LSN | 1,38 | 8.09, p=0.0071 | 11.24, p=0.0018 |
| PVN | 1,38 | 5.0, p=0.0313 | 4.39, p=0.0428 |
| AH | 1,38 | 4.72, p=0.0362 | 5.78, p=0.0212 |
| DM | 1,37 | 9.76, p=0.0035 | 7.59, p=0.0091 |
| BST | 1,38 | 8.43, p=0.0061 | 5.0, p=0.0313 |
| MPO | 1,38 | 7.71, p=0.0085 | 5.39, p=0.0258 |
2.2.1 Behavioral variation and Fos induction in male NR1+/+ mice in response to social challenge
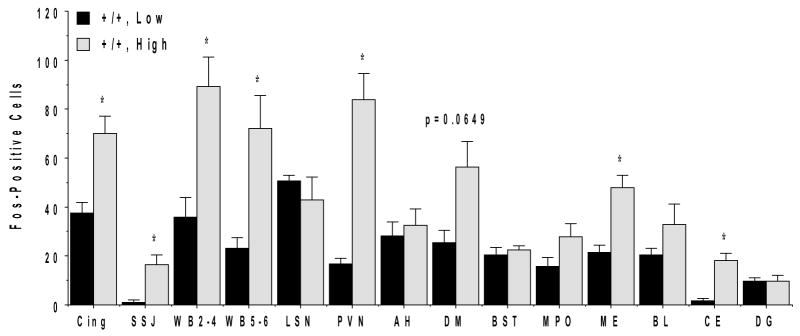
As noted above, the male wild type mice showed substantial variability in the behavioral response to the social challenge. Two of the four pairs tested exhibited high levels of aggressive responses, while the other two pairs had low levels of aggression, with no attack behavior involving biting. In order to determine whether reactivity to the social challenge affected Fos induction, comparisons were made between the low and high aggression male NR1+/+ mice (Figure 7). A repeated measures ANOVA confirmed a significant effect of level of aggression on Fos across brain regions [main effect of phenotype, F(1,5)=65.07, p=0.0005, and phenotype × region interaction, F(9,45)=3.83, p=0.0012]. The induction of Fos was significantly greater in the high-aggression compared to the low-aggression males in some regions, including limbic and sensory cortical structures, medial amygdala, and the paraventricular nucleus of the hypothalamus. In other regions, such as the lateral septum and anterior hypothalamic area, the two groups showed equivalent Fos induction. Levels of Fos were particularly reduced in the PVN of the low-aggression mice, suggesting that neuronal activation in this region is dependent on overt aggression during the social encounter. Although the study was not sufficiently powered to detect subtle differences in Fos induction between resident and intruder subjects, there were no obvious differences between the resident and intruder mice. The significant blunting of Fos expression in the NR1-deficient males when compared to the entire set of wild type males was still clearly apparent even when compared only to the low-aggression wild type males [repeated measures ANOVA, main effect of genotype, F(1,9)=17.87, p=0.0022, and genotype × brain region interaction, F(13,117)=4.0, p<0.0001].
Figure 7.
Cell counts of Fos induction in male NR1+/+ mice that had either low or high levels of aggressive responses in the resident-intruder test. Data are means + SEM for 4 low-aggression and 4 high-aggression mice. See Figures 2, 4, and 6 for abbreviations. *p<0.05. Repeated measures ANOVA confirmed a significant effect of level of aggression on Fos across brain regions [main effect of phenotype, F(1,5)=54.92, p=0.0007, and phenotype × region interaction, F(13,65)=4.68, p<0.0001].
2.3.1 Behavior in the forced swim test
A separate set of mice was tested in the forced swim test, a non-social stress challenge, to determine the potential role of general arousal and motor activation in Fos induced by the resident-intruder test. All mice exhibited vigorous swimming behavior, alternated by periods of floating, during the 1st 5–6 min of the 10 min test. During the last 4 min of the test the swimming behavior was less vigorous and more floating with minimal movements was observed for both genotypes. Immobility times (mean ± SEM) measured during the last 4 min of the test were 130 ± 33 sec for the NR1+/+ and 101 ± 32 sec for the NR1neo/neo mice.
2.3.2 Induction of Fos in the forced swim test
The forced swim test induced robust Fos staining in some, but not all, of the same regions where Fos induction was observed for the resident-intruder test, including the lateral septum, PVN, and anterior hypothalamic area (Figure 7). As in the resident-intruder test, very little Fos was observed after swim stress in ventral hypothalamic regions. In contrast to the results with the social challenge, significant deficits in Fos induction were observed in the mutant mice for only two brain regions, the lateral septum [main effect of genotype, F(1,18)=6.88, p=0.0172, and genotype × test condition (control or swim stress) interaction, F(1,18)=6.81, p=0.0177] and anterior hypothalamus [main effect of genotype, F(1,18)=6.2, p=0.0228] (Figure 6C).
Certain regions where Fos was markedly induced in the resident-intruder test showed minimal Fos induction in response to swim stress. The most striking differences in activation patterns for the two tests were in cerebral cortical regions and amygdala. In those regions Fos expression was substantially less in mice exposed to swim stress in comparison to those following a social challenge (Figures 8 and 9).
Figure 8.
Photomicrographs of Fos induction in the hypothalamus in NR1+/+ mice following the forced swim test (left) and resident-intruder test (right). See Figure 5 for abbreviations.
Figure 9.
Photomicrographs of Fos induction in the neocortex in NR1+/+ mice following the forced swim test and resident-intruder test. S1BF, primary sensory cortex, barrel field. S2, secondary somatosensory cortex; Au, auditory cortex. The bottom panels show the indicated region in the top panels at higher magnification.
3. Discussion
The NR1 hypomorphic mouse represents a developmental model of endogenous NMDA receptor hypofunction. Behavioral phenotypes of the model (see Introduction) are consistent with the hypothesis that behavioral phenotypes of schizophrenia could relate to reduced NMDA receptor function. Although the lack of social aggression in the mutant mice is not directly analogous to social dysfunction in schizophrenia, the NR1 hypomorphic mice clearly exhibit marked deficits in fundamental species-typical social behavior. As such, the model could have heuristic value for understanding the neurobiology of social interaction deficits in schizophrenia, which could also be construed as deficits in species-typical social behavior.
Induction of Fos associated with behavioral activation in the resident intruder paradigm was significantly blunted in almost all brain regions assessed in the NR1neo/neo compared to the NR1+/+ mice. Wild type male mice evaluated in the resident-intruder test showed a robust and consistent pattern of Fos induction. Some of the most striking expression of Fos in the NR1+/+ mice was in neocortical regions. The widespread neocortical activation suggests that the resident-intruder test engages high level processing of the environmental stimuli. Within the wild type group, induction of Fos in most brain regions was substantially greater in mice that exhibited robust aggressive behavior, compared to the sub-set showing less aggressive interactions. However, with the exceptions of the PVN and central nucleus of the amygdala, the specific brain regions where Fos was induced were almost identical in the high and low aggression mice. These data suggest that the regions activated are related to circuits involved in the social interactions and not just the stress of fighting.
During the resident-intruder test, the NR1neo/neo mice of both sexes engaged in active social sniffing even though no fighting or mounting behavior was observed. Reduced induction of Fos in the NR1neo/neo mice was found even when compared to the low-aggression NR1+/+ sub-group. These data indicate that the NR1neo/neo mice have attenuated brain activation responses to the social stimuli.
The NR1+/+ female mice assessed in the resident-intruder test engaged in dominant mounting behavior accompanied by rapid male-like pelvic thrusting. This behavior was observed in each of the 5 pair of NR1+/+ females tested. The aggressive mounting behavior displayed by the NR1+/+ mice was not observed in the female NR1neo/neo mice. The neuroanatomical patterns of Fos induction were almost identical in the NR1+/+ female and NR1+/+ male mice following the resident-intruder test. As found for the male mutants, Fos induction was blunted in all brain regions assessed in the female NR1neo/neo mice. These findings indicated that adequate NMDA receptor function is required for the species-typical behavioral responses and brain activation in the resident-intruder test.
Although the NR1neo/neo mice showed marked deficits in social-aggressive behavior, the mice did not have deficits in motor function during the forced swim test. Other reports have noted increased locomotor activity and reduced habituation in a novel environment, and normal performance on a rotarod in NR1 mutant mice (Duncan et al. 2004; Mohn et al. 1999). Therefore, the behavioral deficits observed in the resident-intruder test are likely due to disruption in circuits involved the species-typical social-aggressive behavior.
There is inherent general arousal and stress associated with the resident-intruder test. Interpreting induction of Fos in specific brain regions with regard to involvement in social behavior is complicated by the fact that a variety of stressors induce Fos. We therefore examined neuroanatomical patterns of Fos induction in a behavioral test that produces arousal and stress, the forced swim test. Although the forced swim test induced a robust Fos induction in the lateral septum and hypothalamic regions, responses in cerebral cortical regions and the amygdala were much less than seen following the social challenge.
The minimal Fos induction in neocortical regions and amygdala in response to swim stress indicates the robust activation observed in the resident-intruder test was not simply a stress response. In comparison to NR1+/+ mice, NR1neo/neo mice displayed significantly reduced Fos response in lateral septum and anterior hypothalamic area following the forced swim. These data suggest that NMDA receptor activation is involved in the stress-induced Fos in those regions.
Regionally selective deficits in Fos induction have been observed in NR1 mutant in response to other types of challenges. For example, the Fos response to amphetamine in the NR1neo/neo mice was reduced in cingulate cortex, basolateral amygdala, and lateral septum (Miyamoto et al., 2004). However, amphetamine-induced Fos was not different in the caudate and accumbens in NR1neo/neo compared to NR1+/+ mice (Miyamoto et al., 2004; Ramsey et al., 2008). Fos responses to the direct NMDA agonist tetrazol-5yl glycine were markedly reduced in hippocampus, cingulate cortex, and amygdala in the NR1 hypomorphic mice (Inada et al., 2007; Duncan et al., 2008). Surprisingly, induction of Fos in the NR1+/+and NR1neo/neo mice by the NMDA agonist was similar in a number of subcortical brain regions, including lateral septum, nucleus accumbens, bed nucleus of the stria terminalis, ventromedial hypothalamus, arcuate nucleus of the hypothalamus, and nucleus of the solitary tract (Duncan et al., 2008). Furthermore, there was no difference between the wild type and mutant animals in seizure sensitivity to tetrazol-5yl glycine. These data suggest that the reduced NMDA receptor function in the NR1neo/neo mice is not neuroanatomically global.
Following the social challenge, NR1neo/neo mice had significant deficits in Fos responses in the lateral septum and the bed nucleus of the stria terminalis. However, no deficits in Fos induction were observed in these regions following tetrazol-5yl glycine treatment (Duncan et al., 2008). It is therefore possible that some of the activation deficits in these regions in mice tested in the resident-intruder procedure are not directly related to reduced NMDA receptor sensitivity in those regions.
A limitation of the present study is that assessment of Fos induction provides a restricted window into neural activation and lack of induction of the protein cannot be interpreted as a lack of functional change. It is possible that some of the neurons in brain regions that showed reduced Fos induction in the NR1neo/neo mice did exhibit increased activity that was not reflected by induction of the protein. Indeed, specific hippocampal and thalamic regions that exhibited marked increases in 14C-2-deoxyglucose uptake in response to ketamine did not show any induction of Fos (Duncan et al., 1998).
Whether the deficits in social-aggressive behavior and Fos induction in the mutant mice result from ineffective developmental of species-typical neural circuits or result directly from reduced NMDA receptor function in adults remains to be established. NMDA receptors are known to provide developmental signals for normal brain maturation (Rudhard et al., 2003; Adams et al., 2004; Lee et al., 2005). If the social deficits of the NR1neo/neo mice result from altered development associated with NMDA receptor hypofunction, the NR1 hypomorphic mice could represent a model system to explore pharmacologic strategies to prevent the emergence of behavioral phenotypes relevant to schizophrenia.
4. Experimental Procedure
4.1 Animals
NR1neo/neo mice were generated by incorporating a neomysin resistance gene (neo) into an intron 20 of the NR1 (Grin1) locus as described in detail (Mohn et al., 1999). The initial mutation was inserted in mice on a mixed genetic background consisting of alleles derived from 129/SvEv, C57BL/6, and DBA/2 (Mohn et al., 1999). This insertion mutation greatly reduced expression of the NR1 gene in all brain regions thus far examined. To obtain mice that differ genetically only at the NR1 locus, a strategy was devised to generate NR1 hypomorphic mice and genetically identical wild type populations (Duncan et al., 2004). C57BL/6 heterozygous animals (NR1+/neo) were intercrossed with 129S6 heterozygous animals. All of the F1 offspring of these litters are genetically identical at all loci except at the NR1 gene. The 129S6 (Taconic) mice were used as breeding females and were co-isogenic. C57BL/6 mice were backcrossed > 14 generations from C57BL/6J mice purchased from Jackson Laboratories. The NR1 mutation was maintained on the C57BL/6J and 129S6 genetic backgrounds by breeding heterozygous animals. Resulting heterozygous offspring from these crosses were used to maintain the lines and to provide heterozygous breeders for the generation of the F1 hybrid homozygous mice (NR1neo/neo) and their control populations (NR1+/+). Both male and female mice 7–8 months of age were used in these studies.
4.2 Resident-Intruder Paradigm
Wild type and NR1neo/neo mice of both sexes were assessed in a resident-intruder paradigm. Mice were housed individually for 7–8 days before an unfamiliar intruder mouse of the same sex and genotype was introduced to the resident’s home cage. Behavior was monitored for the first fifteen minutes after introduction of the intruder for the presence of aggressive responses (overt biting attacks), dominant mounting, and investigatory sniffing (sniffing directed toward the partner), as previously described (Duncan et al. 2004). The pairs of mice remained together for a total period of 2 hours, and were then anesthetized and perfused as described below. Control mice remained in their home cages before perfusion.
4.3 Forced Swim Procedure
Mice were placed in a 4 L Pyrex beaker (No. 1000) containing 2500 ml of tap water at 25°C for a period of 10 min. The time that the mice were floating and immobile was scored during the last 4 min of the test.
4.4 Immunohistochemistry
Immunocytochemical procedures were performed according to the previously published protocols (Duncan et al., 1993; Miyamoto et al., 2004). Mice were anesthetized with chloral hydrate (400 mg/kg, i.p.), then perfused through the left cardiac ventricle with ice-cold 100mM sodium phosphate-buffered saline (PBS, pH=7.4) for 1 min, at a rate of 3ml/min, followed by 4% paraformaldehyde for 3 min at the same rate of perfusion. The brains were removed and fixed with 4% paraformaldehyde overnight. Coronal sections (50 μm) of the forebrain were cut with a vibratome and placed in PBS. Sections were treated with 5% normal goat serum [Vector Laboratories, Burlingame, CA] and 0.1% Triton X-100 in PBS for 30 min. and then were incubated for 72 hours at 4 degrees C with a Fos antibody [gift from Dr. Peter Petruz]. After incubation with the Fos antibody, sections were incubated for 1h with biotinylated anti-rabbit IgG [Vector Laboratories, Burlingame, CA]. Sections were then incubated with avidin-biotin complex [Vecstain Elite ABC kit; Vector Laboratories] for 1h. Sections were then placed in a solution containing 0.05% 3,3′-diamino-benzidene tetra-hydrochloride, 0.005% cobalt chloride, 0.008% nickel ammonium sulfate, and 0.02% hydrogen peroxide.
4.5 Quantification of Fos expression
Cells exhibiting nuclear staining for Fos in selected brain regions were counted at a magnification of 200x by an experimenter blind to the treatment group. The location of the areas used within each brain region for assessing Fos cells was guided by the atlas of (Franklin and Paxinos, 1997). Darkly stained cells in defined areas of varying sizes of an eyepiece grid were counted depending on the size of the regions of interest. The brain regions assessed and grid areas measured (in microns) were: lateral septal nucleus (250 × 500), cingulate cortex (250 × 500), jaw region of the somatosensory cortex layers 5 and 6 (500 × 350), whisker barrel region of somatosensory cortex layers 2–4 (500 × 350), whisker barrel region of somatosensory cortex layers 5 and 6 (500 × 350), medial nucleus of amygdala (250 × 500), dorsomedial nucleus of hypothalamus (250 × 500), granule cell layer of dentate gyrus (50 × 500), anterior hypothalamic area (400 × 400), bed nucleus of the stria terminalis (200 × 400), medial preoptic area (300 × 500). The entire nuclear areas were measured for the basolateral nucleus of amygdala, central nucleus of amygdala, and magnocellular paraventricular nucleus of hypothalamus.
4.6 Statistical Analyses
Data for each brain region were separately analyzed using 2-way or 3-way Analysis of Variance (ANOVA), with the factors genotype, sex (only for the resident-intruder test), and test condition. For the resident-intruder experiment, separate ANOVAs were then conducted for the male and female mice, to determine the effects of genotype and test condition within each sex. A repeated measures ANOVA was conducted on the data from the wild type male mice, with the factors behavioral phenotype (low or high aggression) and brain region (the repeated measure). Fishers Protected Least Significant Difference (PLSD) tests were conducted between group means only when a significant F value was found in the 2-way, 3-way, or repeated measures ANOVA. For all comparisons, significance was set at p<0.05.
Figure 4.
Counts of Fos-positive cells in the amygdala and dentate gyrus of NR1+/+ and NR1neo/neo mice following the resident-intruder paradigm. Abbreviations: ME, medial nucleus; BL, basolateral nucleus; CE, central nucleus; and DG, dentate gyrus of the hippocampal formation. *p<0.05.
Figure 10.
Cell counts of Fos induction following the forced swim test for NR1+/+ and NR1neo/neo mice. Data are means + SEM. See Figures 2, 4, and 6 for abbreviations. P value is result from a post-hoc test following a significant genotype × test condition interaction in a 2-way ANOVA [F(1,18)=7.93, p=0.0114].
Acknowledgments
This research was supported by MH063398, MH080069, and HD03110 from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams SM, Vaccari JCD, Corriveau RA. Pronounced cell death in the absence of NMDA receptors in the developing somatosensory thalamus. Journal of Neuroscience. 2004;24:9441–9450. doi: 10.1523/JNEUROSCI.3290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S, Lipp HP, Umbricht D. Impaired attentional modulation of auditory evoked potentials in N-methyl-D-aspartate NR1 hypomorphic mice. Genes Brain and Behavior. 2007;6:558–568. doi: 10.1111/j.1601-183X.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- Bickel S, Lipp HP, Umbricht D. Early auditory sensory processing deficits in mouse mutants with reduced NMDA receptor function. Neuropsychopharmacology. 2008;33:1680–1689. doi: 10.1038/sj.npp.1301536. [DOI] [PubMed] [Google Scholar]
- Davis ES, Marler CA. C-fos changes following an aggressive encounter in female California mice: A synthesis of behavior, hormone changes and neural activity. Neuroscience. 2004;127:611–624. doi: 10.1016/j.neuroscience.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behavior and Evolution. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Inada K, Farrington JS, Koller BH. Seizure responses and induction of Fos by the NMDA Agonist (tetrazol-5-yl)glycine in a genetic model of NMDA receptor hypofunction. Brain Research. 2008;1221:41–48. doi: 10.1016/j.brainres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Johnson KB, Breese GRT. Topographic patterns of brain activity in response to swim stress: Assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. J Neuroscience. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Knapp DJ, Mueller RA, Breese GR. Metabolic mapping of the rat brain after subanesthetic doses of ketamine: potential relevance to schizophrenia. Brain Research. 1998;787:181–190. doi: 10.1016/s0006-8993(97)01390-5. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH. Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology. 2006;184:190–200. doi: 10.1007/s00213-005-0214-1. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Fradley RL, O’Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behavioural Brain Research. 2005;163:257–264. doi: 10.1016/j.bbr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1997. [Google Scholar]
- Goodson JL. The vertebrate social behavior network: Evolutionary themes and variations. Hormones and Behavior. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Toth M, Halasz J, De Boer SF. Patterns of violent aggression-induced brain c-fos expression in male mice selected for aggressiveness. Physiology & Behavior. 2006;88:173–182. doi: 10.1016/j.physbeh.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Inada K, Farrington JS, Moy SS, Koller BH, Duncan GE. Assessment of NMDA receptor activation in vivo by Fos induction after challenge with the direct NMDA agonist (tetrazol-5-yl)glycine: effects of clozapine and haloperidol. Journal of Neural Transmission. 2007;114:899–908. doi: 10.1007/s00702-007-0628-5. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. American Journal of Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Kollackwalker S, Newman SW. Mating and Agonistic Behavior Produce Different Patterns of Fos Immunolabeling in the Male Syrian-Hamster Brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara MB, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Lee LJ, Lo FS, Erzurumlu RS. NMDA receptor-dependent regulation of axonal and dendritic branching. Journal of Neuroscience. 2005;25:2304–2311. doi: 10.1523/JNEUROSCI.4902-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Snouwaert JN, Koller BH, Moy SS, Lieberman JA, Duncan GE. Amphetamine-induced Fos is reduced in limbic cortical regions but not in the caudate or accumbens in a genetic model of NMDA receptor hypofunction. Neuropsychopharmacology. 2004;29:2180–2188. doi: 10.1038/sj.npp.1300548. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Moy SS, Perez A, Koller BH, Duncan GE. Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain Research. 2006;1089:186–194. doi: 10.1016/j.brainres.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nature Reviews Neuroscience. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Archives of General Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Laakso A, Cyr M, Sotnikova TD, Salahpour A, Medvedev IO, Dykstra LA, Gainetdinov RR, Caron MG. Genetic NMDA receptor deficiency disrupts acute and chronic effects of cocaine but not amphetamine. Neuropsychopharmacology. 2008;33:2701–2714. doi: 10.1038/sj.npp.1301663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudhard Y, Kneussel M, Nassar MA, Rast GF, Annala AJ, Chen PE, Tigaret CM, Dean I, Roes J, Gibb AJ, Hunt SP, Schoepfer R. Absence of whisker-related pattern formation in mice with NMDA receptors lacking coincidence detection properties and calcium signaling. Journal of Neuroscience. 2003;23:2323–2332. doi: 10.1523/JNEUROSCI.23-06-02323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Neurobiological mechanisms of aggression and stress coping: A comparative study in mouse and rat selection lines. Brain Behavior and Evolution. 2007;70:274–285. doi: 10.1159/000105491. [DOI] [PubMed] [Google Scholar]
- Veening JG, Coolen LM, de Jong TR, Joosten HW, de Boer SF, Koolhaas JN, Olivier B. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. European Journal of Pharmacology. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]